Assessment of a 16-Channel Ambulatory Dry Electrode EEG for Remote Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. EEG System Modelling
2.1.1. Hardware Development
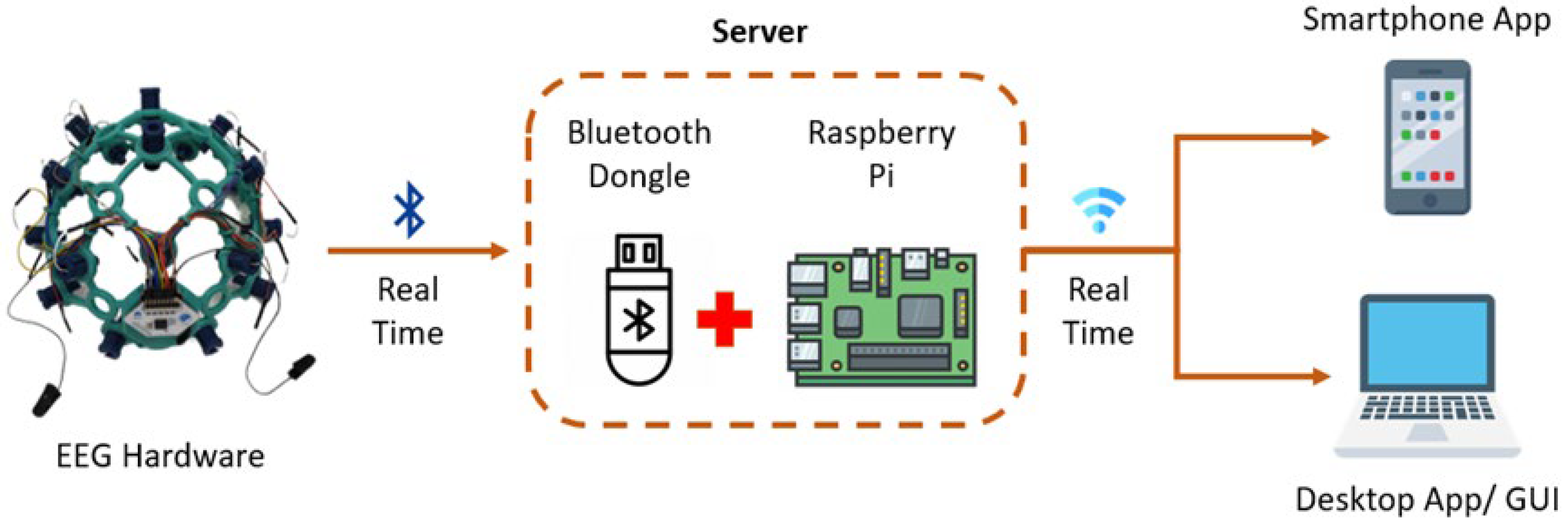
2.1.2. Communication Gateway Development
2.1.3. Software Integration
2.1.4. Device Integration
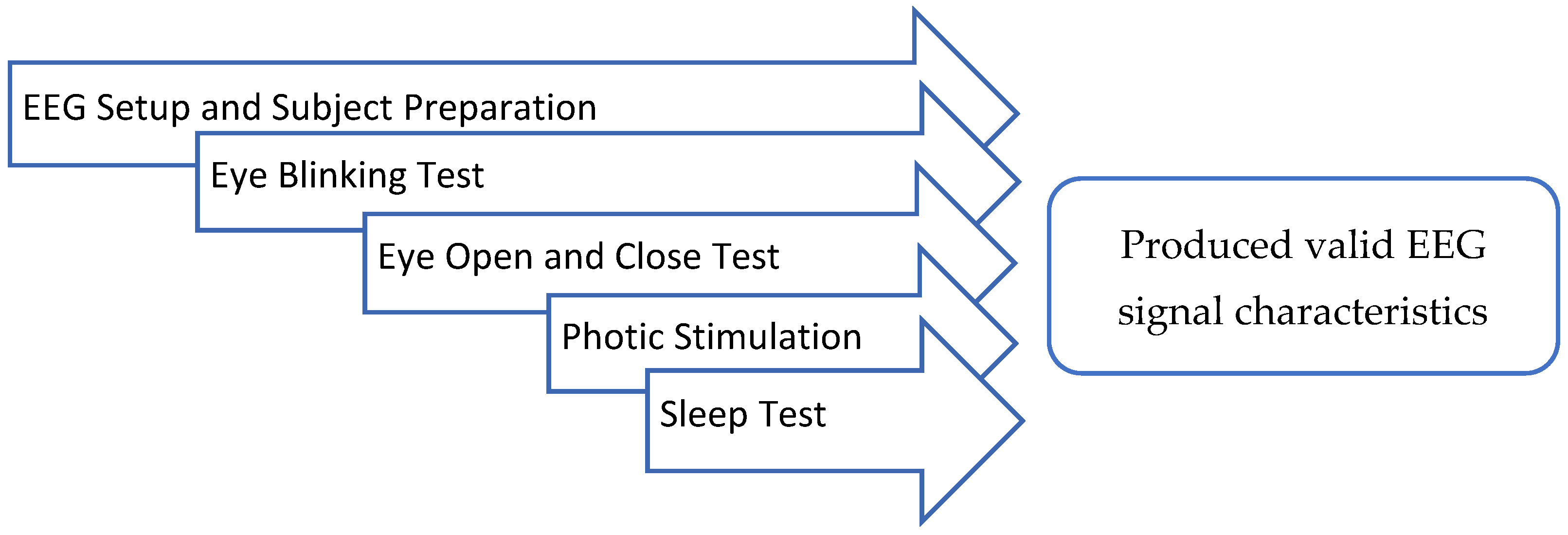
2.2. Device Verification
- I.
- EEG Setup and Subject Preparation
- II.
