Enzymatic Glucose Fiber Sensor for Glucose Concentration Measurement with a Heterodyne Interferometry
Abstract
:1. Introduction
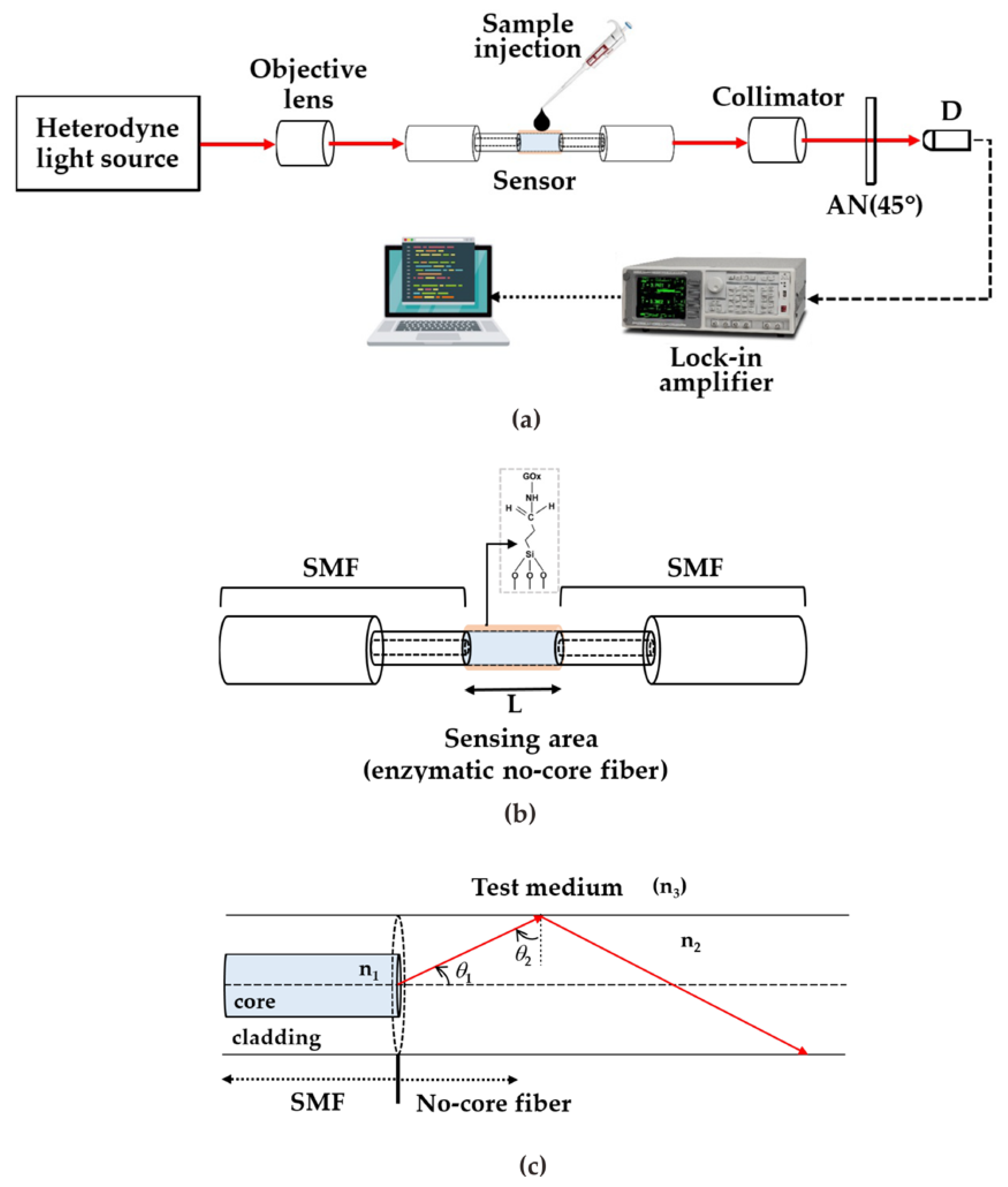
2. Principles
3. Experimental Results
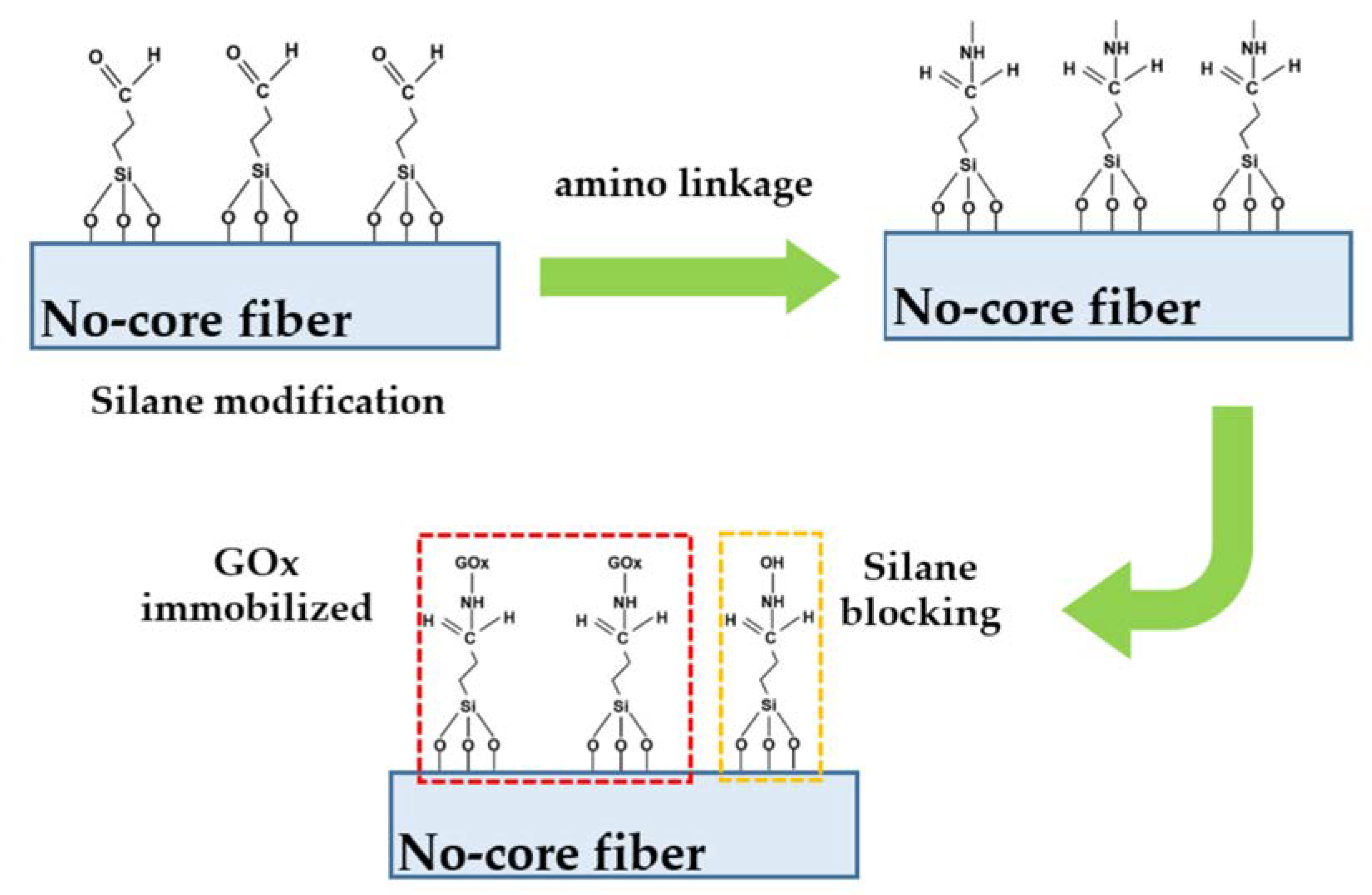
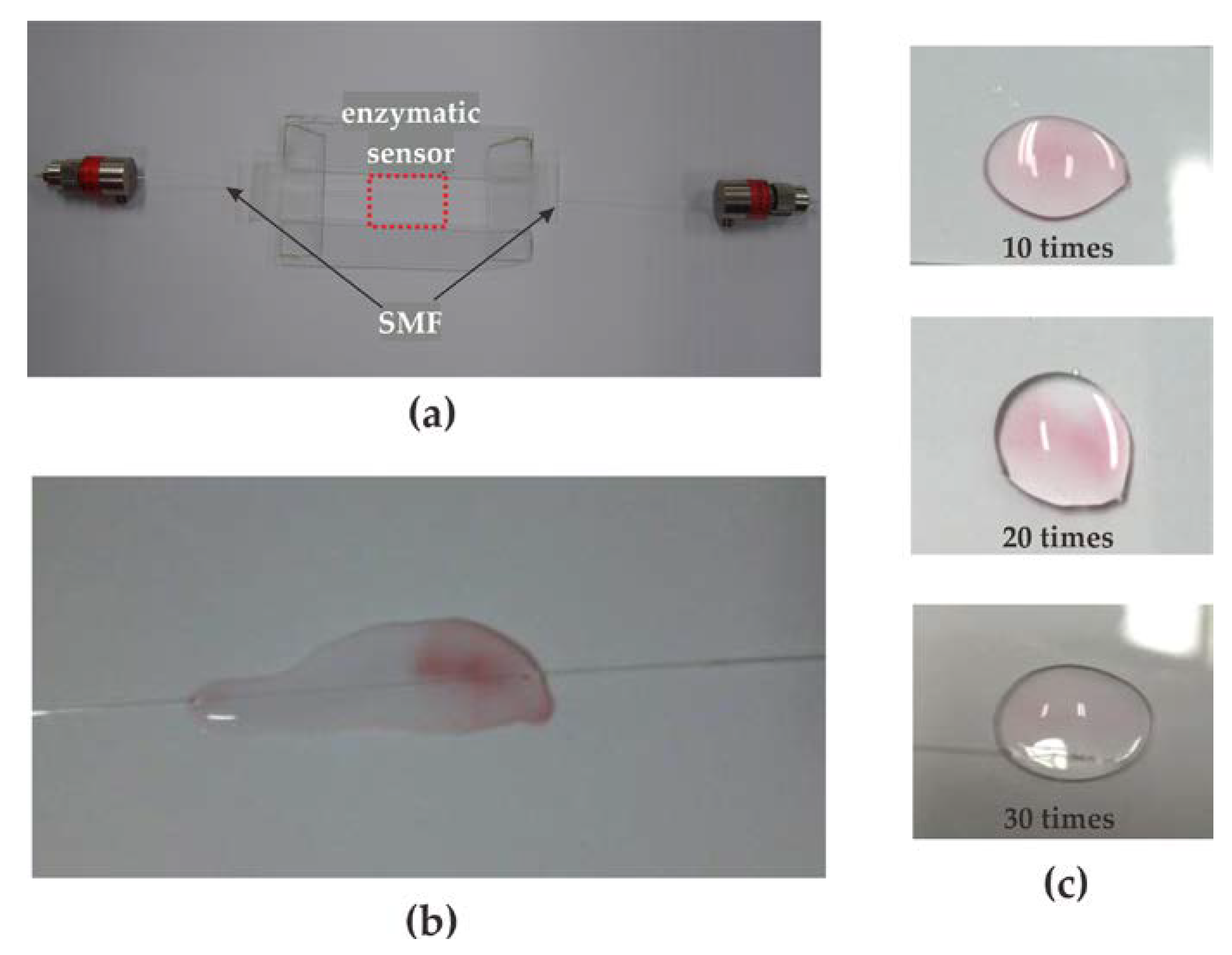
3.1. Sensor Fabrication
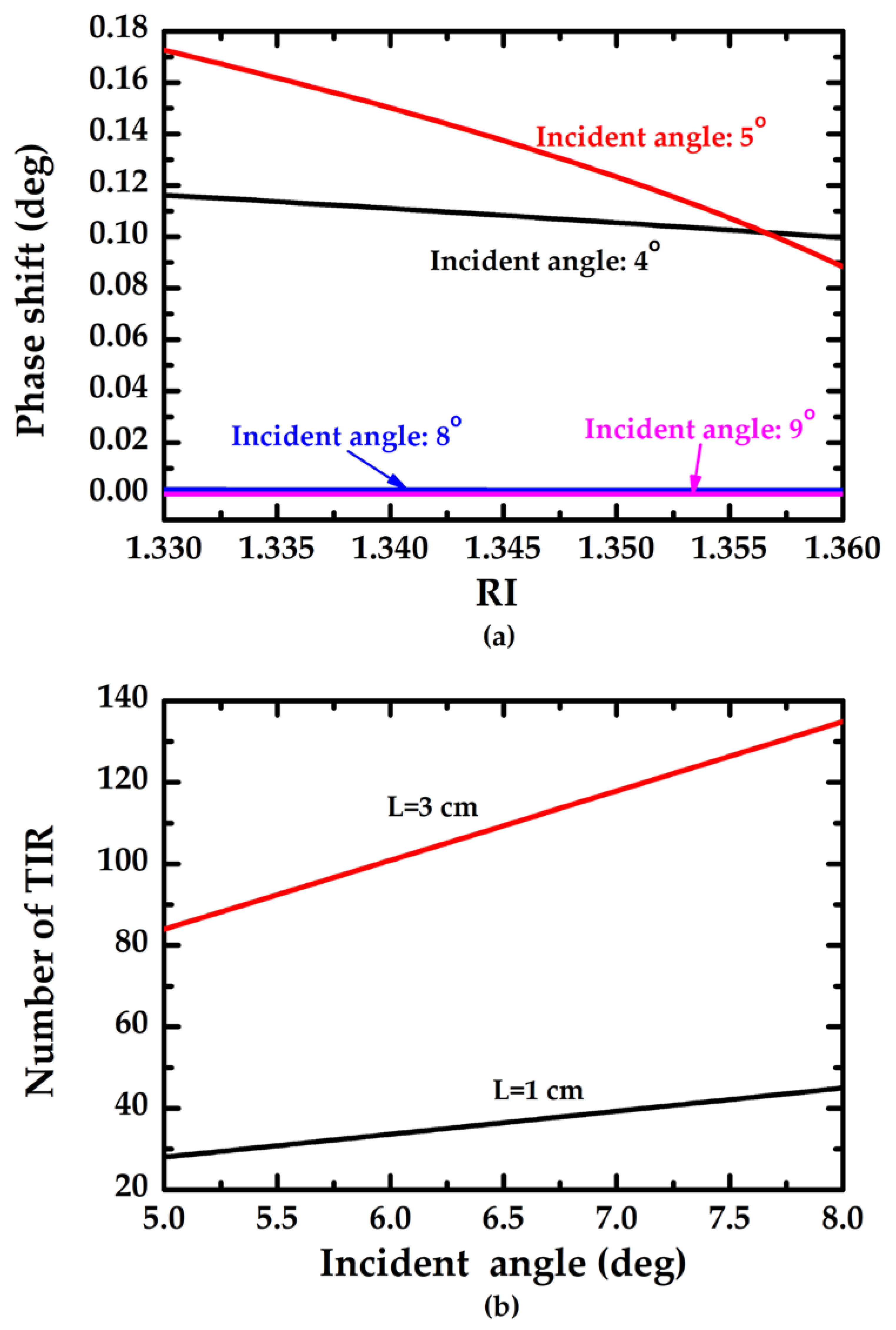
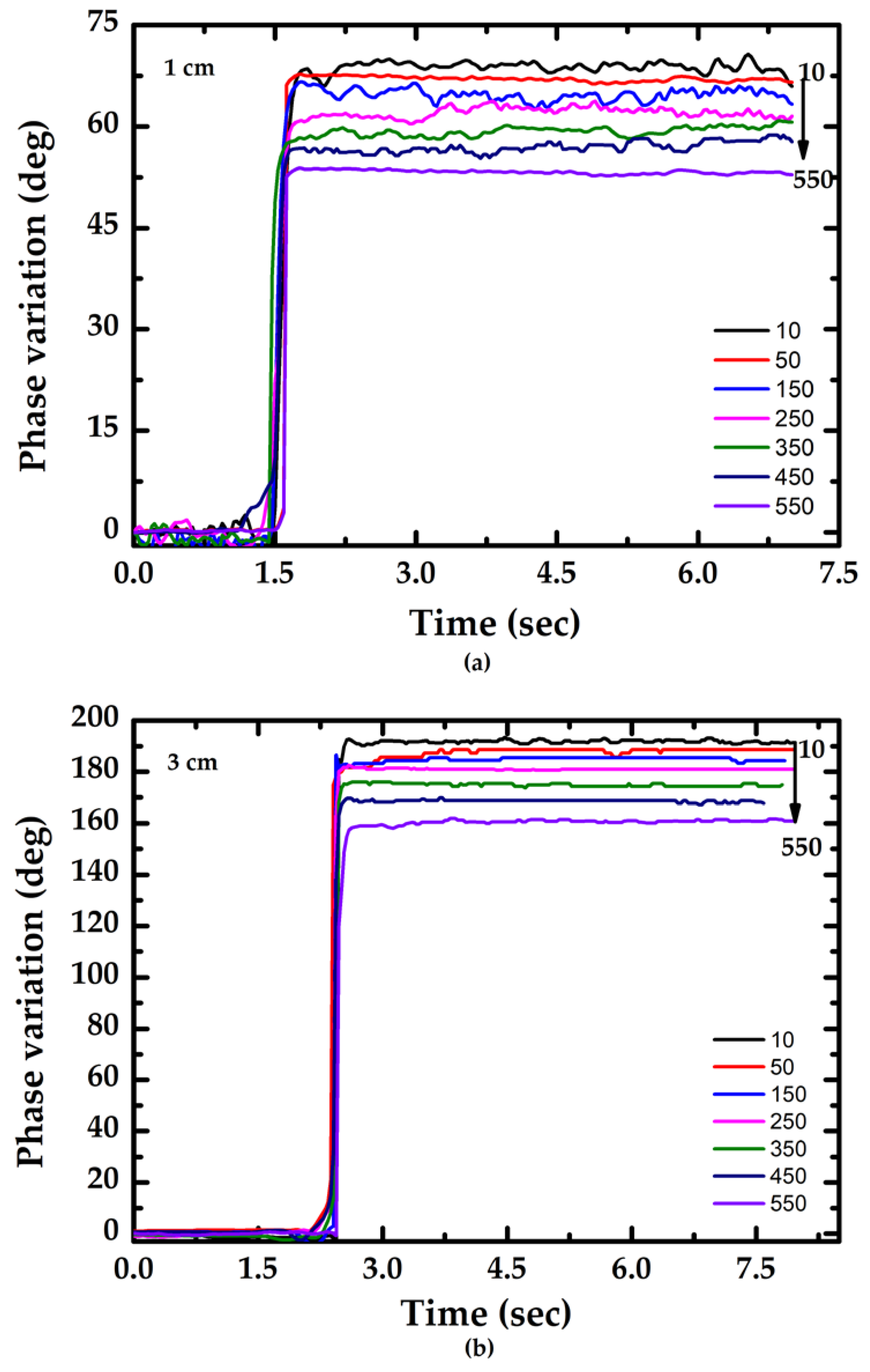
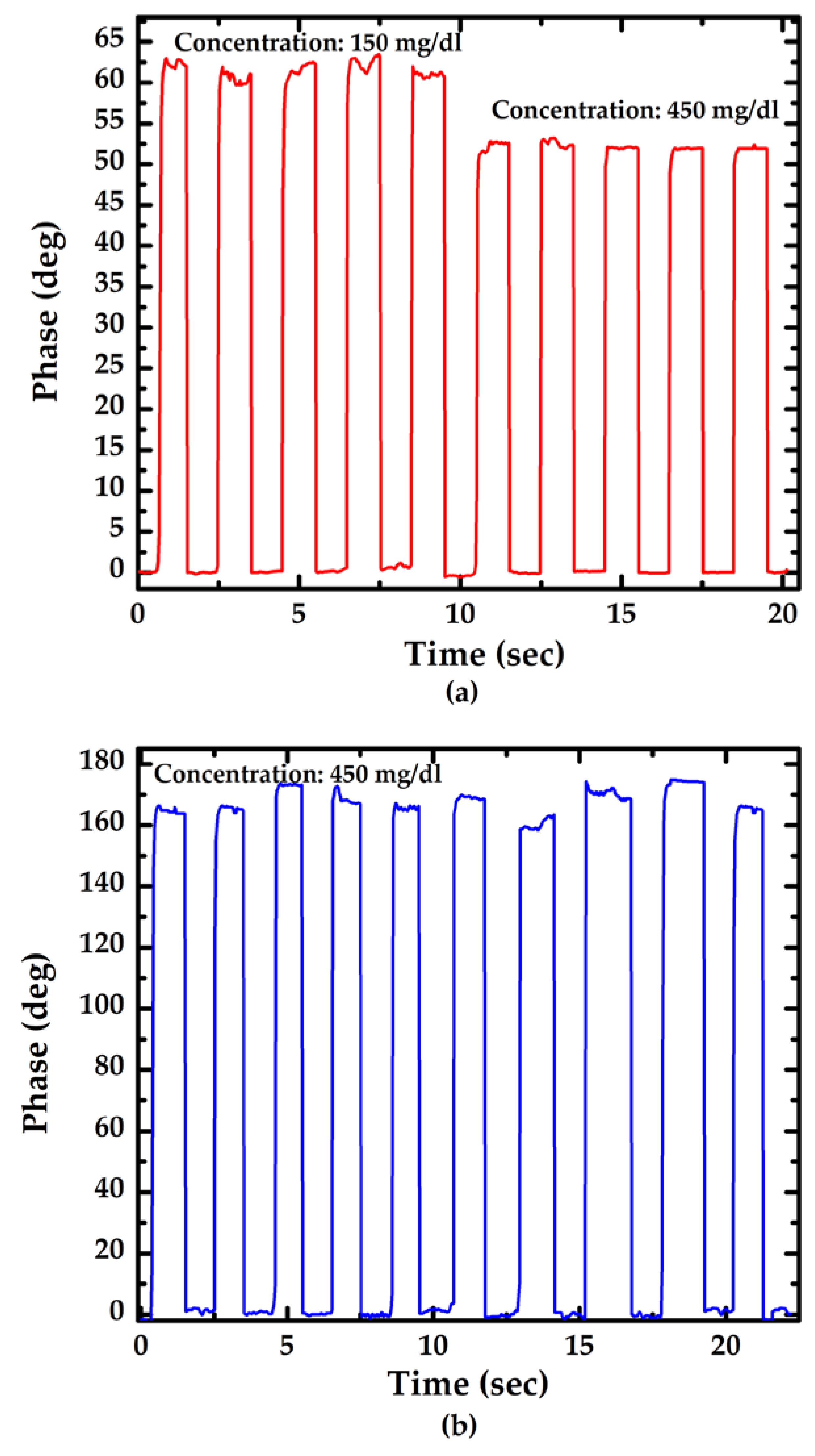
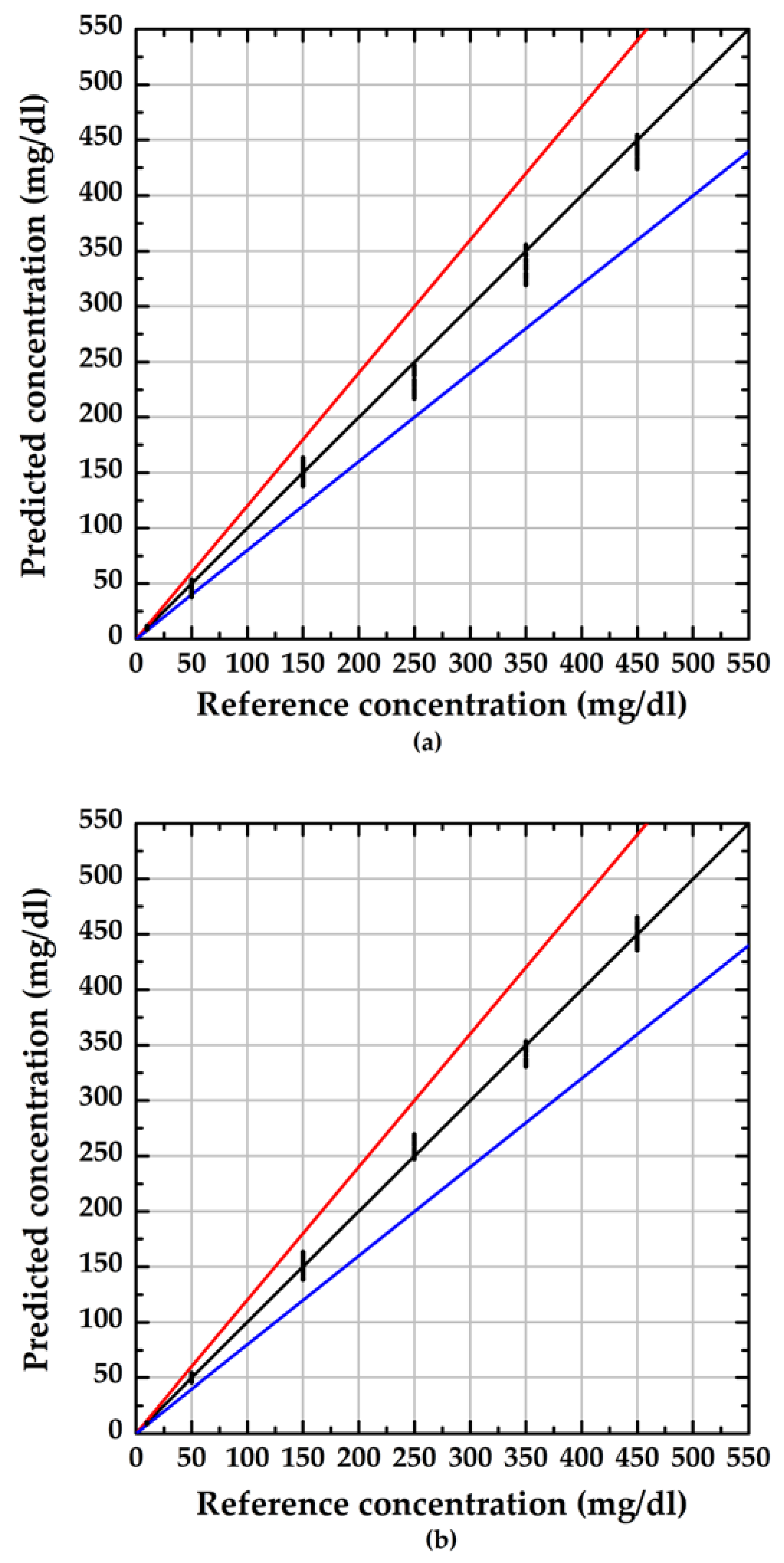
3.2. Performance of the Proposed Method
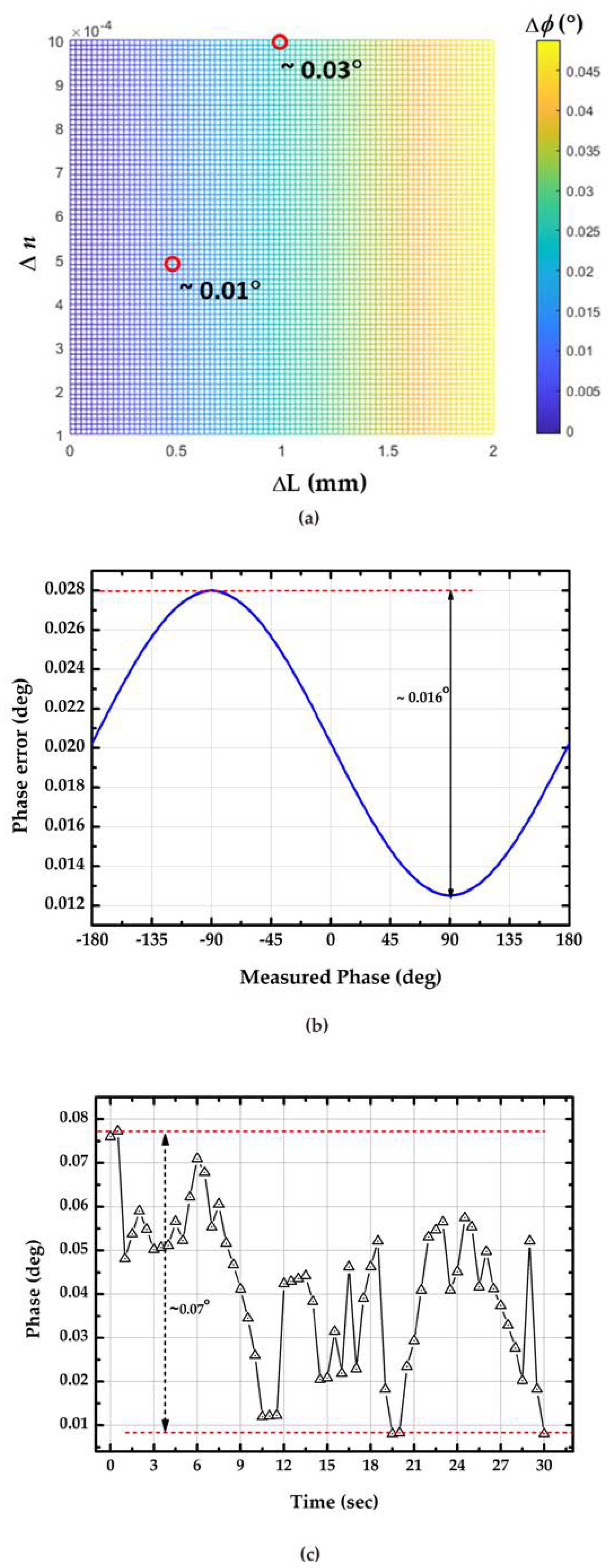
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Diabetes Statistics Report, 2020 Edition. Available online: http:chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf (accessed on 3 November 2022).
- IDF Diabetes Atlas, 2015 Edition. Available online: http:chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.diabetesatlas.org/upload/resources/previous/files/7/IDF%20Diabetes%20Atlas%207th.pdf (accessed on 3 November 2022).
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chang, S.J.; Chen, C.J.; Liu, J.T. Non-invasive blood glucose monitoring technology: A review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef] [PubMed]
