A Novel IoT-Enabled Healthcare Monitoring Framework and Improved Grey Wolf Optimization Algorithm-Based Deep Convolution Neural Network Model for Early Diagnosis of Lung Cancer
Abstract
1. Introduction
- We developed an IoT platform, inferred the mechanism for acquiring lung disease data, and investigated the process for extracting the most significant attributes employing the Tasmanian Devil Optimization (TDO) algorithm, which enables high accuracy in the diagnosis of lung cancer.
- We investigate the mechanism of the Grey-Wolf Optimization algorithm and modify its convergence rates, resulting in an improved GWO algorithm that is employed to fine-tune the parameters of the deep convolutional neural network model. Eventually, we presented an IoT-enabled platform with an IGWO-based DCNN model for lung cancer detection.
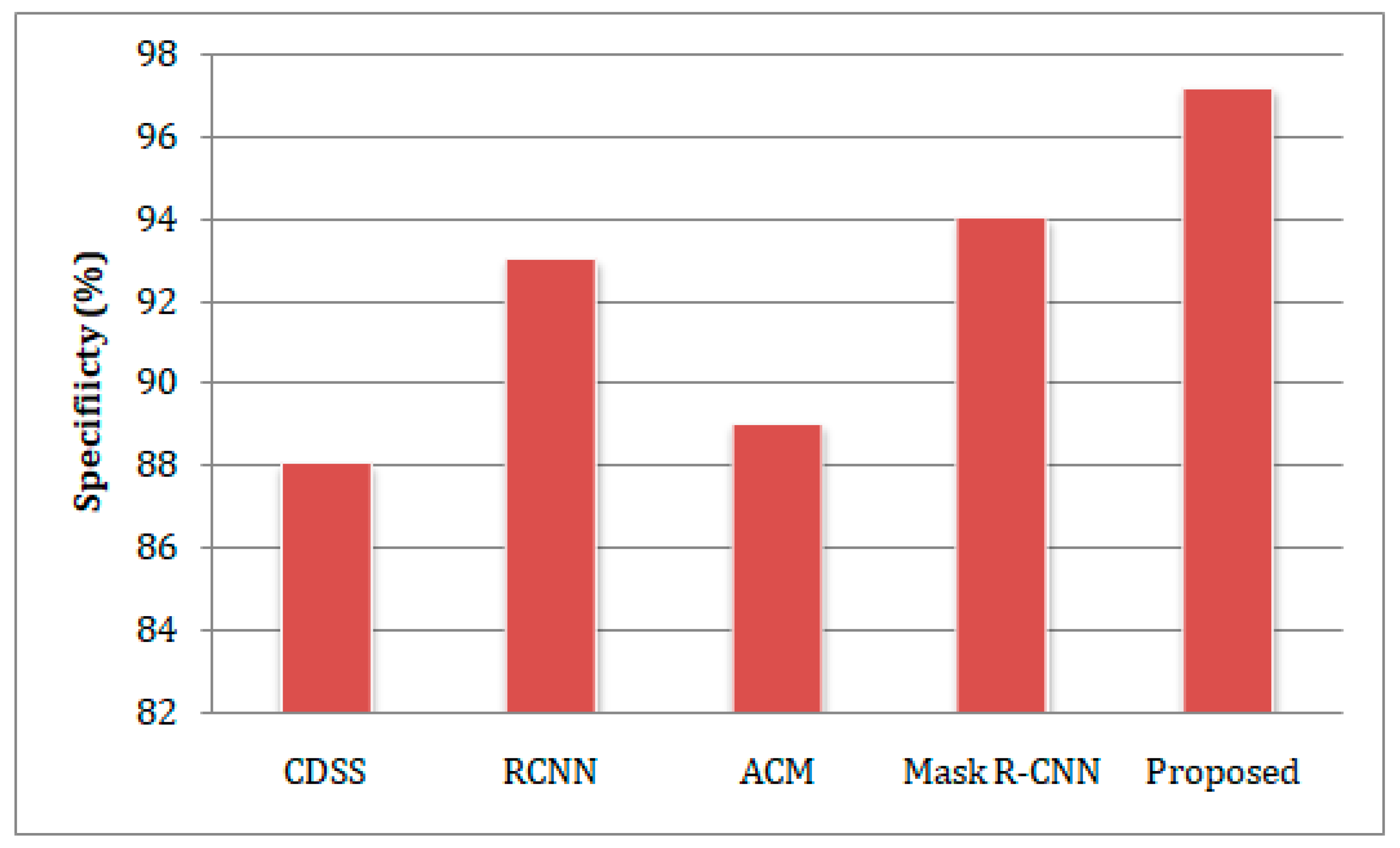
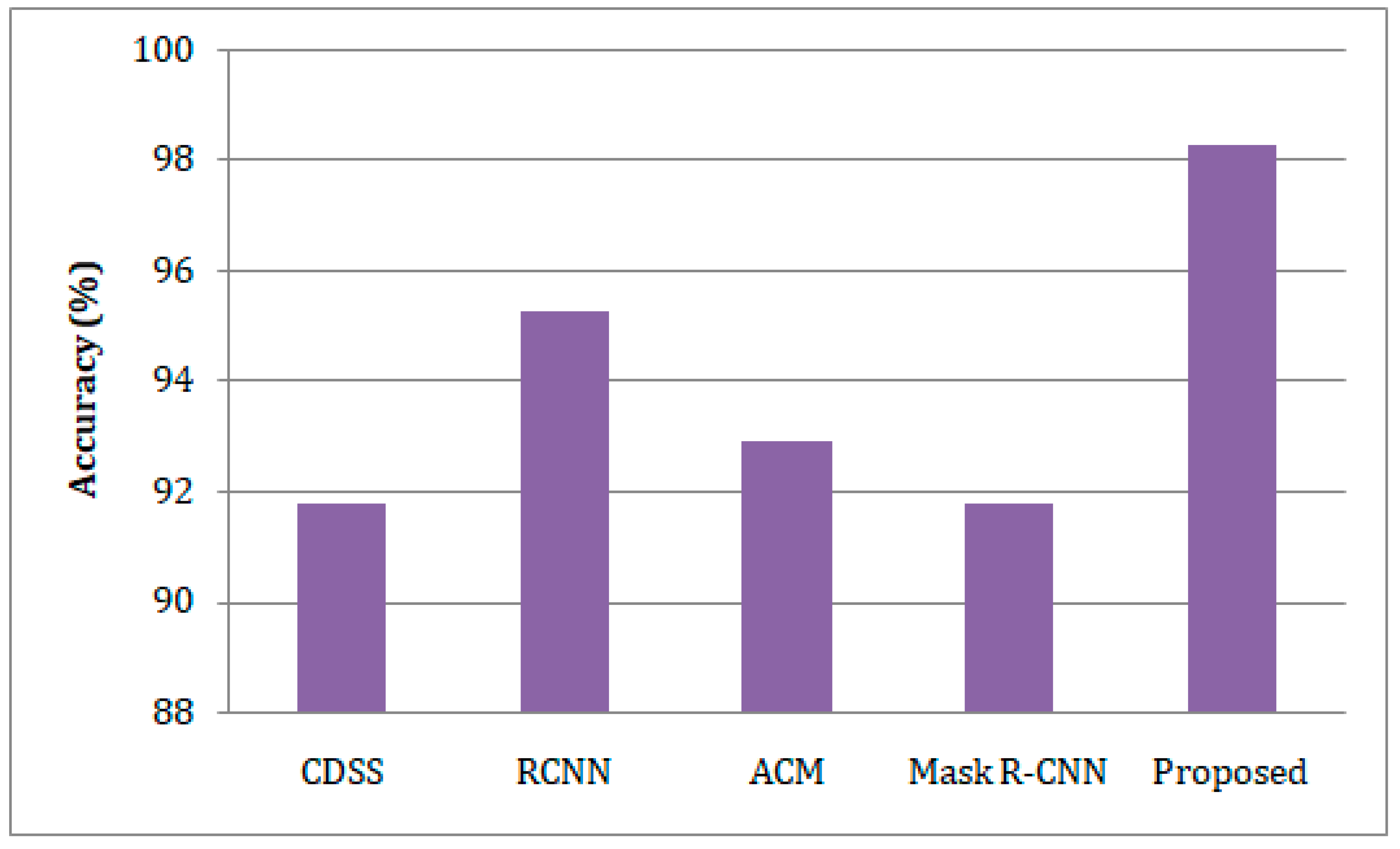
- The developed model was trained and tested on the benchmark Exasens dataset, and its accuracy, sensitivity, specificity, and precision were evaluated against state-of-the-art clinical decision support systems (CDSS), regional-based convolutional neural networks (RCNN), active contour method (ACM), and Mask Region-Convolutional Neural Networks (Mask R-CNN) models for lung cancer detection.
