Combining Dipole and Loop Coil Elements for 7 T Magnetic Resonance Studies of the Human Calf Muscle
Abstract
1. Introduction
2. Materials and Methods
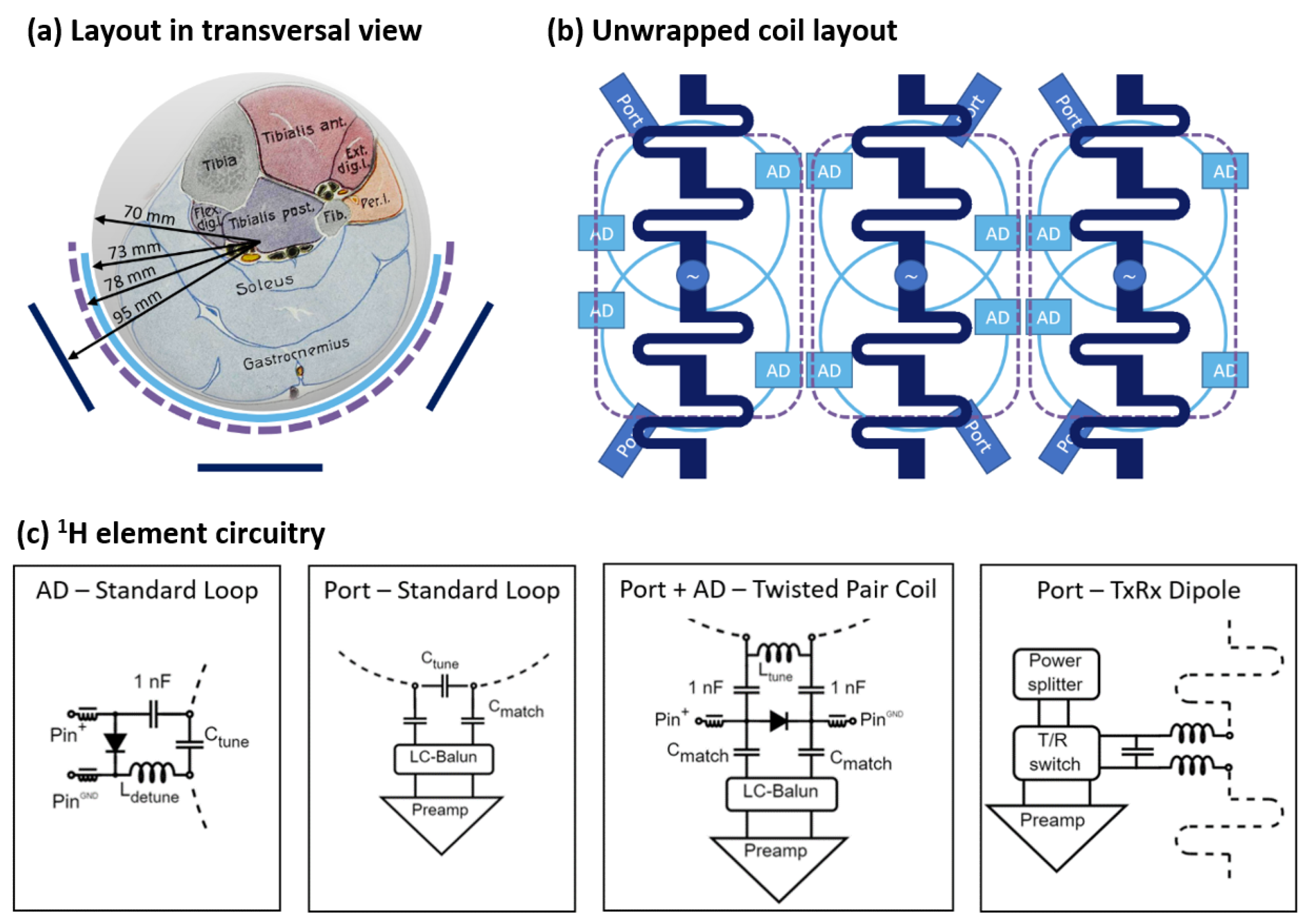
2.1. Coil Design and Implementation
2.2. Bench Measurements
2.3. MR Measurements
2.4. Electromagnetic Simulations
3. Results and Discussion
3.1. Bench Measurements
3.2. Transmit Performance
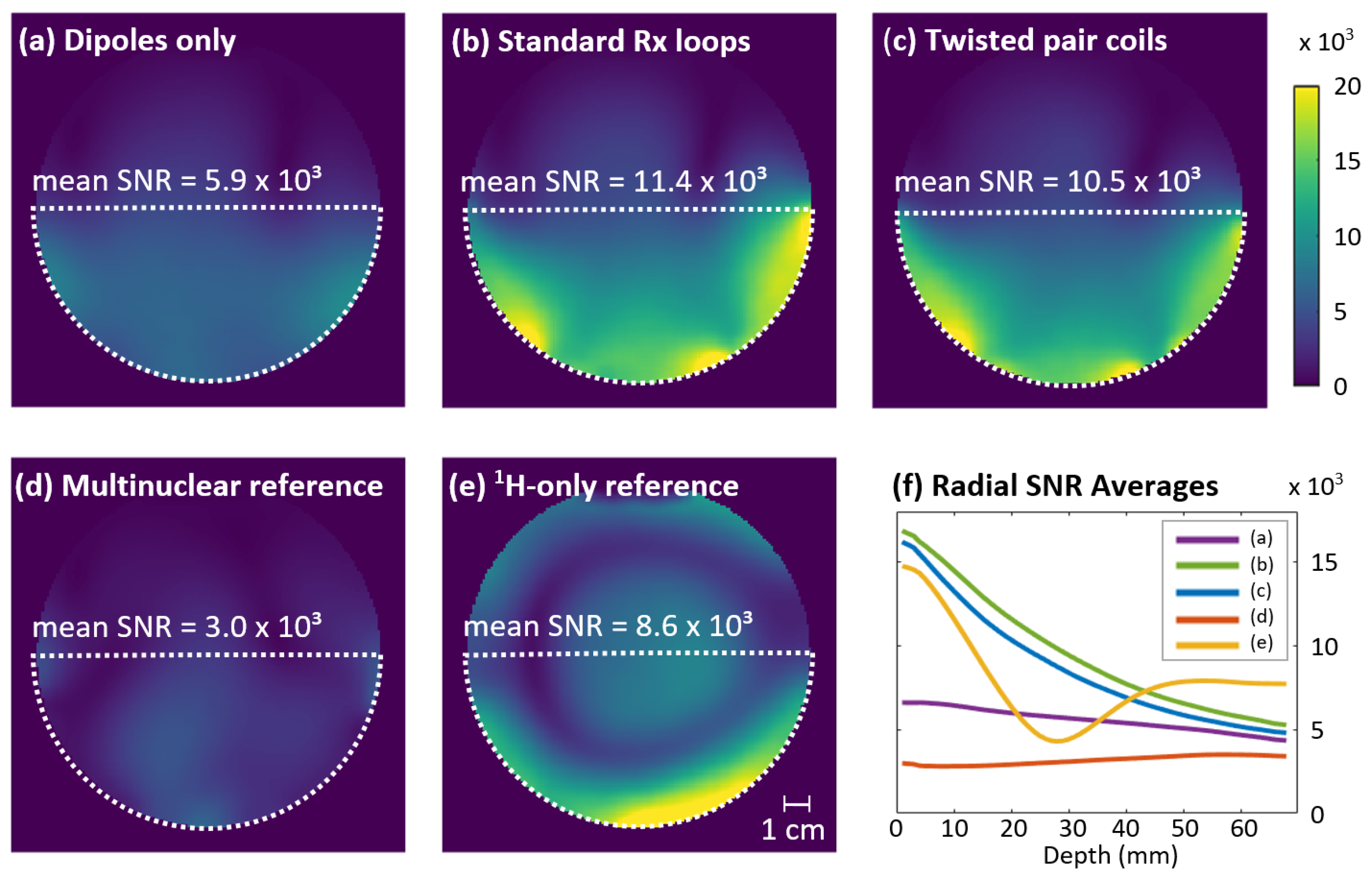
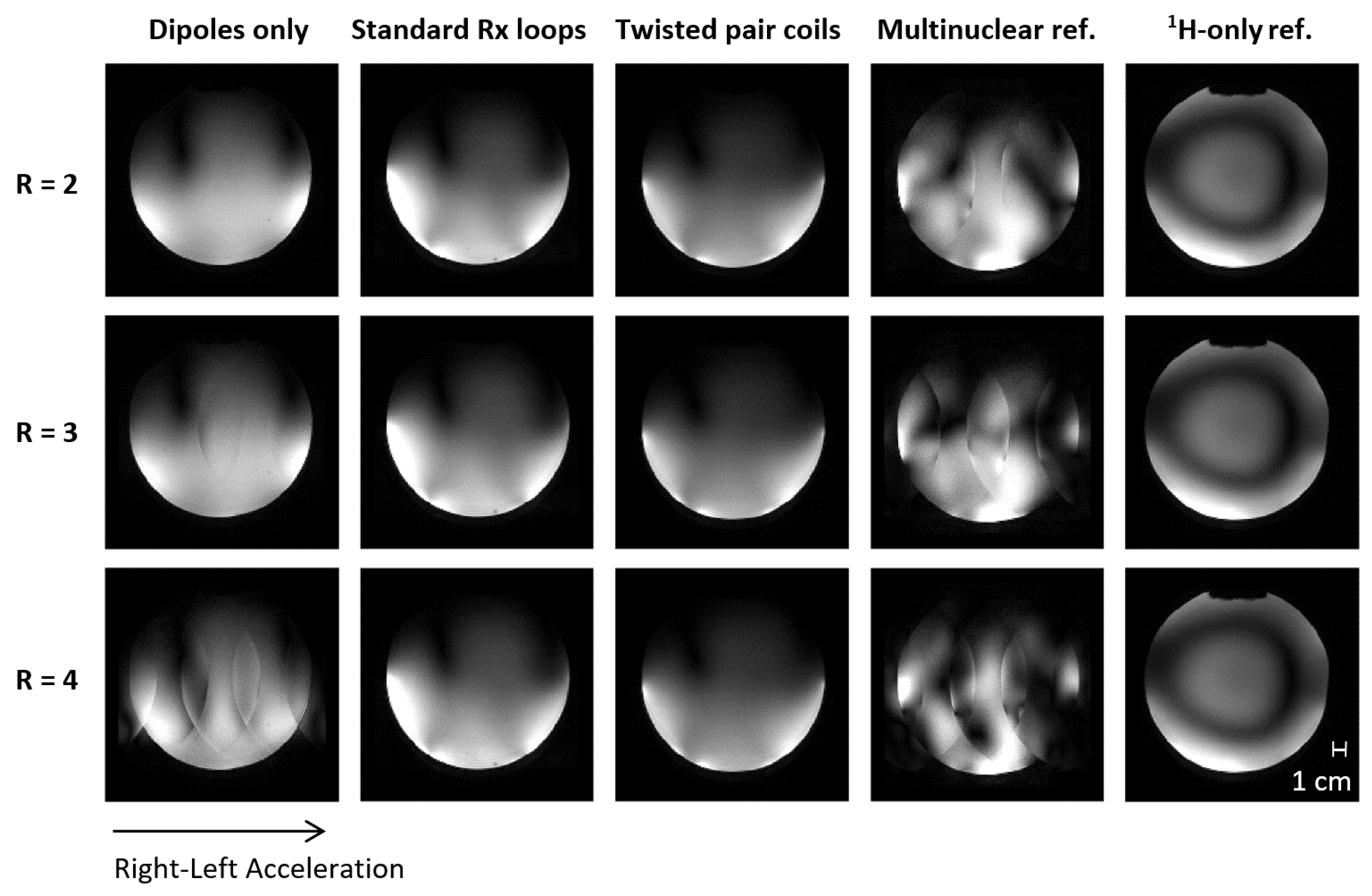
3.3. Receive Performance
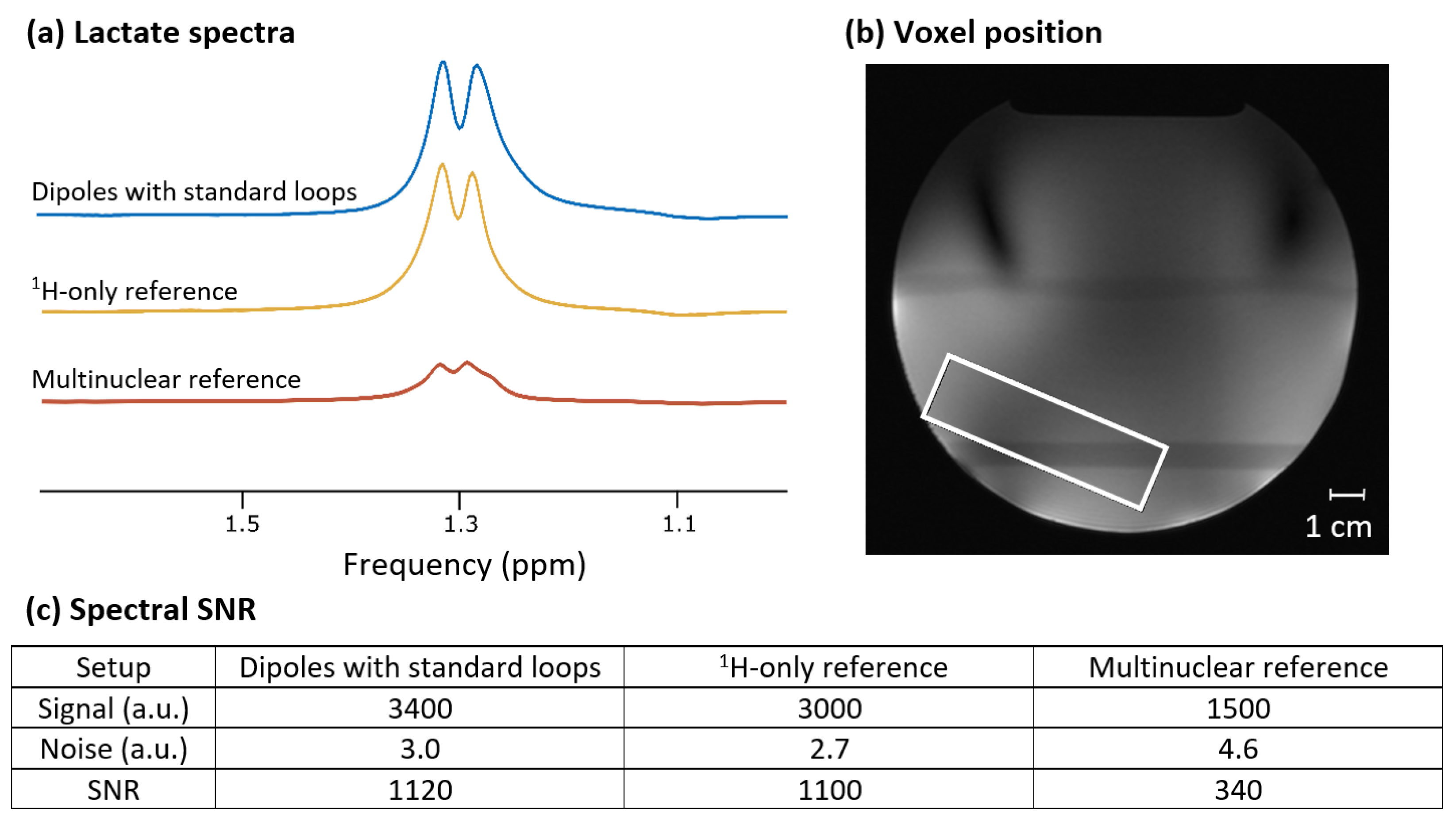
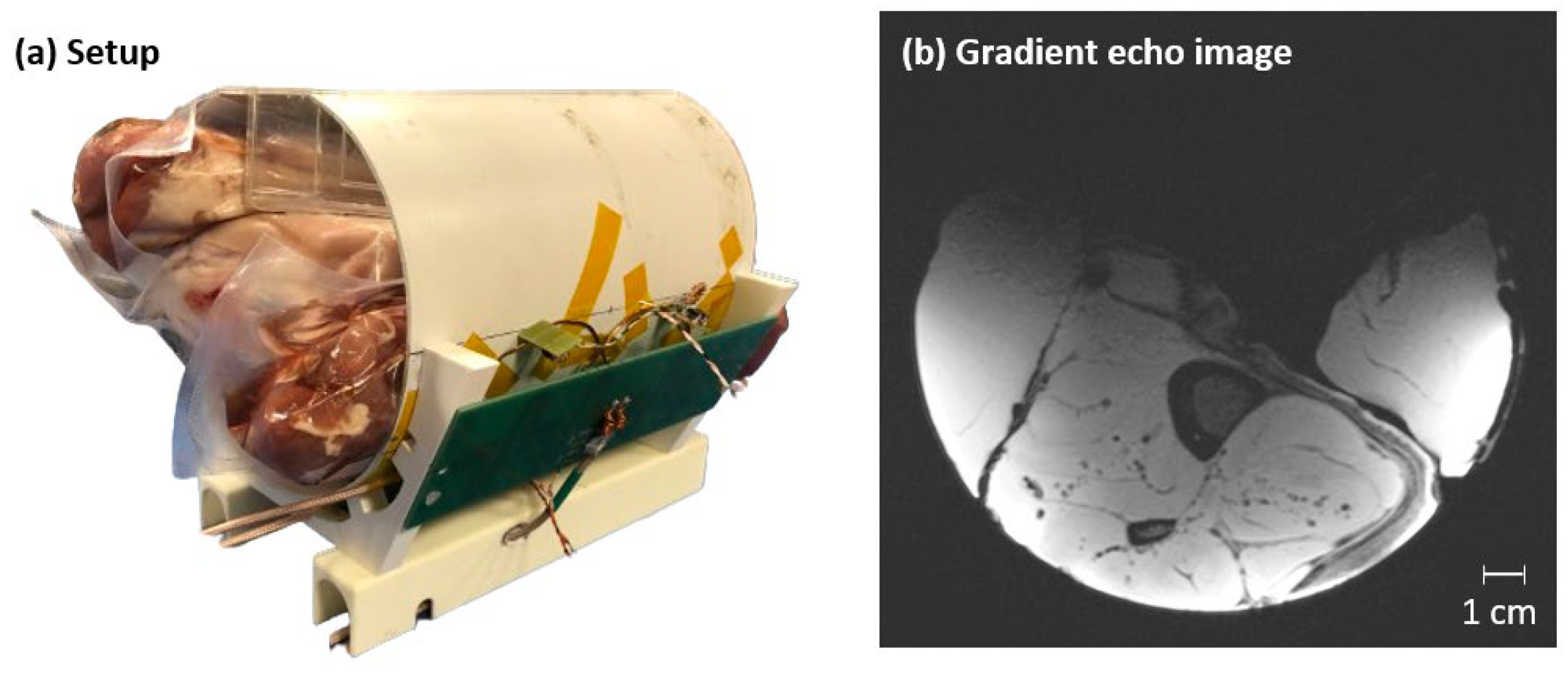
3.4. Lactate Phantom Measurements and Anatomical Image
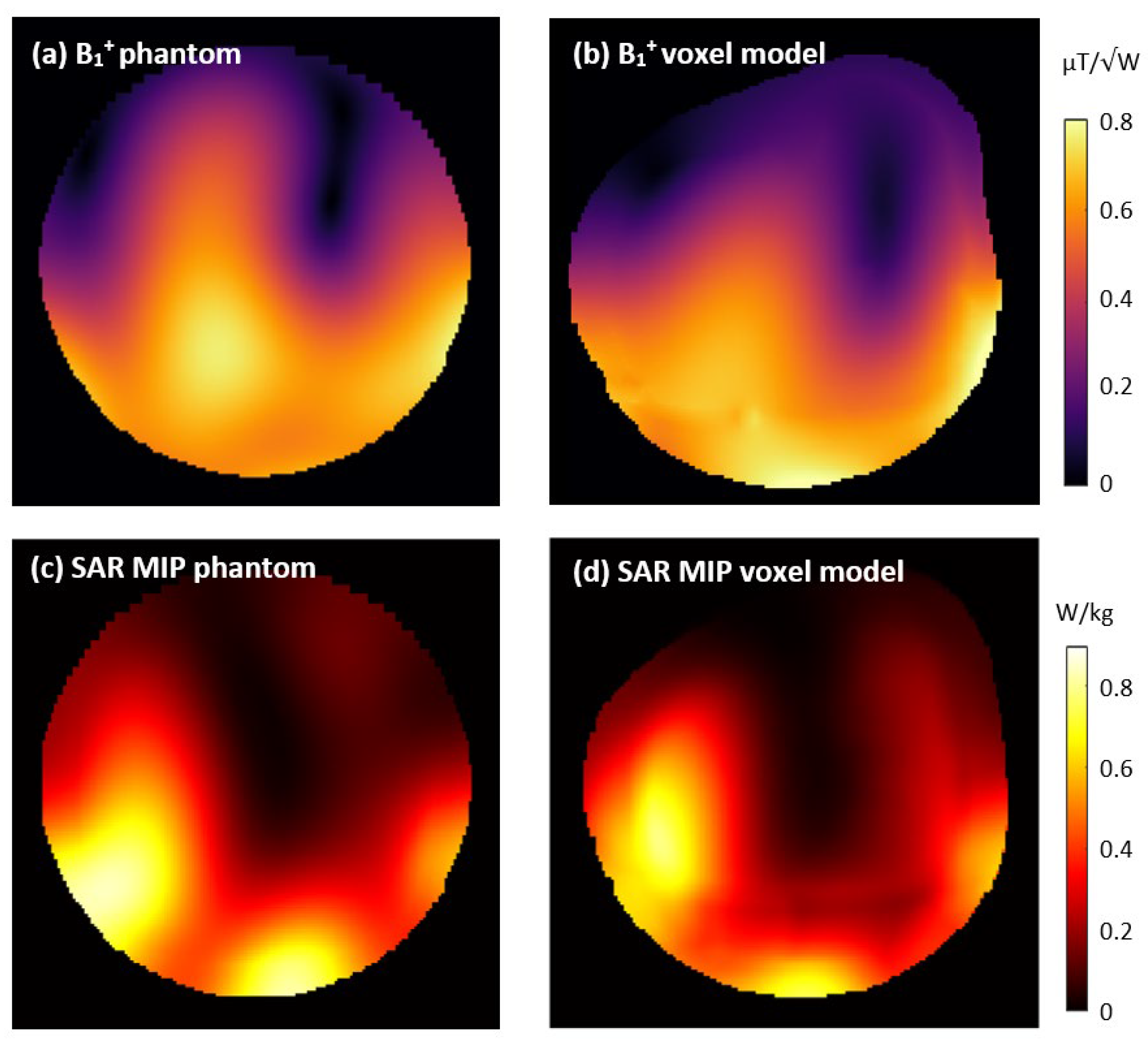
3.5. Simulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meyerspeer, M.; Boesch, C.; Cameron, D.; Dezortová, M.; Forbes, S.C.; Heerschap, A.; Jeneson, J.A.L.; Kan, H.E.; Kent, J.; Layec, G.; et al. 31P Magnetic Resonance Spectroscopy in Skeletal Muscle: Experts’ Consensus Recommendations. NMR Biomed. 2021, 34, e4246. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Chang, H.; Reiter, D.A.; Bradley, D.P.; Belury, M.; McCormack, S.E.; Raman, S.V. Phosphorus-31 Magnetic Resonance Spectroscopy: A Tool for Measuring In Vivo Mitochondrial Oxidative Phosphorylation Capacity in Human Skeletal Muscle. J. Vis. Exp. 2017, 119, 54977. [Google Scholar] [CrossRef] [PubMed]
- Šedivý, P.; Christina Kipfelsberger, M.; Dezortová, M.; Krššák, M.; Drobný, M.; Chmelík, M.; Rydlo, J.; Trattnig, S.; Hájek, M.; Valkovič, L. Dynamic 31 P MR Spectroscopy of Plantar Flexion: Influence of Ergometer Design, Magnetic Field Strength (3 and 7 T), and RF-coil Design. Med. Phys. 2015, 42, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.I.; Meyerspeer, M.; Robinson, S.D.; Goluch, S.; Wolzt, M.; Fiedler, G.B.; Bogner, W.; Laistler, E.; Krššák, M.; Moser, E.; et al. Dynamic PCr and pH Imaging of Human Calf Muscles during Exercise and Recovery Using 31 P gradient-Echo MRI at 7 Tesla. Magn. Reson. Med. 2016, 75, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Krššák, M.; Lindeboom, L.; Schrauwen-Hinderling, V.; Szczepaniak, L.S.; Derave, W.; Lundbom, J.; Befroy, D.; Schick, F.; Machann, J.; Kreis, R.; et al. Proton Magnetic Resonance Spectroscopy in Skeletal Muscle: Experts’ Consensus Recommendations. NMR Biomed. 