- John, P.; Vasa, N.J.; Sujatha, N. Glucose sensing in the anterior chamber of the human eye model using supercontinuum source based dual wavelength low coherence interferometry. Sens. Biosens. Res. 2019, 23, 100277. [Google Scholar] [CrossRef]
- Liao, Y.T.; Yao, H.; Lingly, A.; Parviz, B. A 3-μW CMOS glucose sensor for wireless contact-lens tear glucose monitoring. IEEE J. Solid-State Circuits 2012, 47, 335–344. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.H. Enzyme-based glucose sensor: From invasive to wearable device. Adv. Healthcare Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.Y. Determination of the refractive index and the chiral parameter of chiral solution based on chiral reflection equations and heterodyne interferometry. Appl. Opt. 2008, 47, 3828–3834. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Singh, V.K. Fabrication and characterization of cascaded tapered Mach-Zehnder interferometer for refractive index sensing. Sens. Actuator A Phys. 2016, 244, 30–34. [Google Scholar] [CrossRef]
- Chiu, M.H.; Tan, C.T.; Wang, C.; He, J.N. Phase sensitive optical rotation measurement using the common-path heterodyne interferometry and a half-eave plate at a specific azimuth angle. OSA Contin. 2021, 4, 239–251. [Google Scholar] [CrossRef]
- Upadhyay, C.; Dhawan, D. Fiber Bragg grating refractive index sensor based on double D-shaped fiber. Opt. Quant. Electron. 2023, 55, 271. [Google Scholar] [CrossRef]
- Zhong, J.; Liu, S.; Zou, T.; Yan, W.; Chen, P.; Liu, B.; Sun, Z.; Wang, Y. High-sensitivity optical fiber-based glucose sensor using helical intermediate-period fiber grating. Sensors 2022, 22, 6824. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W. S-shaped long period fiber grating glucose concentration biosensor based on immobilized glucose oxidase. Optik 2020, 203, 163960. [Google Scholar] [CrossRef]
- Azkune, M.; Ruiz-Rubio, L.; Aldabaldetreku, G.; Arrospide, E.; Pérez-Álvarez, L.; Bikandi, I.; Zubia, J.; Vilas-Vilela, J.L. U-shaped and surface functionalized polymer optical fiber probe for glucose detection. Sensors 2018, 18, 34. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.C.; Hung, S.H.; Lin, Y.H.; Wu, M.R. In vitro glucose concentration measurement by a reusable enzymatic glucose sensor and a highly stable circular heterodyne polarimeter. Opt. Lett. 2021, 46, 5004–5007. [Google Scholar] [CrossRef]
- Badmos, A.A.; Sun, Q.; Sun, Z.; Zhang, J.; Yan, Z.; Lutsyk, P.; Rozhin, A.; Zhang, L. Enzyme-functionalized thin-cladding long-period fiber grating in transition mode at dispersion turning point for sugar-level and glucose detection. J. Biomed. Opt. 2017, 22, 027003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Yan, Y.; Fan, M.; Xu, C.; Wang, Y.; Shen, D.; Liu, Y.; Ma, L.; Sun, X.; Kost, A. Phenylboronic acid functionalized helical long period grating for glucose sensing. Opt. Fiber Technol. 2021, 64, 102557. [Google Scholar] [CrossRef]
- Lee, S.L.; Kim, J.; Choi, S.; Han, J.; Lee, Y.W. Optical glucose detection using birefringent long-period fiber grating functionalized with graphene oxide. Opt. Eng. 2021, 60, 087102. [Google Scholar] [CrossRef]
- Zhang, J.; Mai, X.; Hong, X.; Chen, Y.; Li, X. Optical fiber SPR biosensor with a solid-phase enzymatic reaction device for glucose detection. Sens. Actuator B Chem. 2022, 366, 131984. [Google Scholar] [CrossRef]