2. Literature Survey
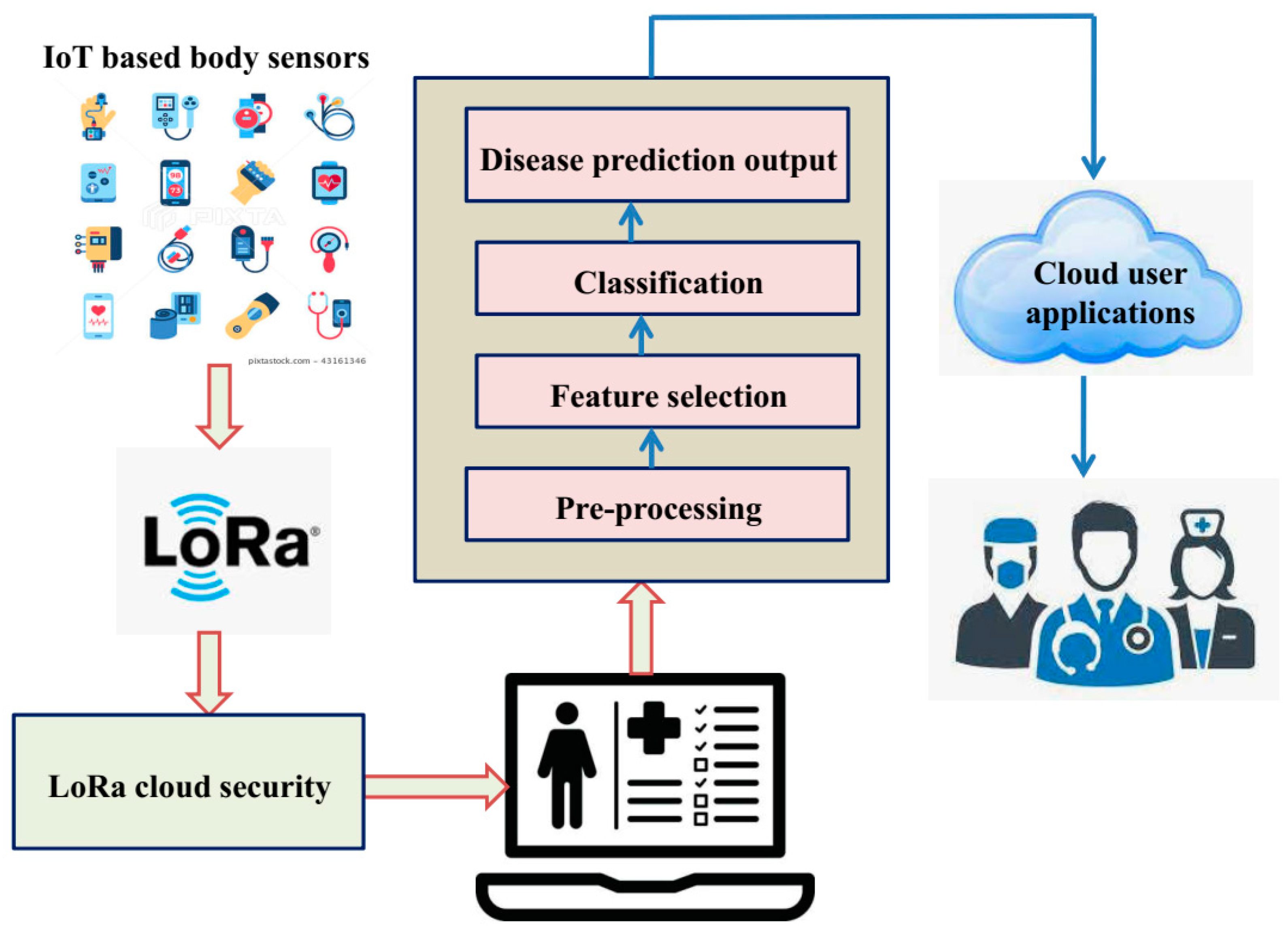
3. The Proposed IoT-Enabled Platform with IGWO-Based DCNN Model
3.1. Pre-Processing
3.2. Feature Selection
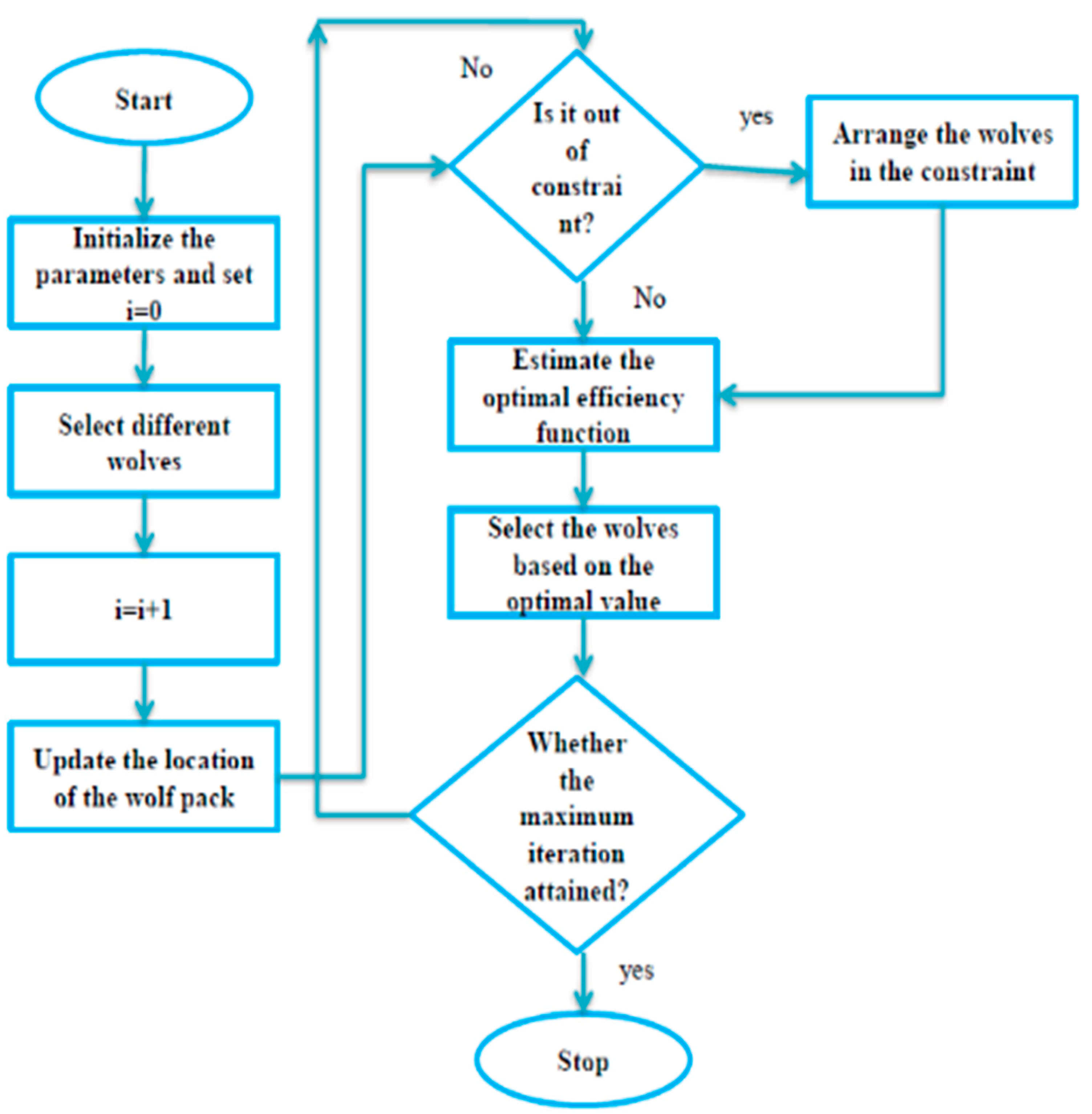
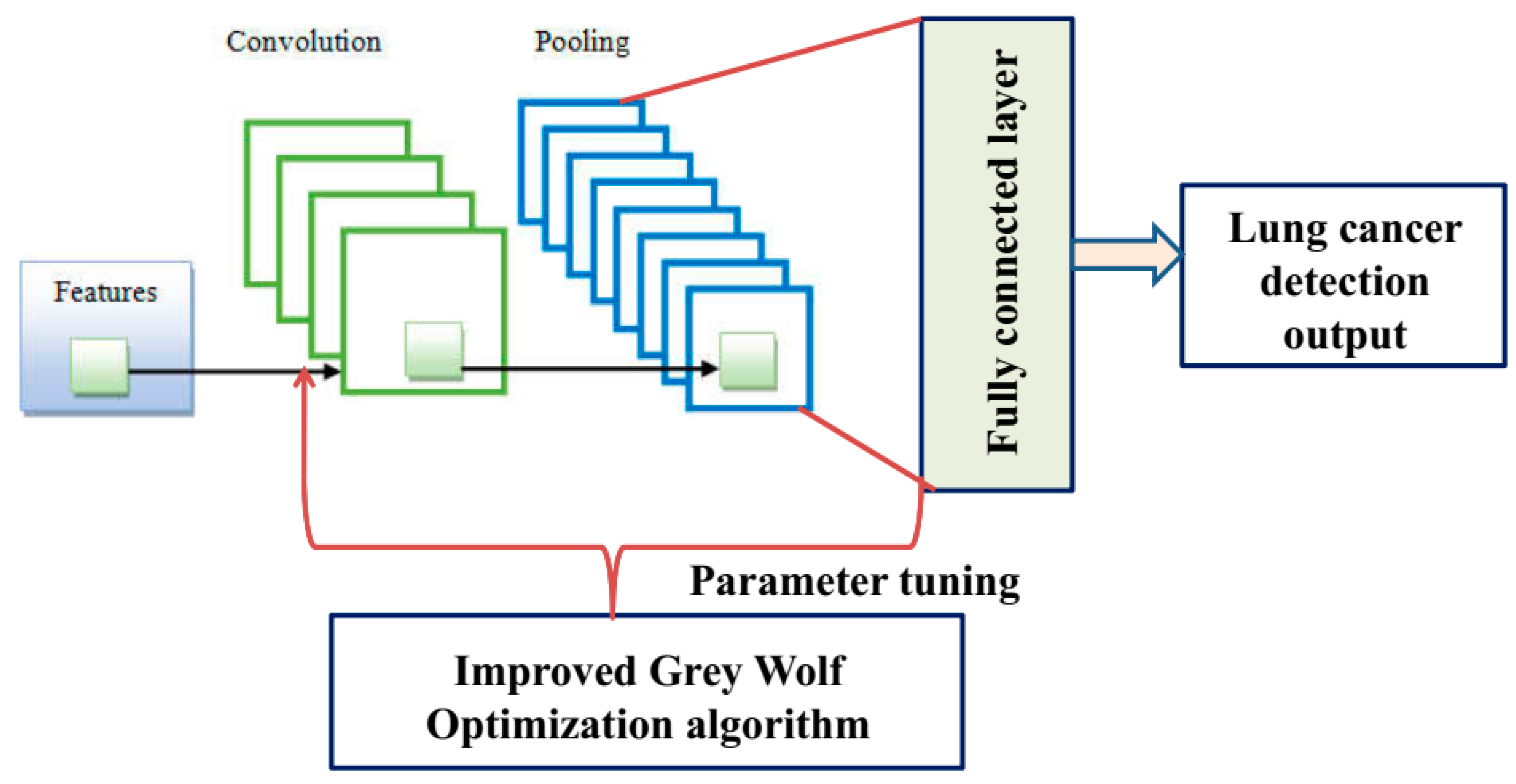
3.3. Improved Grey Wolf Optimization Algorithm-Based Deep-CNN for Lung Cancer Detection
- Improved Grey Wolf Optimization
- B.
- Improved grey wolf optimization algorithm-based deep convolution neural network model
4. Results and Discussion
4.1. Dataset Explanation
4.2. Experimental Setup
4.3. Performance Metrics
- (i)
- Sensitivity
- (ii)
- Specificity
- (iii)
- Accuracy
- (iv)
- Precision (Negative Predict Value)
- (v)
- Disease Prevalence
- (vi)
- Negative Predict Value
4.4. Comparative Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yang, X.; Zhang, X.; Curran, W.J.; Liu, T. Ultrasound elastography for lung disease assessment. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Demir, F.; Sengur, A.; Bajaj, V. Convolutional neural networks-based efficient approach for classification of lung diseases. Health Inf. Sci. Syst. 2020, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, R.; Kloer, P.; Lewis, K.E. A review of novel biological tools used in screening for the early detection of lung cancer. Postgrad. Med. J. 2009, 85, 358–363. [Google Scholar] [CrossRef]
- Feng, F.; Wu, Y.; Wu, Y.; Nie, G.; Ni, R. The effect of artificial neural network model combined with six tumor markers in auxiliary diagnosis of lung cancer. J. Med. Syst. 2012, 36, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.K.; Lee, M.S. Biomarkers for lung cancer: Clinical uses. Curr. Opin. Pulm. Med. 2007, 13, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.C.; Blaszkowsky, L.; Bharti, A.; Ladanyi, A.; Kraeft, S.K.; Bruno, A.; Salgia, R. Circulating tumor cells and serum tumor biomarkers in small cell lung cancer. Anticancer Res. 2003, 23, 49–62. [Google Scholar] [PubMed]