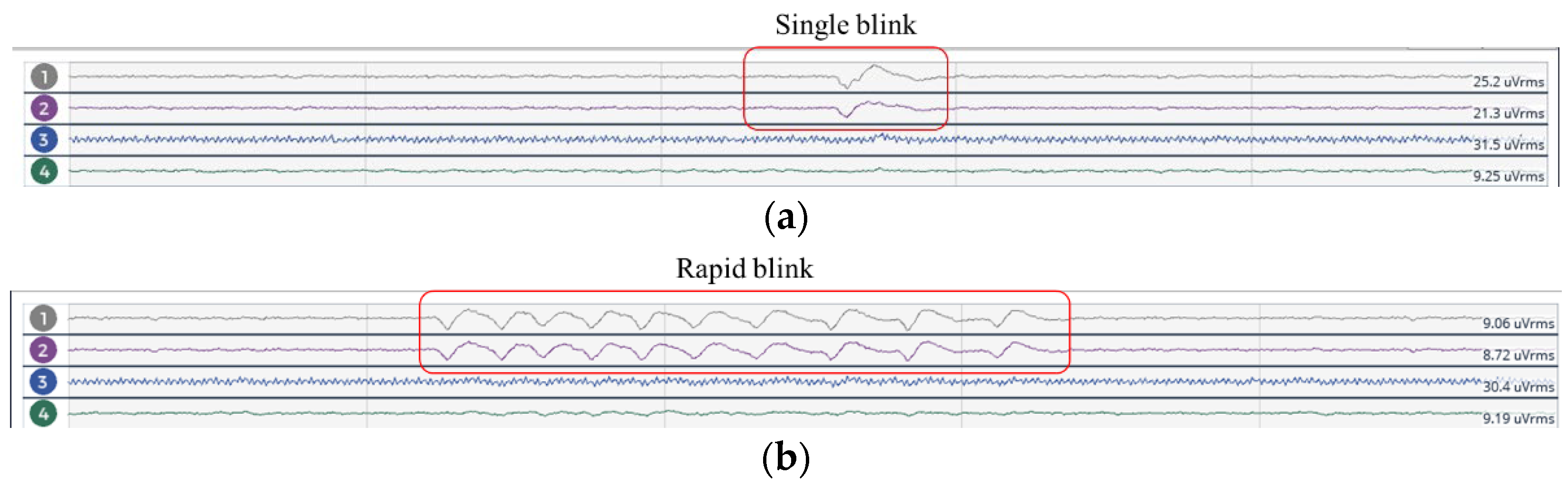
- Eye Blinking Test
- III.
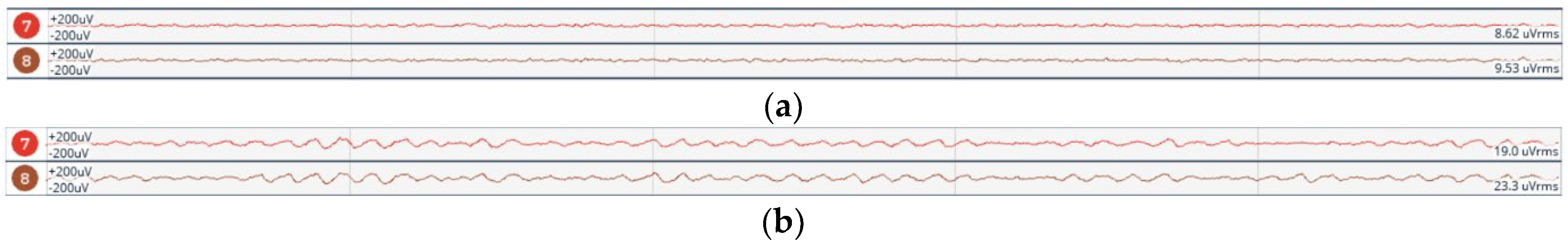
- Eye Open and Close Test
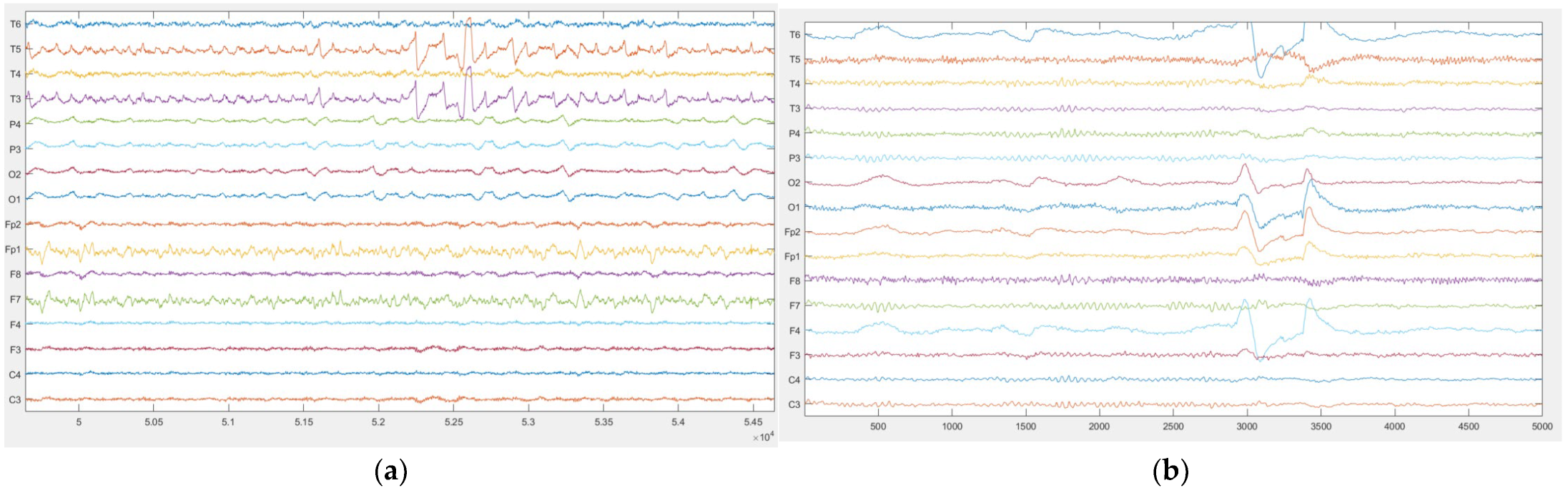
- IV. Photic Simulation Test
- V. Sleep Test
2.3. Clinical Device Validation
2.3.1. Signal Quality Experiment Design