- Hsu, C.C.; Chen, Y.C.; Lee, J.Y.; Wu, C.C. Reusable glucose fiber sensor for measuring glucose concentration in serum. Chin. Opt. Lett. 2011, 10, 100608. [Google Scholar]
- Lin, T.Q.; Lu, Y.L.; Hsu, C.C. Fabrication of glucose fiber sensor based on immobilized GOD technique for rapid measurement. Opt. Express 2010, 18, 27560–27566. [Google Scholar] [CrossRef]
- Yeh, P. Optical Waves in Layered Media, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 1991. [Google Scholar]
- Trinder, P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969, 6, 24–27. [Google Scholar] [CrossRef]
- Barham, D.; Trinder, P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst 1972, 97, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Clarke, W.L.; Cox, D.; Gonder-Frederick, L.A.; Carter, W.; Pohl, S.L. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care 1987, 10, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Self-Monitoring Blood Glucose Test Systems for Over-the-Counter Use: Guidance for Industry and Food and Drug Administration Staff; FDA U.S.: Silver Spring, MD, USA, 2018.
- Wu, C.M.; Lawall, J.; Deslattes, R.D. Heterodyne interferometer with subatomic period nonlinearity. Appl. Opt. 1999, 38, 4089–4094. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.A.; Gylfason, K.B.; Sánchez, B.; Griol, A.; Sohlström, H.; Holgado, M. Slot-waveguide biochemical sensor. Opt. Lett. 2007, 32, 3080–3082. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Deka, B.; Sahu, P.P. Modeling and fabrication of evanescent waveguide-based optical sensor for sensitivity enhancement using silicon oxynitride technology. Opt. Eng. 2013, 52, 077101. [Google Scholar] [CrossRef]
- Wu, X.J.; Choi, M.M.F. An optical glucose biosensor based on entrapped-glucose oxidase in silicate xerogel hybridized with hydroxyethyl carboxymethyl cellulose. Anal. Chim. Acta 2004, 514, 219–226. [Google Scholar] [CrossRef]
- Certificate of Analysis of Standard Reference Material 965; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1996.
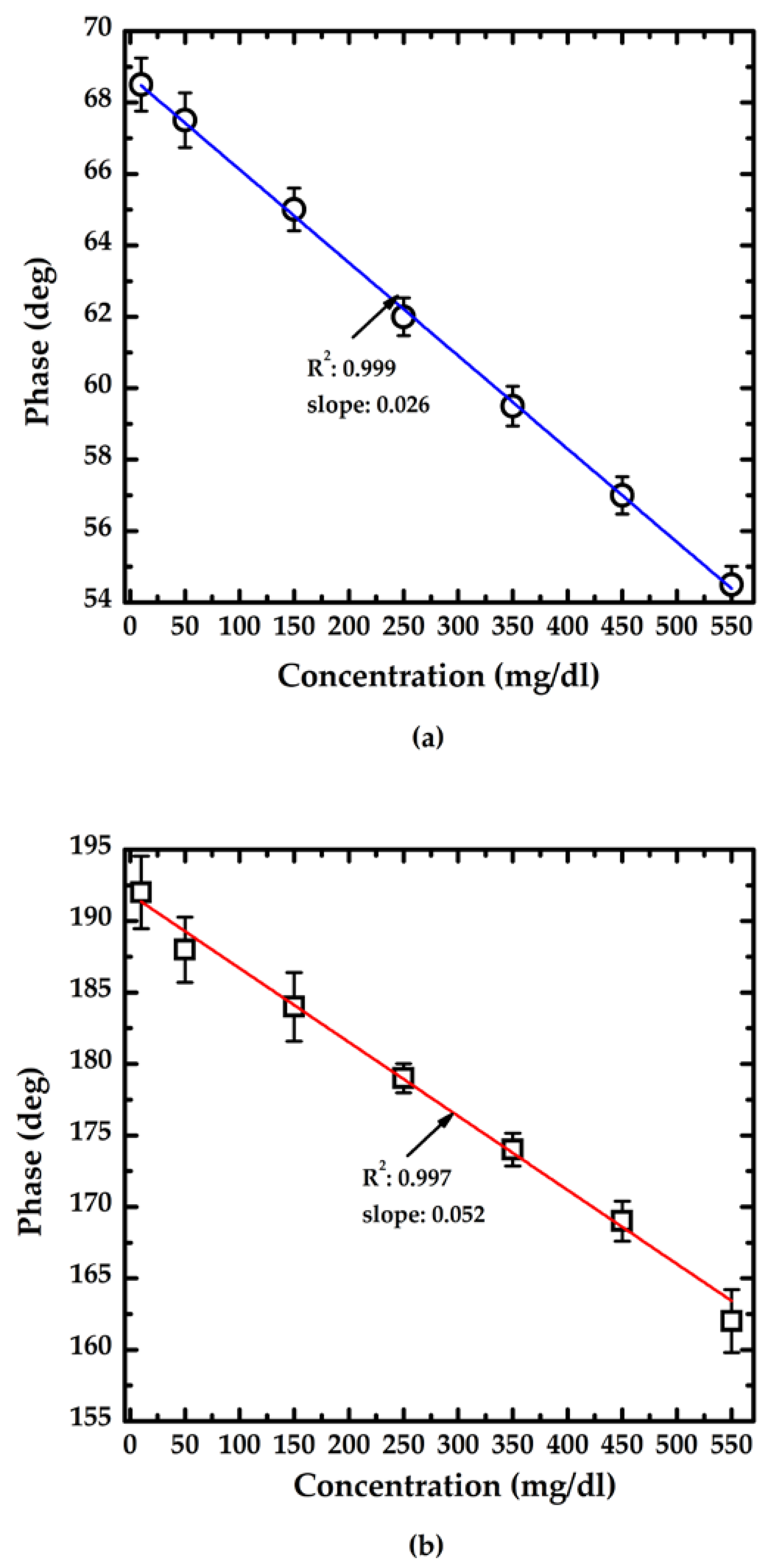
| L | |S| (°/(mg/dL)) | Theoretical | Practical | ||