2021, 34, e4266. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Lakoski, S.; Haller, R.G.; Sherry, A.D.; Malloy, C.R. Dynamic Monitoring of Carnitine and Acetylcarnitine in the Trimethylamine Signal after Exercise in Human Skeletal Muscle by 7T 1H-MRS. Magn. Reson. Med. 2013, 69, 7–17. [Google Scholar] [CrossRef]
- Klepochová, R.; Niess, F.; Meyerspeer, M.; Slukova, D.; Just, I.; Trattnig, S.; Ukropec, J.; Ukropcová, B.; Kautzky-Willer, A.; Leutner, M.; et al. Correlation between Skeletal Muscle Acetylcarnitine and Phosphocreatine Metabolism during Submaximal Exercise and Recovery: Interleaved 1H/31P MRS 7 T Study. Sci. Rep. 2024, 14, 3254. [Google Scholar] [CrossRef] [PubMed]
- Boesch, C. Musculoskeletal Spectroscopy. J. Magn. Reson. Imaging 2007, 25, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Kreis, R.; Bruegger, K.; Skjelsvik, C.; Zwicky, S.; Ith, M.; Jung, B.; Baumgartner, I.; Boesch, C. Quantitative 1H Magnetic Resonance Spectroscopy of Myoglobin de- and Reoxygenation in Skeletal Muscle: Reproducibility and Effects of Location and Disease. Magn. Reson. Med. 2001, 46, 240–248. [Google Scholar] [CrossRef]
- Kreis, R.; Jung, B.; Slotboom, J.; Felblinger, J.; Boesch, C. Effect of Exercise on the Creatine Resonances in 1H MR Spectra of Human Skeletal Muscle. J. Magn. Reson. 1999, 137, 350–357. [Google Scholar] [CrossRef]
- Meyerspeer, M.; Kemp, G.J.; Mlynárik, V.; Krššák, M.; Szendroedi, J.; Nowotny, P.; Roden, M.; Moser, E. Direct Noninvasive Quantification of Lactate and High Energy Phosphates Simultaneously in Exercising Human Skeletal Muscle by Localized Magnetic Resonance Spectroscopy. Magn. Reson. Med. 2007, 57, 654–660. [Google Scholar] [CrossRef]