2.3.2. EEG Pre-Processing
2.3.3. Statistical Analysis
2.3.4. Wavelet Analysis
3. Results
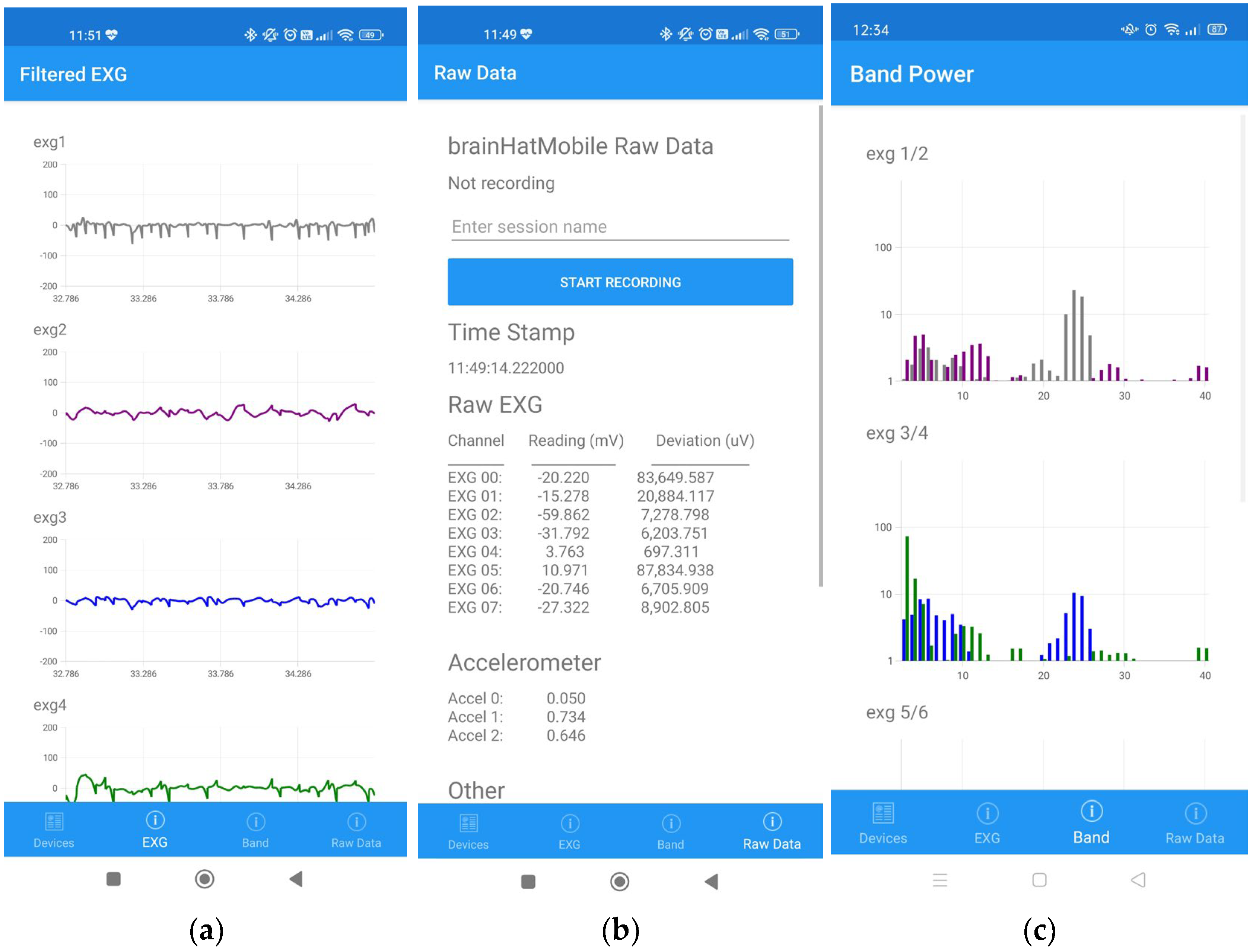
3.1. EEG Device
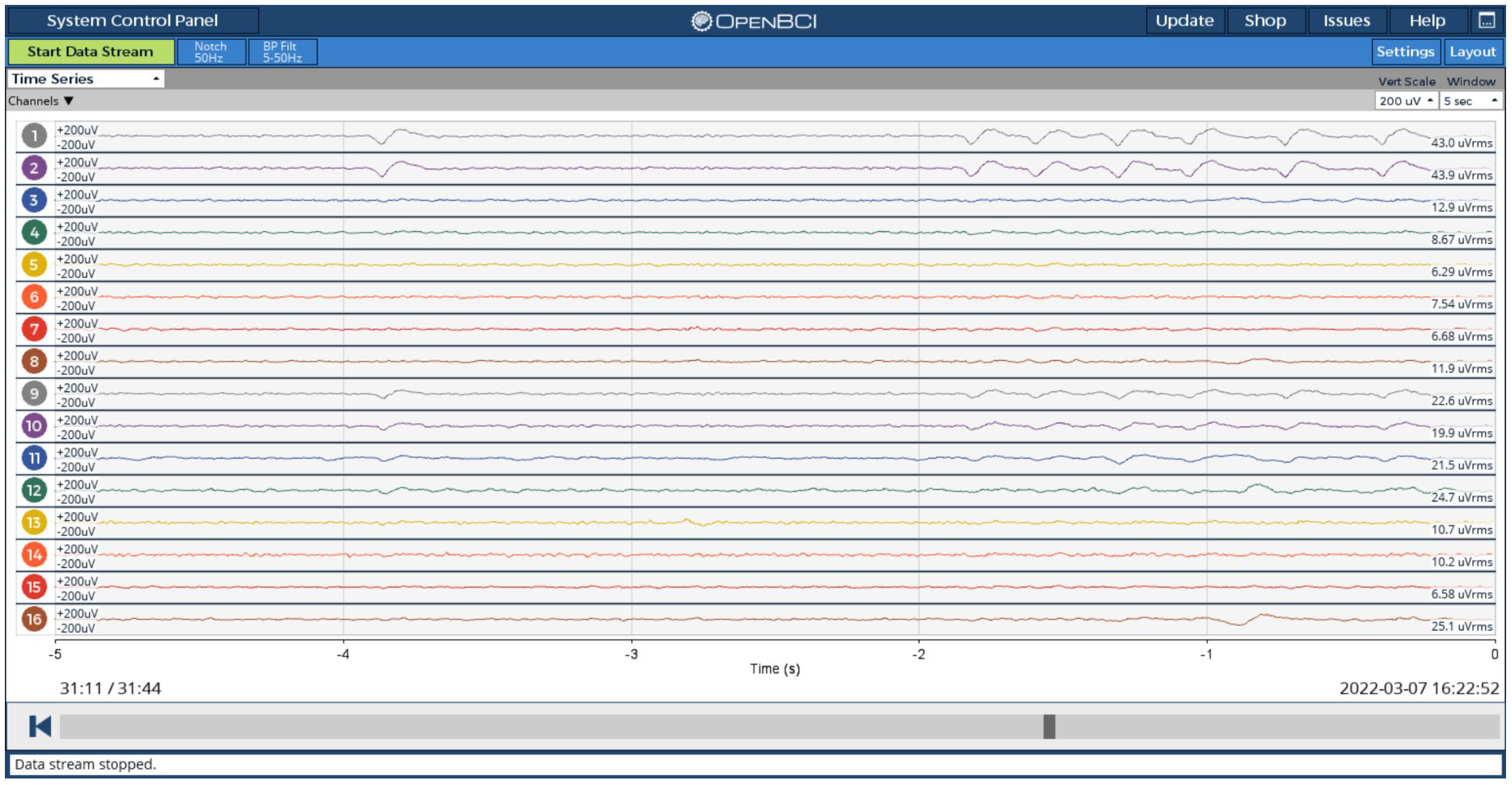
3.2. Device Verification Observations
- I.
- Eye Blinking Test
- II.
- Eye Open and Close Test
- III.