- Chu, X.Y.; Hou, X.B.; Song, W.A.; Xue, Z.Q.; Wang, B.; Zhang, L.B. Diagnostic values of SCC, CEA, Cyfra21-1 and NSE for lung cancer in patients with suspicious pulmonary masses: A single center analysis. Cancer Biol. Ther. 2011, 11, 995–1000. [Google Scholar] [CrossRef]
- Prakash, N.; Kumar, A.; Kumar, P. Neuron-specific enolase as a biomarker: Biochemical and clinical aspects. Sensors 2015, 15, 24375–24396. [Google Scholar] [CrossRef]
- Park, S.; Ock, C.-Y.; Kim, H.; Pereira, S.; Park, S.; Ma, M.; Choi, S.; Kim, S.; Shin, S.; Aum, B.J.; et al. Artificial Intelligence–Powered Spatial Analysis of Tumor-Infiltrating Lymphocytes as Complementary Biomarker for Immune Checkpoint Inhibition in Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1916. [Google Scholar] [CrossRef]
- Caron, J.; Mangé, A.; Guillot, B.; Solassol, J. Highly sensitive detection of melanoma based on serum proteomic profiling. J. Cancer Res. Clin. Oncol. 2009, 135, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Shafique, K.; Khawaja, B.A.; Sabir, F.; Qazi, S.; Mustaqim, M. Internet of things (IoT) for next-generation smart systems: A review of current challenges, future trends and prospects for emerging 5G-IoT scenarios. IEEE Access 2020, 8, 23022–23040. [Google Scholar] [CrossRef]
- Booij, T.M.; Chiscop, I.; Meeuwissen, E.; Moustafa, N.; den Hartog, F.T. ToN_IoT: The Role of Heterogeneity and the Need for Standardization of Features and Attack Types in IoT Network Intrusion Data Sets. IEEE Internet Things J. 2021, 9, 485–496. [Google Scholar] [CrossRef]
- Ratnaparkhi, S.; Khan, S.; Arya, C.; Khapre, S.; Singh, P.; Diwakar, M.; Shankar, A. Smart agriculture sensors in IOT: A review. Mater. Today Proc. 2020. [CrossRef]
- Alam, M.M.; Malik, H.; Khan, M.I.; Pardy, T.; Kuusik, A.; Le Moullec, Y. A survey on the roles of communication technologies in IoT-based personalized healthcare applications. IEEE Access 2018, 6, 36611–36631. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, H.; Li, Z.; Liu, X. A survey on multimodal data-driven smart healthcare systems: Approaches and applications. IEEE Access 2019, 7, 133583–133599. [Google Scholar] [CrossRef]