- Niess, F.; Roat, S.; Bogner, W.; Krššák, M.; Kemp, G.J.; Schmid, A.I.; Trattnig, S.; Moser, E.; Zaitsev, M.; Meyerspeer, M. 3D Localized Lactate Detection in Muscle Tissue Using Double-Quantum Filtered 1H MRS with Adiabatic Refocusing Pulses at 7T. Magn. Reson. Med. 2022, 87, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Damon, B.M.; Gore, J.C. Physiological Basis of Muscle Functional MRI: Predictions Using a Computer Model. J. Appl. Physiol. 2005, 98, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Dooley, K.; Snodgrass, S.J.; Stanwell, P.; Birse, S.; Schultz, A.; Drew, M.K.; Edwards, S. Spatial Muscle Activation Patterns during Different Leg Exercise Protocols in Physically Active Adults Using Muscle Functional MRI: A Systematic Review. J. Appl. Physiol. 2020, 129, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Niess, F.; Schmid, A.I.; Bogner, W.; Wolzt, M.; Carlier, P.; Trattnig, S.; Moser, E.; Meyerspeer, M. Interleaved 31P MRS/1H ASL for Analysis of Metabolic and Functional Heterogeneity along Human Lower Leg Muscles at 7T. Magn. Reson. Med. 2020, 83, 1909–1919. [Google Scholar] [CrossRef]
- Englund, E.K.; Langham, M.C.; Li, C.; Rodgers, Z.B.; Floyd, T.F.; Mohler, E.R.; Wehrli, F.W. Combined Measurement of Perfusion, Venous Oxygen Saturation, and Skeletal Muscle T2* during Reactive Hyperemia in the Leg. J. Cardiovasc. Magn. Reson. 2013, 15, 70. [Google Scholar] [CrossRef]
- Damon, B.M.; Froeling, M.; Buck, A.K.W.; Oudeman, J.; Ding, Z.; Nederveen, A.J.; Bush, E.C.; Strijkers, G.J. Skeletal Muscle Diffusion Tensor-MRI Fiber Tracking: Rationale, Data Acquisition and Analysis Methods, Applications and Future Directions. NMR Biomed. 2017, 30, e3563. [Google Scholar] [CrossRef]
- Fouré, A.; Ogier, A.C.; Le Troter, A.; Vilmen, C.; Feiweier, T.; Guye, M.; Gondin, J.; Besson, P.; Bendahan, D. Diffusion Properties and 3D Architecture of Human Lower Leg Muscles Assessed with Ultra-High-Field-Strength Diffusion-Tensor MR Imaging and Tractography: Reproducibility and Sensitivity to Sex Difference and Intramuscular Variability. Radiology 2018, 287, 592–607. [Google Scholar] [CrossRef]
- Englund, E.K.; Elder, C.P.; Xu, Q.; Ding, Z.; Damon, B.M. Combined Diffusion and Strain Tensor MRI Reveals a Heterogeneous, Planar Pattern of Strain Development during Isometric Muscle Contraction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1079–R1090. [Google Scholar] [CrossRef]