- Photic Stimulation Test
- IV. Sleep Test
3.3. Device Validation Results
3.3.1. Statistical Analysis
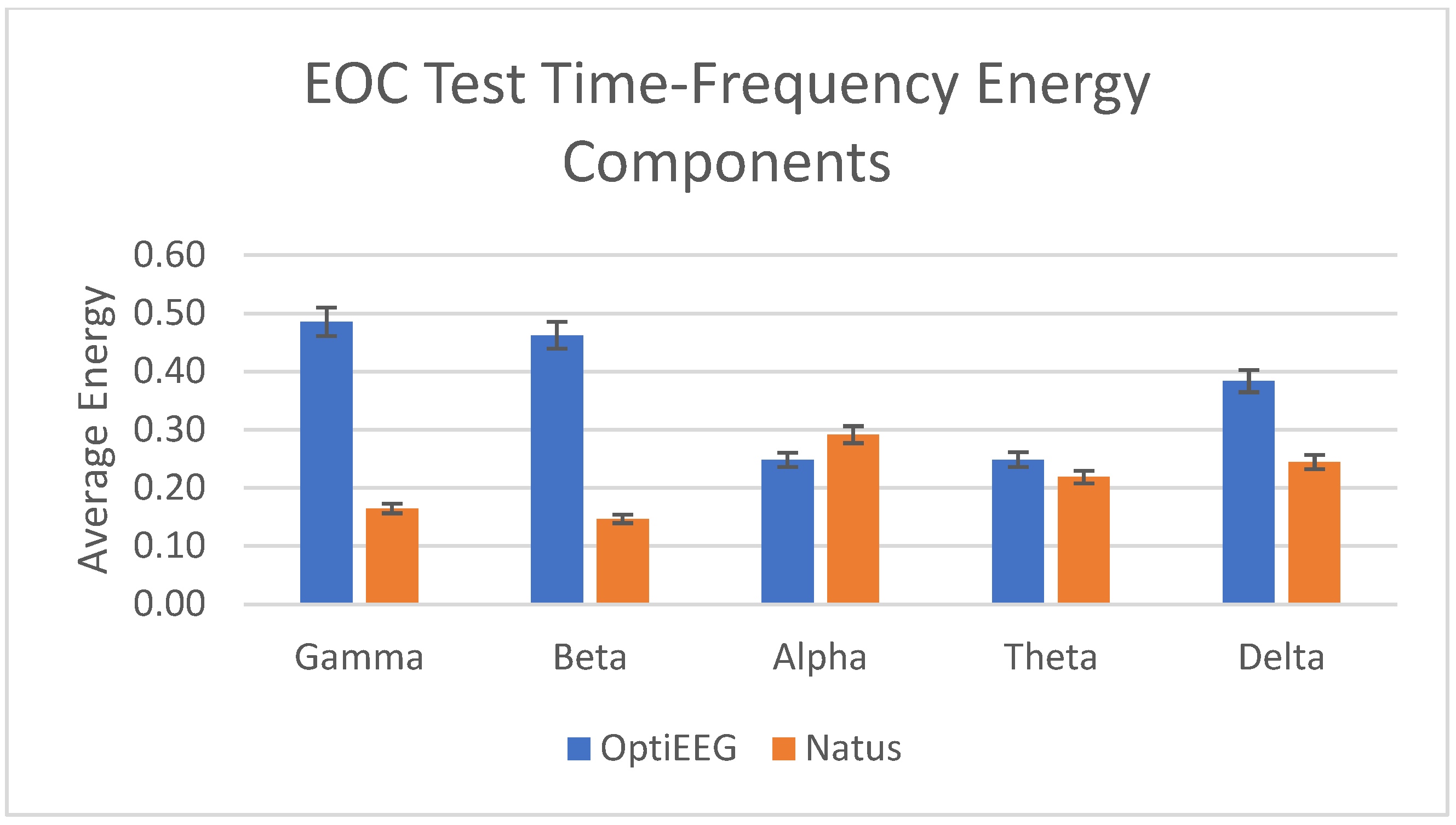
3.3.2. Wavelet Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, S.; Sinha, J.K.; Khan, T.; Devaraju, K.S.; Singh, P.; Vaibhav, K.; Gaur, P. Pharmacological and Therapeutic Approaches in the Treatment of Epilepsy. Biomedicines 2021, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Najafi, T.; Jaafar, R.; Remli, R.; Zaidi, A.W.; Chellappan, K. The Role of Brain Signal Processing and Neuronal Modelling in Epilepsy—A Review. J. Kejuruter. 2021, 33, 801–815. [Google Scholar] [CrossRef]
- Epilepsy. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 12 December 2022).
- Jackson, G.L.; Powers, B.J.; Chatterjee, R.; Prvu Bettger, J.; Kemper, A.R.; Hasselblad, V.; Dolor, R.J.; Julian Irvine, R.; Heidenfelder, B.L.; Kendrick, A.S.; et al. The Patient-Centered Medical Home A Systematic Review. Ann. Intern. Med. 2013, 158, 169–178. [Google Scholar] [CrossRef]
- McKinsey. From Facility to Home: How Healthcare Could Shift by 2025. Available online: https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/from-facility-to-home-how-healthcare-could-shift-by-2025 (accessed on 15 December 2022).
- Cannard, C.; Brandmeyer, T.; Wahbeh, H.; Delorme, A. Self-Health Monitoring and Wearable Neurotechnologies. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 168, pp. 207–232. [Google Scholar]
- Rahmani, A.M.; Gia, T.N.; Negash, B.; Anzanpour, A.; Azimi, I.; Jiang, M.; Liljeberg, P. Exploiting Smart E-Health Gateways at the Edge of Healthcare Internet-of-Things: A Fog Computing Approach. Future Gener. Comput. Syst. 2018, 78, 641–658. [Google Scholar] [CrossRef]
- Hassanalieragh, M.; Page, A.; Soyata, T.; Sharma, G.; Aktas, M.; Mateos, G.; Kantarci, B.; Andreescu, S. Health Monitoring and Management Using Internet-of-Things (IoT) Sensing with Cloud-Based Processing: Opportunities and Challenges. In Proceedings of the 2015 IEEE International Conference on Services Computing, SCC 2015, New York, NY, USA, 27 June–2 July 2015; Institute of Electrical and Electronics Engineers Inc.: New York, NY, USA, 2015; pp. 285–292. [Google Scholar]
- Kelly, S.D.T.; Suryadevara, N.K.; Mukhopadhyay, S.C. Towards the Implementation of IoT for Environmental Condition Monitoring in Homes. IEEE Sens. J. 2013, 13, 3846–3853. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Majumder, S.; Mondal, T.; Deen, M.J. Wearable Sensors for Remote Health Monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef]
- Athavipach, C.; Pan-Ngum, S.; Israsena, P. A Wearable In-Ear EEG Device for Emotion Monitoring. Sensors 2019, 19, 4014. [Google Scholar] [CrossRef]
- Biondi, A.; Laiou, P.; Bruno, E.; Viana, P.F.; Schreuder, M.; Hart, W.; Nurse, E.; Pal, D.K.; Richardson, M.P. Remote and Long-Term Self-Monitoring of Electroencephalographic and Noninvasive Measurable Variables at Home in Patients with Epilepsy (EEG@HOME): Protocol for an Observational Study. JMIR Res. Protoc. 2021, 10, e25309. [Google Scholar] [CrossRef]
- Noachtar, S.; Rémi, J. The Role of EEG in Epilepsy: A Critical Review. Epilepsy Behav. 2009, 15, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Askamp, J.; van Putten, M.J.A.M. Mobile EEG in Epilepsy. Int. J. Psychophysiol. 2014, 91, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Mohd Mohid, I.A.; Khamis, N.K.; Shamsuddin, M.A.; Kabilmahir, N.; Noh, N.A. Driver’s Performance Under Different Secondary Tasks and Disruptions on Rural Road Environment. J. Kejuruter. 2022, 34, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, G.; Hossain, M.S.; Kumar, N. EEG-Based Pathology Detection for Home Health Monitoring. IEEE J. Sel. Areas Commun. 2021, 39, 603–610. [Google Scholar] [CrossRef]
- Fathillah, M.S.; Chellappan, K.; Jaafar, R.; Remli, R.; Asyraf, W.; Zaidi, W. Time-Frequency Analysis in Ictal and Interictal Seizure Epilepsy Patients using Electroencephalogram. J. Theor. Appl. Inf. Technol. 2018, 15, 3426–3431. [Google Scholar]
- Electroencephalogram (EEG)–NHS. Available online: https://www.nhs.uk/conditions/electroencephalogram/ (accessed on 12 December 2022).
