Photoacoustic Imaging of COVID-19 Vaccine Site Inflammation of Autoimmune Disease Patients
Abstract
1. Introduction
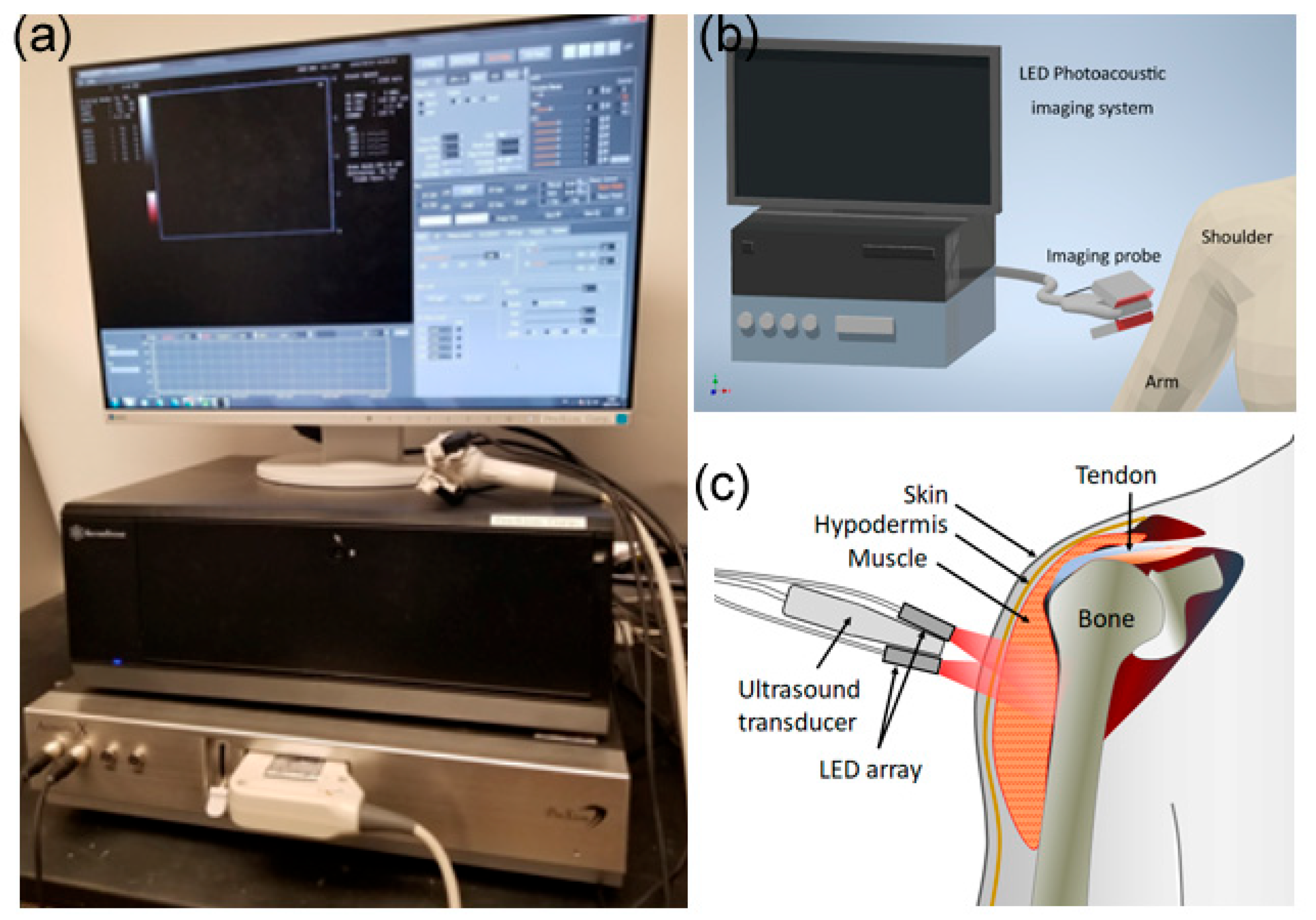
2. Materials and Methods
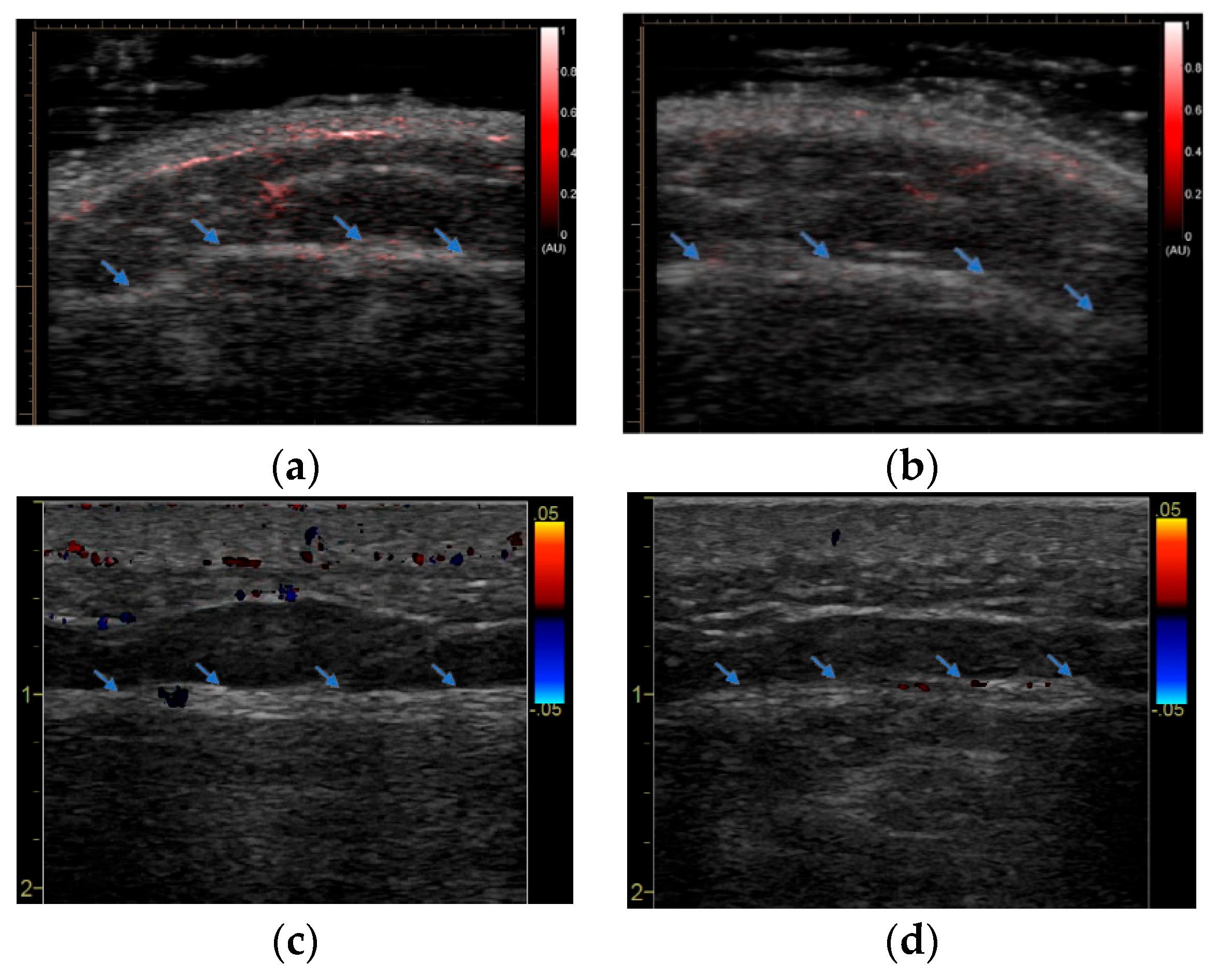
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foster, R.R.P. Immunocompromised May Need Fourth COVID Shot: CDC. HealthDay Report 27 October 2021. Available online: https://edition.cnn.com/2021/10/26/health/covid-19-fourth-dose-for-the-immunocompromised/index.html (accessed on 30 November 2022).