- Ladd, M.E.; Bachert, P.; Meyerspeer, M.; Moser, E.; Nagel, A.M.; Norris, D.G.; Schmitter, S.; Speck, O.; Straub, S.; Zaiss, M. Pros and Cons of Ultra-High-Field MRI/MRS for Human Application. Prog. Nucl. Magn. Reson. Spectrosc. 2018, 109, 1–50. [Google Scholar] [CrossRef]
- Choi, C.-H.; Hong, S.-M.; Felder, J.; Shah, N.J. The State-of-the-Art and Emerging Design Approaches of Double-Tuned RF Coils for X-Nuclei, Brain MR Imaging and Spectroscopy: A Review. Magn. Reson. Imaging 2020, 72, 103–116. [Google Scholar] [CrossRef]
- Isaac, G.; Schnall, M.D.; Lenkinski, R.E.; Vogele, K. A Design for a Double-Tuned Birdcage Coil for Use in an Integrated MRI/MRS Examination. J. Magn. Reson. 1990, 89, 41–50. [Google Scholar] [CrossRef]
- Schnall, M.D.; Harihara Subramanian, V.; Leigh, J.S.; Chance, B. A New Double-Tuned Probed for Concurrent 1H and 31P NMR. J. Magn. Reson. 1985, 65, 122–129. [Google Scholar] [CrossRef]
- Lim, H.; Thind, K.; Martinez-Santiesteban, F.M.; Scholl, T.J. Construction and Evaluation of a Switch-tuned 13C-1H Birdcage Radiofrequency Coil for Imaging the Metabolism of Hyperpolarized 13C-enriched Compounds. Magn. Reson. Imaging 2014, 40, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-H.; Hong, S.-M.; Ha, Y.; Shah, N.J. Design and Construction of a Novel 1H/19F Double-Tuned Coil System Using PIN-Diode Switches at 9.4 T. J. Magn. Reson. 2017, 279, 11–15. [Google Scholar] [CrossRef]
- Raaijmakers, A.J.E.; Italiaander, M.; Voogt, I.J.; Luijten, P.R.; Hoogduin, J.M.; Klomp, D.W.J.; Van Den Berg, C.A.T. The Fractionated Dipole Antenna: A New Antenna for Body Imaging at 7 Tesla. Magn. Reson. Med. 2016, 75, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Steensma, B.R.; Voogt, I.J.; Leiner, T.; Luijten, P.R.; Habets, J.; Klomp, D.W.J.; van den Berg, C.A.T.; Raaijmakers, A.J.E. An 8-Channel Tx/Rx Dipole Array Combined with 16 Rx Loops for High-Resolution Functional Cardiac Imaging at 7T. Magn. Reson. Mater. Phys. Biol. Med. 2018, 31, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Avdievich, N.I.; Solomakha, G.; Ruhm, L.; Nikulin, A.V.; Magill, A.W.; Scheffler, K. Folded-End Dipole Transceiver Array for Human Whole-Brain Imaging at 7T. NMR in Biomed. 2021, 34, e4541. [Google Scholar] [CrossRef] [PubMed]