- Engel, J., Jr. A Practical Guide for Routine EEG Studies in Epilepsy. J. Clin. Neurophysiol. 1984, 1, 109–142. [Google Scholar] [CrossRef]
- Titgemeyer, Y.; Surges, R.; Altenmüller, D.M.; Fauser, S.; Kunze, A.; Lanz, M.; Malter, M.P.; Nass, R.D.; von Podewils, F.; Remi, J.; et al. Can Commercially Available Wearable EEG Devices Be Used for Diagnostic Purposes? An Explorative Pilot Study. Epilepsy Behav. 2020, 103, 106507. [Google Scholar] [CrossRef]
- Gottlibe, M.; Rosen, O.; Weller, B.; Mahagney, A.; Omar, N.; Khuri, A.; Srugo, I.; Genizi, J. Stroke Identification Using a Portable EEG Device—A Pilot Study. Neurophysiol. Clin. 2020, 50, 21–25. [Google Scholar] [CrossRef]
- Gao, Z.; Cui, X.; Wan, W.; Qin, Z.; Gu, Z. Signal Quality Investigation of a New Wearable Frontal Lobe EEG Device. Sensors 2022, 22, 1898. [Google Scholar] [CrossRef]
- Sintotskiy, G.; Hinrichs, H. In-Ear-EEG—A Portable Platform for Home Monitoring. J. Med. Eng. Technol. 2020, 44, 26–37. [Google Scholar] [CrossRef]
- Lin, S.K.; Istiqomah; Wang, L.C.; Lin, C.Y.; Chiueh, H. An Ultra-Low Power Smart Headband for Real-Time Epileptic Seizure Detection. IEEE J. Transl. Eng. Health Med. 2018, 6, 2700410. [Google Scholar] [CrossRef]
- Valentin, O.; Viallet, G.; Delnavaz, A.; Cretot-Richert, G.; Ducharme, M.; Monsarat-Chanon, H.; Voix, J. Custom-Fitted in-and around-the-Ear Sensors for Unobtrusive and on-the-Go Eeg Acquisitions: Development and Validation. Sensors 2021, 21, 2953. [Google Scholar] [CrossRef]
- Mai, N.D.; Lee, B.G.; Chung, W.Y. Affective Computing on Machine Learning-Based Emotion Recognition Using a Self-Made Eeg Device. Sensors 2021, 21, 5135. [Google Scholar] [CrossRef]
- Grosselin, F.; Navarro-Sune, X.; Vozzi, A.; Pandremmenou, K.; Fallani, F.D.V.; Attal, Y.; Chavez, M. Quality Assessment of Single-Channel EEG for Wearable Devices. Sensors 2019, 19, 601. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.; Temko, A.; Bocchino, A.; O’Mahony, C.; Boylan, G.; Popovici, E. Analysis of a Low-Cost Eeg Monitoring System and Dry Electrodes toward Clinical Use in the Neonatal Icu. Sensors 2019, 19, 2637. [Google Scholar] [CrossRef]
- Ko, L.-W.; Lai, W.-K.; Liang, W.-G.; Chuang, C.-H.; Lu, S.-W.; Lu, Y.-C.; Hsiung, T.-Y.; Wu, H.-H.; Lin, C.-T. Single Channel Wireless EEG Device for Real-Time Fatigue Level Detection. In Proceedings of the 2015 IEEE International Joint Conference on Neural Networks (IJCNN), Killarney, Ireland, 12–17 July 2015; pp. 1–5. [Google Scholar]
- Saadi, H.; Ferroukhi, M.; Attari, M. Development of Wireless High Immunity EEG Recording System. In Proceedings of the 2011 IEEE International Conference on Electronic Devices, Systems and Applications (ICEDSA), Kuala Lumpur, Malaysia, 25–27 April 2011; pp. 120–124. [Google Scholar]
- Suzuki, A.; Ito, H.; Ishii, M.; Dohsaka, K. Emotional Recognition with Wearable EEG Device. In Proceedings of the 2019 IEEE 1st Global Conference on Life Sciences and Technologies (LifeTech), Osaka, Japan, 12–14 March 2019; pp. 214–215. [Google Scholar]
- AltexSoft. Internet of Things (IoT) Architecture: Key Layers and Components. Available online: https://www.altexsoft.com/blog/iot-architecture-layers-components/ (accessed on 13 December 2022).
- Oscillator SPI Test Signals and Monitors Spi Patient Bias and Reference ADS1299-x Low-Noise, 4-, 6-, 8-Channel, 24-Bit, Analog-to-Digital Converter for EEG and Biopotential Measurements; Texas Instruments: Dallas, TX, USA, 2012.
- Microchip Technology. PIC32MX250F128B. Available online: https://www.microchip.com/en-us/product/PIC32MX250F128B (accessed on 13 December 2022).
- Uktveris, T.; Jusas, V. Development of a Modular Board for EEG Signal Acquisition. Sensors 2018, 18, 2140. [Google Scholar] [CrossRef]
- Balim, M.A.; Acir, N. 8 Channel Mobile EEG Measurement Device Design. In Proceedings of the 2018 IEEE Medical Technologies National Congress (TIPTEKNO), Magusa, Cyprus, 8–10 November 2018; pp. 1–4. [Google Scholar]
- Crowd Supply. HackEEG. Available online: https://www.crowdsupply.com/starcat/hackeeg (accessed on 12 February 2023).
- TI.Com. ADS1299EEGFE-PDK Evaluation Board. Available online: https://www.ti.com/tool/ADS1299EEGFE-PDK?keyMatch=&tisearch=search-everything&usecase=hardware#description (accessed on 12 February 2023).
- WallySci. E3K. Available online: https://www.wallysci.com/product-page/e3k (accessed on 12 February 2023).
- Cyton + Daisy Biosensing Boards (16-Channels)—OpenBCI Online Store. Available online: https://shop.openbci.com/products/cyton-daisy-biosensing-boards-16-channel (accessed on 12 February 2023).