- Pablos, J.L.; Abasolo, L.; Alvaro-Gracia, J.M.; Blanco, F.J.; Blanco, R.; Castrejon, I.; Fernandez-Fernandez, D.; Fernandez-Gutierrez, B.; Galindo-Izquierdo, M.; Gonzalez-Gay, M.A.; et al. Prevalence of hospital PCR-confirmed COVID-19 cases in patients with chronic inflammatory and autoimmune rheumatic diseases. Ann. Rheum. Dis. 2020, 79, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, S.; Hamdeh, S.; Micic, D.; Sakuraba, A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: A systematic review and meta-analysis. Ann. Rheum. Dis. 2021, 80, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Deepak, P.; Kim, W.; Paley, M.A.; Yang, M.; Carvidi, A.B.; Demissie, E.G.; El-Qunni, A.A.; Haile, A.; Huang, K.; Kinnett, B.; et al. Effect of Immunosuppression on the Immunogenicity of mRNA Vaccines to SARS-CoV-2: A Prospective Cohort Study. Ann. Intern. Med. 2021, 174, 1572–1585. [Google Scholar] [CrossRef]
- Negahdaripour, M.; Shafiekhani, M.; Moezzi, S.M.I.; Amiri, S.; Rasekh, S.; Bagheri, A.; Mosaddeghi, P.; Vazin, A. Administration of COVID-19 vaccines in immunocompromised patients. Int. Immunopharmacol. 2021, 99, 108021. [Google Scholar] [CrossRef]
- Haidar, G.; Agha, M.; Lukanski, A.; Linstrum, K.; Troyan, R.; Bilderback, A.; Rothenberger, S.; McMahon, D.K.; Crandall, M.; Enick, P.N.; et al. Immunogenicity of COVID-19 Vaccination in Immunocompromised Patients: An Observational, Prospective Cohort Study Interim Analysis. medRxiv 2021. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef] [PubMed]
- Herve, C.; Laupeze, B.; Del Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines 2019, 4, 39. [Google Scholar] [CrossRef]
- Mitchell, T.C.; Casella, C.R. No pain no gain? Adjuvant effects of alum and monophosphoryl lipid A in pertussis and HPV vaccines. Curr. Opin. Immunol. 2017, 47, 17–25. [Google Scholar] [CrossRef]
- Burny, W.; Callegaro, A.; Bechtold, V.; Clement, F.; Delhaye, S.; Fissette, L.; Janssens, M.; Leroux-Roels, G.; Marchant, A.; van den Berg, R.A.; et al. Different Adjuvants Induce Common Innate Pathways That Are Associated with Enhanced Adaptive Responses against a Model Antigen in Humans. Front. Immunol. 2017, 8, 943. [Google Scholar] [CrossRef]
- Gotthardt, M.; Bleeker-Rovers, C.P.; Boerman, O.C.; Oyen, W.J. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J. Nucl. Med. 2010, 51, 1937–1949. [Google Scholar] [CrossRef]
- Boesen, M.; Kubassova, O.; Cimmino, M.A.; Ostergaard, M.; Taylor, P.; Danneskiold-Samsoe, B.; Bliddal, H. Dynamic Contrast Enhanced MRI Can Monitor the Very Early Inflammatory Treatment Response upon Intra-Articular Steroid Injection in the Knee Joint: A Case Report with Review of the Literature. Arthritis 2011, 2011, 578252. [Google Scholar] [CrossRef]
- McQueen, F.M. Magnetic resonance imaging in early inflammatory arthritis: What is its role? Rheumatology 2000, 39, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Rooney, M.E.; McAllister, C.; Burns, J.F. Ankle disease in juvenile idiopathic arthritis: Ultrasound findings in clinically swollen ankles. J. Rheumatol. 2009, 36, 1725–1729. [Google Scholar] [CrossRef]
- Schmidt, W.A. Technology Insight: The role of color and power Doppler ultrasonography in rheumatology. Nat. Clin. Pract. Rheumatol. 2007, 3, 35–42, quiz 59. [Google Scholar] [CrossRef]
- Goldie, I. The synovial microvascular derangement in rheumatoid arthritis and osteoarthritis. Acta Orthop. Scand. 1969, 40, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Chamberland, D.L.; Wang, X.; Roessler, B.J. Photoacoustic tomography of carrageenan-induced arthritis in a rat model. J. Biomed. Opt. 2008, 13, 011005. [Google Scholar] [CrossRef] [PubMed]
- Chamberland, D.L.; Taurog, J.D.; Richardson, J.A.; Wang, X. Photoacoustic tomography: A new imaging technology for inflammatory arthritis—As applied to tail spondylitis in rats. Clin. Exp. Rheumatol. 2009, 27, 387–388. [Google Scholar]
- Chamberland, D.; Jiang, Y.; Wang, X. Optical imaging: New tools for arthritis. Integr. Biol. 2010, 2, 496–509. [Google Scholar] [CrossRef]
- Rajian, J.R.; Girish, G.; Wang, X. Photoacoustic tomography to identify inflammatory arthritis. J. Biomed. Opt. 2012, 17, 096013. [Google Scholar] [CrossRef] [PubMed]