|---|---|---|---|---|---|
| Δϕ (°) | |ΔC| (mg/dL) | Δϕ (°) | |ΔC| (mg/dL) | ||
| 1 cm | 0.026 | 0.03 | 1.154 | 0.07 | 2.692 |
| 3 cm | 0.052 | 0.577 | 1.346 | ||
| Ref. | Detection Limit | Linear Range | Response Time | Reusability | Method | Enzyme Adopted |
|---|---|---|---|---|---|---|
| [9] | Better than 50% | X | X | X | Polarimeter | X |
| [10] | 2% | 0%–10% | X | X | Double tapped Mach-Zehnder interferometer | X |
| [11] | Better than 10 mg/dL | 10–1000 mg/dL | X | X | Polarimeter | X |
| [12] | 10% | 0%–50% | X | X | Double D-shaped FBG | X |
| [13] | 1 mg/mL | 0.02–200 mg/mL | X | X | HIPFG | X |
| [14] | 0.25 wt% | 0 wt%–1 wt% | X | X | S-shaped LPFG | GOx |
| [15] | 0.1 M | X | 10 min | X | U-shaped fiber | PBA-ARS |
| [16] | 1.41 mg/dL | 1–450 mg/dL | <10 s | >100 times | Circular polarimeter | GOx |
| [17] | X | 0.1–3.2 mg/mL | X | X | LPFG | GOx |
| [18] | 0.037 mg/mL | 0.18–3 mg/mL | X | X | HLPG | 4-VPBA |
| [19] | 5 mM | 5–25 mM | X | X | PM-LPFG | GOx |
| [20] | X | 20–400 mg/dL | ~8 min | 10 times | SPR fiber | GOx |
| [21] | 0.139 mg/dL | SRM 965a [32] | <2 s | 13 times | SMF | GOx |