- Clément, J.; Gruetter, R.; Ipek, Ö. A Combined 32-channel Receive-loops/8-channel Transmit-dipoles Coil Array for Whole-brain MR Imaging at 7T. Magn. Reson. Med. 2019, 82, 1229–1241. [Google Scholar] [CrossRef]
- Avdievich, N.I.; Nikulin, A.V.; Ruhm, L.; Magill, A.W.; Glang, F.; Henning, A.; Scheffler, K.; Society, M.P. A 32-Element Loop/Dipole Hybrid Array for Human Head Imaging at 7T. Magn. Reson. Med. 2022, 88, 1912–1916. [Google Scholar] [CrossRef]
- Dai, J.; Gosselink, M.; van der Velden, T.A.; Meliadò, E.F.; Raaijmakers, A.J.E.; Klomp, D.W.J. An RF Coil Design to Enable Quintuple Nuclear Whole-Brain MRI. Magn. Reson. Med. 2023, 89, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Ohliger, M.A.; Sodickson, D.K. An Introduction to Coil Array Design for Parallel MRI. NMR Biomed. 2006, 19, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.; Franson, D.; Seiberlich, N. Recent Advances in Parallel Imaging for MRI. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 101, 71–95. [Google Scholar] [CrossRef]
- Maravilla, J.A.; Gopalan, K.; Arias, A.C.; Lustig, M. Transmission Line Receiver Coils (TLCs) for MRI. In Proceedings of the the 30th Scientific Meeting, International Society for Magnetic Resonance in Medicine, London, UK, 7–12 May 2022; Volume 30. [Google Scholar]
- Vliem, J.; Xiao, Y.; Wenz, D.; Xin, L.; Teeuwise, W.; Ruytenberg, T.; Webb, A.; Zivkovic, I. Twisted Pair Transmission Line Coil—A Flexible, Self-Decoupled and Robust Element for 7 T MRI. Magn. Reson. Imaging 2024, 108, 146–160. [Google Scholar] [CrossRef]
- Czerny, R.; Frass-Kriegl, R.; Cap, V.; Laistler, E.; Nohava, L. Twisted Pair Coils as Flexible Receive Elements for 7 T-SNR and Active Detuning Efficiency. In Proceedings of the 31st Scientific Meeting, International Society for Magnetic Resonance in Medicine, Toronto, ON, Canada, 3–8 June 2023; Volume 30. [Google Scholar]