- Wang, L. Deep Learning Techniques to Diagnose Lung Cancer. Cancers 2022, 14, 5569. [Google Scholar] [CrossRef]
- Almezhghwi, K.; Serte, S.; Al-Turjman, F. Convolutional neural networks for the classification of chest X-rays in the IoT era. Multimed. Tools Appl. 2021, 80, 29051–29065. [Google Scholar] [CrossRef]
- Han, T.; Nunes, V.X.; Souza, L.F.D.F.; Marques, A.G.; Silva, I.C.L.; Junior, M.A.A.F.; Sun, J.; Reboucas Filho, P.P. Internet of medical things—Based on deep learning techniques for segmentation of lung and stroke regions in CT scans. IEEE Access 2020, 8, 71117–71135. [Google Scholar] [CrossRef]
- Rehm, G.B.; Woo, S.H.; Chen, X.L.; Kuhn, B.T.; Cortes-Puch, I.; Anderson, N.R.; Adams, J.Y.; Chuah, C.N. Leveraging IoTs and machine learning for patient diagnosis and ventilation management in the intensive care unit. IEEE Pervasive Comput. 2020, 19, 68–78. [Google Scholar] [CrossRef]
- Ahmed, I.; Ahmad, A.; Jeon, G. An IoT-based deep learning framework for early assessment of COVID-19. IEEE Internet Things J. 2020, 8, 15855–15862. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Yu, L.; Ye, L.; Yao, D.D.; Zhuang, W. Length-of-stay prediction for pediatric patients with respiratory diseases using decision tree methods. IEEE J. Biomed. Health Inform. 2020, 24, 2651–2662. [Google Scholar] [CrossRef]
- Xu, Y.; Holanda, G.; Souza, L.F.D.F.; Silva, H.; Gomes, A.; Silva, I.; Ferreira, M.; Jia, C.; Han, T.; de Albuquerque, V.H.C.; et al. Deep learning-enhanced internet of medical things to analyze brain CT scans of hemorrhagic stroke patients: A new approach. IEEE Sens. J. 2020, 21, 24941–24951. [Google Scholar] [CrossRef]
- Skourt, B.A.; El Hassani, A.; Majda, A. Lung CT image segmentation using deep neural networks. Procedia Comput. Sci. 2018, 127, 109–113. [Google Scholar] [CrossRef]