- Hubs. What’s the Ideal Filament for FDM 3D Printing? 3D Printing Materials Compared. Available online: https://www.hubs.com/knowledge-base/fdm-3d-printing-materials-compared/ (accessed on 20 October 2022).
- Shivaraja, T.R.; Kamal, N.; Zaidi, W.A.W.; Chellappan, K. Adaptable Medical Device with 3D Printing Facilities. J. Phys. Conf. Ser. 2022, 2318, 012020. [Google Scholar] [CrossRef]
- Rojas, G.M.; Alvarez, C.; Montoya, C.E.; de la Iglesia-Vayá, M.; Cisternas, J.E.; Gálvez, M. Study of Resting-State Functional Connectivity Networks Using EEG Electrodes Position as Seed. Front. Neurosci. 2018, 12, 235. [Google Scholar] [CrossRef]
- Radüntz, T. Signal Quality Evaluation of Emerging EEG Devices. Front. Physiol. 2018, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.J.; Ambedkar, B.; Mukta Dhopeshwarkar, I.G.; Pankaj Sathe, I.A. Calculate the Quality Measures on Classification of Continuous EEG without Trial Structure EEG Dataset. Int. J. Comput. Appl. 2016, 147, 32–35. [Google Scholar]
- Shriram, R.; Sundhararajan, M.; Shete, S.; Daimiwal, N. Statistical Features-Based Comparison of Analysis and Synthesis of Normal and Epileptic Electroencephalograms for Various Wavelets. Turk. J. Electr. Eng. Comput. Sci. 2017, 25, 1795–1806. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.D.; Luo, M.L.; Li, K.; Yang, X.F.; Guo, Q. Epileptic Seizure Classification of EEGs Using Time-Frequency Analysis Based Multiscale Radial Basis Functions. IEEE J. Biomed. Health Inform. 2018, 22, 386–397. [Google Scholar] [CrossRef]
- Abdullah, S.; Yates, J.R.; Giacomin, J.A. Fatigue Data Editing Algorithm for Automotive Applications. J. Kejuruter. 2005, 17, 71–84. [Google Scholar]
- Li, M.; Chen, W.; Zhang, T. Classification of Epilepsy EEG Signals Using DWT-Based Envelope Analysis and Neural Network Ensemble. Biomed. Signal Process. Control 2017, 31, 357–365. [Google Scholar] [CrossRef]
- Rhif, M.; ben Abbes, A.; Farah, I.R.; Martínez, B.; Sang, Y. Wavelet Transform Application for/in Non-Stationary Time-Series Analysis: A Review. Appl. Sci. 2019, 9, 1345. [Google Scholar] [CrossRef]
- Ngui, W.K.; Leong, M.S.; Hee, L.M.; Abdelrhman, A.M. Wavelet Analysis: Mother Wavelet Selection Methods. Appl. Mech. Mater. 2013, 393, 953–958. [Google Scholar] [CrossRef]
- Al-Qazzaz, N.K.; Ali, S.H.B.M.; Ahmad, S.A.; Chellappan, K.; Islam, M.S.; Escudero, J. Role of EEG as Biomarker in the Early Detection and Classification of Dementia. Sci. World J. 2014, 2014, 906038. [Google Scholar] [CrossRef] [PubMed]
- Medithe, J.W.C.; Nelakuditi, U.R. Study of Normal and Abnormal EEG. In Proceedings of the ICACCS 2016—3rd International Conference on Advanced Computing and Communication Systems: Bringing to the Table, Futuristic Technologies from Arround the Globe, Coimbatore, India, 22–23 January 2016; Institute of Electrical and Electronics Engineers Inc.: New York, NY, USA, 2016. [Google Scholar]
- Ghosh-Dastidar, S.; Adeli, H.; Dadmehr, N. Mixed-Band Wavelet-Chaos-Neural Network Methodology for Epilepsy and Epileptic Seizure Detection. IEEE Trans. Biomed. Eng. 2007, 54, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Tzallas, A.T.; Tsipouras, M.G.; Fotiadis, D.I. Epileptic Seizure Detection in EEGs Using Time-Frequency Analysis. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 703–710. [Google Scholar] [CrossRef]
- Tsuchimoto, S.; Shibusawa, S.; Iwama, S.; Hayashi, M.; Okuyama, K.; Mizuguchi, N.; Kato, K.; Ushiba, J. Use of Common Average Reference and Large-Laplacian Spatial-Filters Enhances EEG Signal-to-Noise Ratios in Intrinsic Sensorimotor Activity. J. Neurosci. Methods 2021, 353, 109089. [Google Scholar] [CrossRef]
- Qin, Y.; Xu, P.; Yao, D. A Comparative Study of Different References for EEG Default Mode Network: The Use of the Infinity Reference. Clin. Neurophysiol. 2010, 121, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.N.; Acharya, V.J. Overview of EEG Montages and Principles of Localization. J. Clin. Neurophysiol. 2019, 36, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Feyissa, A.M.; Tatum, W.O. Adult EEG. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 160, pp. 103–124. [Google Scholar]
- Marcuse, L.v.; Fields, M.C.; Yoo, J.; Rowan, A.J. Rowan’s Primer of EEG; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780323353878. [Google Scholar]
- Acharya, J.N.; Acharya, V.J. Hyperventilation-Induced EEG Slowing with Altered Awareness: Non-Epileptic, Epileptic or Both? Clin. Neurophysiol. Pract. 2021, 6, 189–190. [Google Scholar] [CrossRef]
- Hussain, L.; Aziz, W.; Alowibdi, J.S.; Habib, N.; Rafique, M.; Saeed, S.; Kazmi, S.Z.H. Symbolic Time Series Analysis of Electroencephalographic (EEG) Epileptic Seizure and Brain Dynamics with Eye-Open and Eye-Closed Subjects during Resting States. J. Physiol. Anthropol. 2017, 36, 21. [Google Scholar] [CrossRef]
- Ríos-Pohl, L.; Franco, M.; Gonzalez, M. Hyperventilation Maneuver during EEG in Children with Epilepsy after the COVID-19 Pandemic. Is a Routine Procedure Necessary? Epilepsia Open 2021, 6, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Kiffin Penry, J.; Dreifuss, F.E. Automatisms Associated with the Absence of Petit Mal Epilepsy. Arch. Neurol. 1969, 21, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Manson, G. EEG Radio Telemetry. Electroencephalogr. Clin. Neurophysiol. 1974, 37, 411–413. [Google Scholar] [CrossRef]
- Ives, J.R.; Woods, J.F. 4-Channel 24 Hour Cassette Recorder for Long-Term EEG Monitoring of Ambulatory Patients. Electroencephalogr. Clin. Neurophysiol. 1975, 39, 88–92. [Google Scholar] [CrossRef]
- Ebersole, J.S. Ambulatory Cassette EEG in Epilepsy Diagnosis. Yale J. Biol. Med. 1987, 60, 85. [Google Scholar] [PubMed]
- Beniczky, S.; Wiebe, S.; Jeppesen, J.; Tatum, W.O.; Brazdil, M.; Wang, Y.; Herman, S.T.; Ryvlin, P. Automated Seizure Detection Using Wearable Devices: A Clinical Practice Guideline of the International League Against Epilepsy and the International Federation of Clinical Neurophysiology. Clin. Neurophysiol. 2021, 132, 1173–1184. [Google Scholar] [CrossRef]
- Biondi, A.; Santoro, V.; Viana, P.F.; Laiou, P.; Pal, D.K.; Bruno, E.; Richardson, M.P. Noninvasive Mobile EEG as a Tool for Seizure Monitoring and Management: A Systematic Review. Epilepsia 2022, 63, 1041–1063. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Clifford, W.; Markham, C.; Ward, T.E.; Deegan, C. Validation of Low-Cost Wireless EEG System for Measuring Event-Related Potentials. In Proceedings of the 2018 29th IEEE Irish Signals and Systems Conference (ISSC), Belfast, UK, 21–22 June 2018; pp. 1–6. [Google Scholar]
- g.Tec Medical Engineering GmbH. G.Nautilus PRO Wearable EEG. Available online: https://www.gtec.at/product/gnautilus-pro/ (accessed on 6 March 2023).