- Goluch, S.; Kuehne, A.; Meyerspeer, M.; Kriegl, R.; Schmid, A.I.; Fiedler, G.B.; Herrmann, T.; Mallow, J.; Hong, S.M.; Cho, Z.H.; et al. A Form-Fitted Three Channel 31P, Two Channel 1H Transceiver Coil Array for Calf Muscle Studies at 7T. Magn. Reson. Med. 2015, 73, 2376–2389. [Google Scholar] [CrossRef]
- Avdievich, N.I.; Solomakha, G.; Ruhm, L.; Henning, A.; Scheffler, K. Unshielded Bent Folded-End Dipole 9.4 T Human Head Transceiver Array Decoupled Using Modified Passive Dipoles. Magn. Reson. Med. 2021, 86, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, Y.; Zhang, Z.; Wang, H. Antenna Decoupling by Common and Differential Modes Cancellation. IEEE Trans. Antennas Propag. 2021, 69, 672–682. [Google Scholar] [CrossRef]
- Roemer, P.B.; Edelstein, W.A.; Hayes, C.E.; Souza, S.P.; Mueller, M. The NMR Phased Array. Magn. Reson. Med. 1990, 16, 192–225. [Google Scholar] [CrossRef] [PubMed]
- ASTM Standard F2182-19e2; Standard Test Method for Measurement of Radio Frequency Induced Heating Near Passive Implants During Magnetic Resonance Imaging. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- Darrasse, L.; Kassab, G. Quick Measurement of NMR-Coil Sensitivity with a Dual-Loop Probe. Rev. Sci. Instrum. 1993, 64, 1841–1844. [Google Scholar] [CrossRef]
- Ginefri, J.-C.; Durand, E.; Darrasse, L. Quick Measurement of Nuclear Magnetic Resonance Coil Sensitivity with a Single-Loop Probe. Rev. Sci. Instrum. 1999, 70, 4730–4731. [Google Scholar] [CrossRef]
- Chung, S.; Kim, D.; Breton, E.; Axel, L. Rapid B1+ Mapping Using a Preconditioning RF Pulse with TurboFLASH Readout. Magn. Reson. Med. 2010, 64, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Robson, P.M.; Grant, A.K.; Madhuranthakam, A.J.; Lattanzi, R.; Sodickson, D.K.; McKenzie, C.A. Comprehensive Quantification of Signal-to-Noise Ratio and g-Factor for Image-Based and k-Space-Based Parallel Imaging Reconstructions. Magn. Reson. Med. 2008, 60, 895–907. [Google Scholar] [CrossRef]