- Medeiros, A.G.; Guimarães, M.T.; Peixoto, S.A.; Santos, L.D.O.; da Silva Barros, A.C.; Rebouças, E.D.S.; de Albuquerque, V.H.C.; Rebouças Filho, P.P. A new fast morphological geodesic active contour method for lung CT image segmentation. Measurement 2019, 148, 106687. [Google Scholar] [CrossRef]
- Cai, L.; Long, T.; Dai, Y.; Huang, Y. Mask R-CNN-based detection and segmentation for pulmonary nodule 3D visualization diagnosis. IEEE Access 2020, 8, 44400–44409. [Google Scholar] [CrossRef]
- Chaudhary, A.; Singh, S.S. Lung cancer detection on CT images by using image processing. In Proceedings of the 2012 International Conference on Computing Sciences, Phagwara, India, 14–15 September 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 142–146. [Google Scholar]
- Polat, K.; Güneş, S. Principles component analysis, fuzzy weighting pre-processing and artificial immune recognition system based diagnostic system for diagnosis of lung cancer. Expert Syst. Appl. 2008, 34, 214–221. [Google Scholar] [CrossRef]
- Dehghani, M.; Hubálovský, Š.; Trojovský, P. Tasmanian Devil Optimization: A New Bio-Inspired Optimization Algorithm for Solving Optimization Algorithm. IEEE Access 2022, 10, 19599–19620. [Google Scholar] [CrossRef]
- Kozakiewicz, C.P.; Ricci, L.; Patton, A.H.; Stahlke, A.R.; Hendricks, S.A.; Margres, M.J.; Ruiz-Aravena, M.; Hamilton, D.G.; Hamede, R.; McCallum, H. Comparative landscape genetics reveals differential effects of environment on host and pathogen genetic structure in Tasmanian devils (Sarcophilus harrisii) and their transmissible tumour. Mol. Ecol. 2020, 29, 3217–3233. [Google Scholar] [CrossRef] [PubMed]
- McCallum, H.; Tompkins, D.M.; Jones, M.; Lachish, S.; Marvanek, S.; Lazenby, B.; Hocking, G.; Wiersma, J.; Hawkins, C.E. Distribution and impacts of Tasmanian devil facial tumor disease. EcoHealth 2007, 4, 318–325. [Google Scholar] [CrossRef]
- Russell, T.; Lisovski, S.; Olsson, M.; Brown, G.; Spindler, R.; Lane, A.; Keeley, T.; Hibbard, C.; Hogg, C.J.; Thomas, F.; et al. MHC diversity and female age underpin reproductive success in an Australian icon; The Tasmanian Devil. Sci. Rep. 2018, 8, 4175. [Google Scholar] [CrossRef]