- TMSi. SAGA 32+/64+–High Density Amplifier. Available online: https://www.tmsi.com/products/saga-32-64/ (accessed on 6 March 2023).
- B-Alert X-Series Wireless & Mobile EEG System. Available online: https://www.advancedbrainmonitoring.com/products/b-alert-x-series (accessed on 6 March 2023).
- Ambati, R.; Raja, S.; Al-Hameed, M.; John, T.; Arjoune, Y.; Shekhar, R. Neuromorphic Architecture Accelerated Automated Seizure Detection in Multi-Channel Scalp EEG. Sensors 2022, 22, 1852. [Google Scholar] [CrossRef] [PubMed]





| Features/Device | HackEEG | Texas Instrument ADS1299EEGFE | WallySci E3K | OpenBCI Cyton and Daisy |
|---|---|---|---|---|
| EEG Channel | 8 to 32 | 8 | 6 | 8 to 16 |
| Sampling Rate (Hz) | 4000 | 250 to 16,000 | 1 to 2000 | 125 or 256 |
| Connectivity | USB | USB | Bluetooth | Bluetooth |
| Raw EEG Data Access | Yes | Yes | Yes | Yes |
| Wireless | No | No | Yes | Yes |
| Cost | High | Low | Low | Low |
| Reference | [38] | [39] | [40] | [41] |
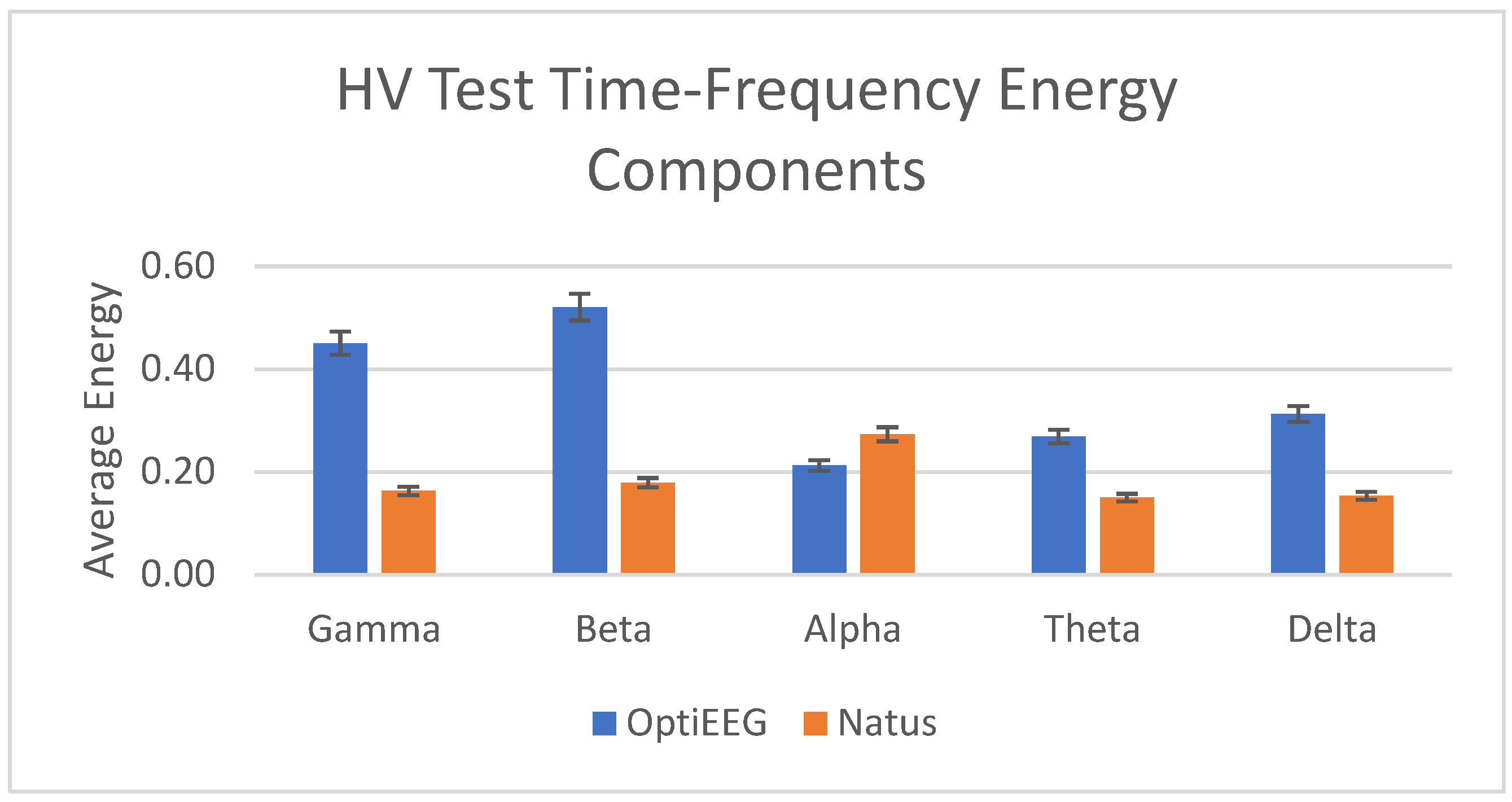
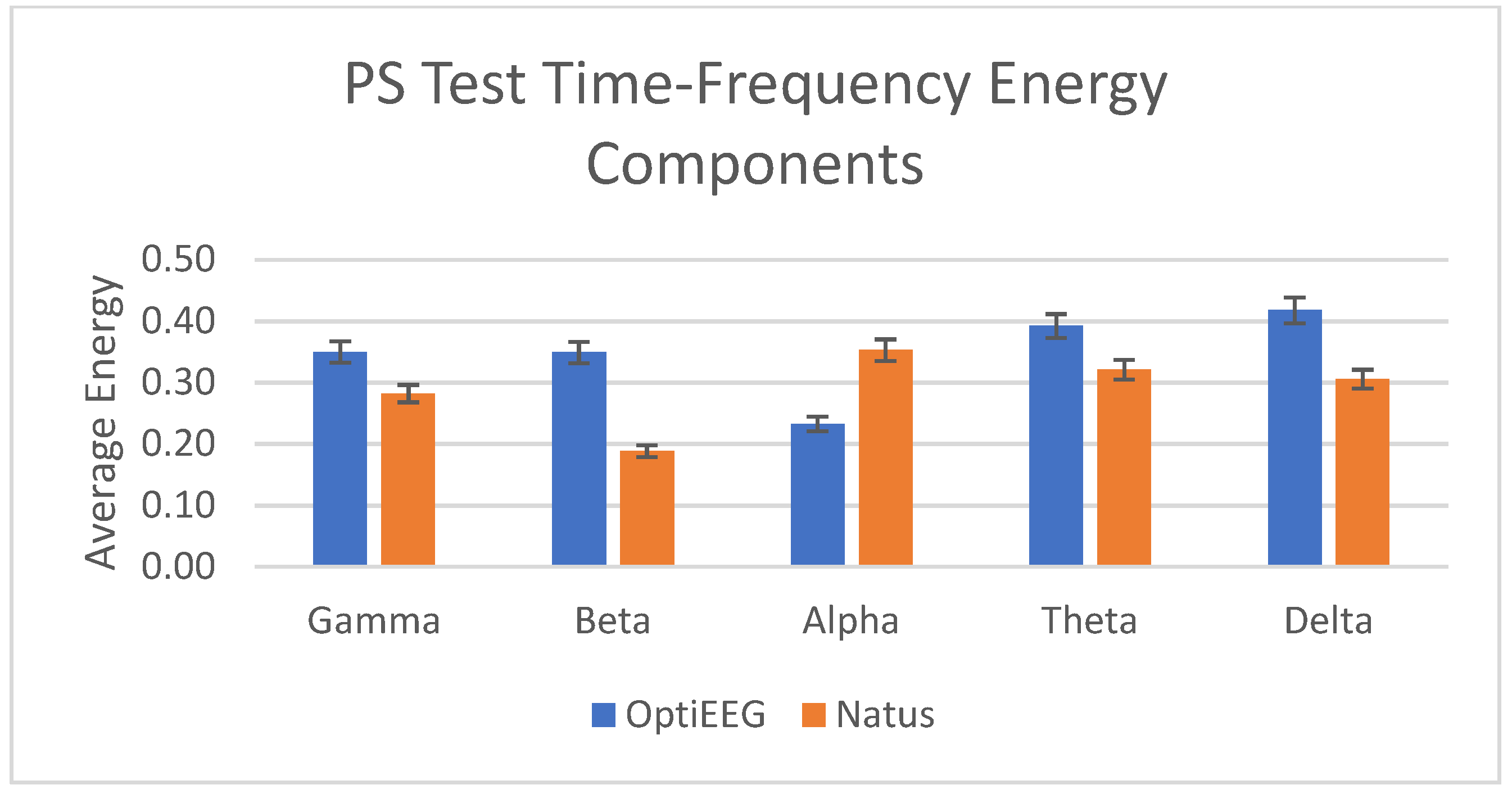
| Frequency Band | Frequency (Hz) | OptiEEG Decomposition Level | Natus Decomposition Level |
|---|---|---|---|
| Gamma | 31.3–62.5 | Detail 2 | Detail 4 |
| Beta | 15.6–31.3 | Detail 3 | Detail 5 |
| Alpha | 7.8–15.6 | Detail 4 | Detail 6 |
| Theta | 3.9–7.8 | Detail 5 | Detail 7 |
| Delta | 0–3.9 | Approximation 5 | Approximation 7 |
| OptiEEG | Natus | ||||||
|---|---|---|---|---|---|---|---|
| SNR | PSNR | MSE | SNR | PSNR | MSE | ||
| Eye Open/close | Average | −2.74 | −2.74 | 1.88 | −0.19 | −0.19 | 1.07 |
| Standard Deviation | 0.24 | 0.24 | 0.12 | 0.71 | 0.71 | 0.17 | |
| Hyperventilation | Average | −2.48 | −2.48 | 1.78 | 1.04 | 1.04 | 0.88 |
| Standard Deviation | 0.46 | 0.46 | 0.19 | 1.26 | 1.26 | 0.21 | |