- Breuer, F.A.; Kannengiesser, S.A.R.; Blaimer, M.; Seiberlich, N.; Jakob, P.M.; Griswold, M.A. General Formulation for Quantitative G-Factor Calculation in GRAPPA Reconstructions. Magn. Reson. Med. 2009, 62, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Meyerspeer, M.; Scheenen, T.; Schmid, A.I.; Mandl, T.; Unger, E.; Moser, E. Semi-LASER Localized Dynamic 31P Magnetic Resonance Spectroscopy in Exercising Muscle at Ultra-High Magnetic Field: Dynamic Semi-LASER Localized 31P MRS in Exercising Muscle. Magn. Reson. Med. 2011, 65, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Kreis, R.; Boer, V.; Choi, I.; Cudalbu, C.; De Graaf, R.A.; Gasparovic, C.; Heerschap, A.; Krššák, M.; Lanz, B.; Maudsley, A.A.; et al. Terminology and Concepts for the Characterization of in Vivo MR Spectroscopy Methods and MR Spectra: Background and Experts’ Consensus Recommendations. NMR Biomed. 2021, 34, e4347. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Kainz, W.; Hahn, E.; Honegger, K.; Zefferer, M.; Neufeld, E.; Rascher, W.; Janka, R.; Bautz, W.; Chen, J.; et al. The Virtual Family-Development of Surface-Based Anatomical Models of Two Adults and Two Children for Dosimetric Simulations. Phys. Med. Biol. 2010, 55, N23. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M.; Turner, R. Fast MRI Coil Analysis Based on 3-D Electromagnetic and RF Circuit Co-Simulation. J. Magn. Reson. 2009, 200, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Lemdiasov, R.A.; Obi, A.A.; Ludwig, R. A Numerical Postprocessing Procedure for Analyzing Radio Frequency MRI Coils. Concepts Magn. Reson. Part A 2011, 38A, 133–147. [Google Scholar] [CrossRef]
- Graesslin, I.; Homann, H.; Biederer, S.; Börnert, P.; Nehrke, K.; Vernickel, P.; Mens, G.; Harvey, P.; Katscher, U. A Specific Absorption Rate Prediction Concept for Parallel Transmission MR. Magn. Reson. Med. 2012, 68, 1664–1674. [Google Scholar] [CrossRef]
- Kuehne, A.; Goluch, S.; Waxmann, P.; Seifert, F.; Ittermann, B.; Moser, E.; Laistler, E. Power Balance and Loss Mechanism Analysis in RF Transmit Coil Arrays. Magn. Reson. Med. 2015, 74, 1165–1176. [Google Scholar] [CrossRef]