- Sun, X.; Hu, C.; Lei, G.; Guo, Y.; Zhu, J. State feedback control for a PM hub motor based on gray wolf optimization algorithm. IEEE Trans. Power Electron. 2019, 35, 1136–1146. [Google Scholar] [CrossRef]
- Novak, E. Deterministic and Stochastic Error Bounds in Numerical Analysis; Springer: Berlin/Heidelberg, Germany, 2006; Volume 1349. [Google Scholar]
- Xie, Q.; Guo, Z.; Liu, D.; Chen, Z.; Shen, Z.; Wang, X. Optimization of heliostat field distribution based on improved Gray Wolf optimization algorithm. Renew. Energy 2021, 176, 447–458. [Google Scholar] [CrossRef]
- Ren, A.; Li, Z.; Ding, C.; Qiu, Q.; Wang, Y.; Li, J.; Qian, X.; Yuan, B. Sc-dcnn: Highly-scalable deep convolutional neural network using stochastic computing. ACM Sigplan Not. 2017, 52, 405–418. [Google Scholar] [CrossRef]
- Jalali SM, J.; Ahmadian, S.; Khodayar, M.; Khosravi, A.; Shafie-khah, M.; Nahavandi, S.; Catalao, J.P. An advanced short-term wind power forecasting framework based on the optimized deep neural network models. Int. J. Electr. Power Energy Syst. 2022, 141, 108143. [Google Scholar] [CrossRef]
- Jakhar, K.; Hooda, N. Big data deep learning framework using keras: A case study of pneumonia prediction. In Proceedings of the 2018 4th International Conference on Computing Communication and Automation (ICCCA), Greater Noida, India, 14–15 December 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–5. [Google Scholar]
- Ma, J.; Du, K.; Zheng, F.; Zhang, L.; Gong, Z.; Sun, Z. A recognition method for cucumber diseases using leaf symptom images based on deep convolutional neural network. Comput. Electron. Agric. 2018, 154, 18–24. [Google Scholar] [CrossRef]
- Chakraborty, R.; Pramanik, A. DCNN-based prediction model for detection of age-related macular degeneration from color fundus images. Med. Biol. Eng. Comput. 2022, 60, 1431–1448. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.kaggle.com/datasets/christopherwsmith/exasens-data-set (accessed on 24 December 2022).