| Photic | Average | −2.71 | −2.71 | 1.86 | −0.05 | -0.05 | 1.05 |
| Standard Deviation | 0.29 | 0.29 | 0.14 | 0.96 | 0.96 | 0.20 | |
| Key Feature | g.Nautilus PRO 16 g.SAHARA | SAGA (TMSI) | B-Alert X-Series | OptiEEG |
|---|---|---|---|---|
| Type | Ambulatory | Ambulatory | Ambulatory | Ambulatory |
| Input Channel | 16 | 32 | 20 | 16 |
| Setup Time | Fast | Slow | Slow | Fast |
| Operator Dependency | Not Dependent | Dependent | Dependent | Not Dependent |
| Electrode Positions | Pre-set 10–20 System | Pre-set 10–20 System | Requires measurement | Pre-set 10–20 System |
| Electrode Type | Hybrid (Dry and Gel) | Gel | Dry | Dry |
| Connectivity | Wireless (Bluetooth) | Wireless (Bluetooth) | Wireless (Bluetooth) | Wireless (Bluetooth) |
| Battery Life | 10 h | 6 h | 8 h | 20 h |
| Cost (US$) | 12,600 | 32,377 | 14,950 | 2520 |
| Reference | [72] | [73] | [74] | Proposed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shivaraja, T.R.; Remli, R.; Kamal, N.; Wan Zaidi, W.A.; Chellappan, K. Assessment of a 16-Channel Ambulatory Dry Electrode EEG for Remote Monitoring. Sensors 2023, 23, 3654. https://doi.org/10.3390/s23073654
Shivaraja TR, Remli R, Kamal N, Wan Zaidi WA, Chellappan K. Assessment of a 16-Channel Ambulatory Dry Electrode EEG for Remote Monitoring. Sensors. 2023; 23(7):3654. https://doi.org/10.3390/s23073654
Chicago/Turabian StyleShivaraja, Theeban Raj, Rabani Remli, Noorfazila Kamal, Wan Asyraf Wan Zaidi, and Kalaivani Chellappan. 2023. "Assessment of a 16-Channel Ambulatory Dry Electrode EEG for Remote Monitoring" Sensors 23, no. 7: 3654. https://doi.org/10.3390/s23073654
APA StyleShivaraja, T. R., Remli, R., Kamal, N., Wan Zaidi, W. A., & Chellappan, K. (2023). Assessment of a 16-Channel Ambulatory Dry Electrode EEG for Remote Monitoring. Sensors, 23(7), 3654. https://doi.org/10.3390/s23073654