- Wang, W.; Sánchez-Heredia, J.D.; Zhurbenko, V.; Ardenkjær-Larsen, J.H. Condition for Optimal Preamplifier Decoupling in One-Turn Single- and Multi-Gap Shielded Loop MRI Detectors. In Proceedings of the 2022 IEEE 2nd Ukrainian Microwave Week (UkrMW), Kharkiv, Ukraine, 14–18 November 2022; pp. 150–154. [Google Scholar]
- Vaughan, J.T.; Garwood, M.; Collins, C.M.; Liu, W.; DelaBarre, L.; Adriany, G.; Andersen, P.; Merkle, H.; Goebel, R.; Smith, M.B.; et al. 7T vs. 4T: RF Power, Homogeneity, and Signal-to-noise Comparison in Head Images. Magn. Reson. Med. 2001, 46, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.M.; Liu, W.; Schreiber, W.; Yang, Q.X.; Smith, M.B. Central Brightening Due to Constructive Interference with, without, and despite Dielectric Resonance. Magn. Reson. Imaging 2005, 21, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Meyerspeer, M.; Roig, E.S.; Gruetter, R.; Magill, A.W. An Improved Trap Design for Decoupling Multinuclear RF Coils. Magn. Reson. Med. 2014, 72, 584–590. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cap, V.; Rocha dos Santos, V.R.; Repnin, K.; Červený, D.; Laistler, E.; Meyerspeer, M.; Frass-Kriegl, R. Combining Dipole and Loop Coil Elements for 7 T Magnetic Resonance Studies of the Human Calf Muscle. Sensors 2024, 24, 3309. https://doi.org/10.3390/s24113309
Cap V, Rocha dos Santos VR, Repnin K, Červený D, Laistler E, Meyerspeer M, Frass-Kriegl R. Combining Dipole and Loop Coil Elements for 7 T Magnetic Resonance Studies of the Human Calf Muscle. Sensors. 2024; 24(11):3309. https://doi.org/10.3390/s24113309
Chicago/Turabian StyleCap, Veronika, Vasco Rafael Rocha dos Santos, Kostiantyn Repnin, David Červený, Elmar Laistler, Martin Meyerspeer, and Roberta Frass-Kriegl. 2024. "Combining Dipole and Loop Coil Elements for 7 T Magnetic Resonance Studies of the Human Calf Muscle" Sensors 24, no. 11: 3309. https://doi.org/10.3390/s24113309
APA StyleCap, V., Rocha dos Santos, V. R., Repnin, K., Červený, D., Laistler, E., Meyerspeer, M., & Frass-Kriegl, R. (2024). Combining Dipole and Loop Coil Elements for 7 T Magnetic Resonance Studies of the Human Calf Muscle. Sensors, 24(11), 3309. https://doi.org/10.3390/s24113309