- Rajian, J.R.; Shao, X.; Chamberland, D.L.; Wang, X. Characterization and treatment monitoring of inflammatory arthritis by photoacoustic imaging: A study on adjuvant-induced arthritis rat model. Biomed. Opt. Express 2013, 4, 900–908. [Google Scholar] [CrossRef]
- Xu, G.; Rajian, J.R.; Girish, G.; Kaplan, M.J.; Fowlkes, J.B.; Carson, P.L.; Wang, X. Photoacoustic and ultrasound dual-modality imaging of human peripheral joints. J. Biomed. Opt. 2013, 18, 10502. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Xu, G.; Yu, Y.; Zhou, Y.; Carson, P.L.; Wang, X.; Liu, X. Real-time photoacoustic and ultrasound dual-modality imaging system facilitated with graphics processing unit and code parallel optimization. J. Biomed. Opt. 2013, 18, 86001. [Google Scholar] [CrossRef]
- Jo, J.; Xu, G.; Cao, M.; Marquardt, A.; Francis, S.; Gandikota, G.; Wang, X. A Functional Study of Human Inflammatory Arthritis Using Photoacoustic Imaging. Sci. Rep. 2017, 7, 15026. [Google Scholar] [CrossRef]
- Jo, J.; Tian, C.; Xu, G.; Sarazin, J.; Schiopu, E.; Gandikota, G.; Wang, X. Photoacoustic tomography for human musculoskeletal imaging and inflammatory arthritis detection. Photoacoustics 2018, 12, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, G.; Yuan, J.; Jo, J.; Gandikota, G.; Demirci, H.; Agano, T.; Sato, N.; Shigeta, Y.; Wang, X. Light Emitting Diodes based Photoacoustic Imaging and Potential Clinical Applications. Sci. Rep. 2018, 8, 9885. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Xu, G.; Zhu, Y.; Burton, M.; Sarazin, J.; Schiopu, E.; Gandikota, G.; Wang, X. Detecting joint inflammation by an LED-based photoacoustic imaging system: A feasibility study. J. Biomed. Opt. 2018, 23, 110501. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Xu, G.; Schiopu, E.; Chamberland, D.; Gandikota, G.; Wang, X. Imaging of enthesitis by an LED-based photoacoustic system. J. Biomed. Opt. 2020, 25, 126005. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.; Lemaster, J.; Wang, J.; Jeevarathinam, A.S.; Chao, D.L.; Jokerst, J.V. The characterization of an economic and portable LED-based photoacoustic imaging system to facilitate molecular imaging. Photoacoustics 2018, 9, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Joseph Francis, K.; Boink, Y.E.; Dantuma, M.; Ajith Singh, M.K.; Manohar, S.; Steenbergen, W. Tomographic imaging with an ultrasound and LED-based photoacoustic system. Biomed. Opt. Express 2020, 11, 2152–2165. [Google Scholar] [CrossRef]


| Study# | Age | Ethnicity/Gender | BMI | AD Diagnosis | Disease Duration (Years) | MyositisActive | Treated |
|---|---|---|---|---|---|---|---|
| 1 | 34 | White/Female | 21 | ||||
| 2 | 52 | White/Male | 27.6 | ||||
| 3 | 45 | White/Female | 21 | ||||
| 4 | 49 | Indian/Male | 23.5 | ||||
| 5 | 36 | Black/Female | 36.1 | JDM | 15 | N | GC, HCQ |
| 6 | 51 | Indian/Male | 24.4 | ||||
| 7 | 63 | White/Male | 26.4 | DM | 9 | N | MTX |
| 8 | 64 | White/Female | 41.5 | DM | 33 | N | GC, MMF, Ustekinumab |
| 9 | 66 | White/Female | 22.2 | DM | 1 | Y | GC, HCQ, RTX |
| 10 | 57 | White/Female | 23 | RA | 19 | Y | MTX |
| 11 | 68 | White/Female | 31.8 | IMNM | 4 | Y | MMF |
| 12 | 24 | White/Female | 31.8 | ||||
| 13 | 85 | White/Female | 25.2 | ||||
| 14 | 86 | White/Male | 30.7 | ||||
| 15 | 79 | White/Male | 27.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, J.; Mills, D.; Dentinger, A.; Chamberland, D.; Abdulaziz, N.M.; Wang, X.; Schiopu, E.; Gandikota, G. Photoacoustic Imaging of COVID-19 Vaccine Site Inflammation of Autoimmune Disease Patients. Sensors 2023, 23, 2789. https://doi.org/10.3390/s23052789
Jo J, Mills D, Dentinger A, Chamberland D, Abdulaziz NM, Wang X, Schiopu E, Gandikota G. Photoacoustic Imaging of COVID-19 Vaccine Site Inflammation of Autoimmune Disease Patients. Sensors. 2023; 23(5):2789. https://doi.org/10.3390/s23052789
Chicago/Turabian StyleJo, Janggun, David Mills, Aaron Dentinger, David Chamberland, Nada M. Abdulaziz, Xueding Wang, Elena Schiopu, and Girish Gandikota. 2023. "Photoacoustic Imaging of COVID-19 Vaccine Site Inflammation of Autoimmune Disease Patients" Sensors 23, no. 5: 2789. https://doi.org/10.3390/s23052789
APA StyleJo, J., Mills, D., Dentinger, A., Chamberland, D., Abdulaziz, N. M., Wang, X., Schiopu, E., & Gandikota, G. (2023). Photoacoustic Imaging of COVID-19 Vaccine Site Inflammation of Autoimmune Disease Patients. Sensors, 23(5), 2789. https://doi.org/10.3390/s23052789