| Author | Methods | Advantages | Limitations |
|---|---|---|---|
| Almezhghwi et al. [18] | support vector machine, Alex Net, and VGG-16-based deep learning models | A robust, rapid, and easy prediction of lung disease | Minimum scalability |
| Han et al. [19] | Medical prediagnostic approach | Higher accuracy, speed, and efficiency | Less aspect of health-of-things domain |
| Ahmed et al. [21] | RCNN | Resulting in accurate and perfect case recognition | Computational; difficulties |
| Ma et al. [22] | Length-of-stay (LOS) | Accurately classified different ailments | Huge cost |
| Xu et al. [23] | A fully automated approach | More precise, reliable, and effective | Not suitable for big dataset |
| Skourt et al. [24] | Deep learning | Minimize the spatial dimension of encoder and decoder objects | Not explored the classification of lung cancer |
| Medeiros et al. [25] | Active Contour Method (ACM) approach | Perform fast, precisely, and sensitively | System lack to support the real-time diagnostic systems. |
| Cai et al. [26] | Mask R-CNN | Capable of detecting other diseases and improving the segmentation network | Higher time taken for execution |
| Hardware | Explanation |
|---|---|
| SX1272 | Act as transmitter and receiver with 900 MHz LoRa |
| AD8232 | The electrocardiographic board used in Analog Devices |
| User Computer | Inter® CoreTM i5-2400CPU@3.10 GHz PC |
| Raspberry Pi-IV | 1.5 GHz quad-core 64-bit ARM Cortex-A72 CPU |
| DP | Records | PPV(%) | ||||
|---|---|---|---|---|---|---|
| CDSS | RCNN | ACM | Mask R-CNN | Proposed | ||
| 67 | 567 | 86.56 | 89.90 | 91.23 | 94.46 | 98.87 |
| 79 | 895 | 92.65 | 91.67 | 95.45 | 93.36 | 99.23 |
| 100 | 1568 | 93.56 | 93.56 | 92.56 | 96.78 | 99.45 |
| 198 | 5000 | 94.34 | 94.89 | 93.66 | 97.78 | 99.78 |
| DP | Records | NPV(%) | ||||
|---|---|---|---|---|---|---|
| CDSS | RCNN | ACM | Mask R-CNN | Proposed | ||
| 67 | 567 | 92.45 | 89.45 | 90.67 | 93.56 | 98.67 |
| 79 | 895 | 89.45 | 90.35 | 91.63 | 94.29 | 98.89 |
| 100 | 1568 | 91.98 | 91.56 | 92.40 | 95.78 | 99.45 |
| 198 | 5000 | 94.78 | 92.11 | 93.00 | 96.28 | 99.69 |
| Algorithms | Time in Seconds |
|---|---|
| Particle swarm optimization (PSO) | 13 (s) |
| Genetic Algorithm (GA) | 10 (s) |
| Gray wolf optimization (GSO) | 8.1 (s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irshad, R.R.; Hussain, S.; Sohail, S.S.; Zamani, A.S.; Madsen, D.Ø.; Alattab, A.A.; Ahmed, A.A.A.; Norain, K.A.A.; Alsaiari, O.A.S. A Novel IoT-Enabled Healthcare Monitoring Framework and Improved Grey Wolf Optimization Algorithm-Based Deep Convolution Neural Network Model for Early Diagnosis of Lung Cancer. Sensors 2023, 23, 2932. https://doi.org/10.3390/s23062932
Irshad RR, Hussain S, Sohail SS, Zamani AS, Madsen DØ, Alattab AA, Ahmed AAA, Norain KAA, Alsaiari OAS. A Novel IoT-Enabled Healthcare Monitoring Framework and Improved Grey Wolf Optimization Algorithm-Based Deep Convolution Neural Network Model for Early Diagnosis of Lung Cancer. Sensors. 2023; 23(6):2932. https://doi.org/10.3390/s23062932
Chicago/Turabian StyleIrshad, Reyazur Rashid, Shahid Hussain, Shahab Saquib Sohail, Abu Sarwar Zamani, Dag Øivind Madsen, Ahmed Abdu Alattab, Abdallah Ahmed Alzupair Ahmed, Khalid Ahmed Abdallah Norain, and Omar Ali Saleh Alsaiari. 2023. "A Novel IoT-Enabled Healthcare Monitoring Framework and Improved Grey Wolf Optimization Algorithm-Based Deep Convolution Neural Network Model for Early Diagnosis of Lung Cancer" Sensors 23, no. 6: 2932. https://doi.org/10.3390/s23062932
APA StyleIrshad, R. R., Hussain, S., Sohail, S. S., Zamani, A. S., Madsen, D. Ø., Alattab, A. A., Ahmed, A. A. A., Norain, K. A. A., & Alsaiari, O. A. S. (2023). A Novel IoT-Enabled Healthcare Monitoring Framework and Improved Grey Wolf Optimization Algorithm-Based Deep Convolution Neural Network Model for Early Diagnosis of Lung Cancer. Sensors, 23(6), 2932. https://doi.org/10.3390/s23062932