| This work | 1.346 mg/dL | 10–550 mg/dL | <3 s | 30 times | No-core fiber with heterodyne interferometry | GOx |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-C.; Chung, W.-Y.; Chang, C.-Y.; Wu, C.-C.; Lee, C.-L. Enzymatic Glucose Fiber Sensor for Glucose Concentration Measurement with a Heterodyne Interferometry. Sensors 2023, 23, 2990. https://doi.org/10.3390/s23062990
Hsu C-C, Chung W-Y, Chang C-Y, Wu C-C, Lee C-L. Enzymatic Glucose Fiber Sensor for Glucose Concentration Measurement with a Heterodyne Interferometry. Sensors. 2023; 23(6):2990. https://doi.org/10.3390/s23062990
Chicago/Turabian StyleHsu, Cheng-Chih, Wan-Yu Chung, Chun-Yi Chang, Chyan-Chyi Wu, and Cheng-Ling Lee. 2023. "Enzymatic Glucose Fiber Sensor for Glucose Concentration Measurement with a Heterodyne Interferometry" Sensors 23, no. 6: 2990. https://doi.org/10.3390/s23062990
APA StyleHsu, C.-C., Chung, W.-Y., Chang, C.-Y., Wu, C.-C., & Lee, C.-L. (2023). Enzymatic Glucose Fiber Sensor for Glucose Concentration Measurement with a Heterodyne Interferometry. Sensors, 23(6), 2990. https://doi.org/10.3390/s23062990






