Development of the New Sensor Based on Functionalized Carbon Nanomaterials for Promethazine Hydrochloride Determination
Abstract
1. Introduction
2. Materials and Methods
2.1. Sensor Preparation
2.2. Reagents and Materials for Measurements
2.3. Apparatus
2.4. Procedure
3. Results
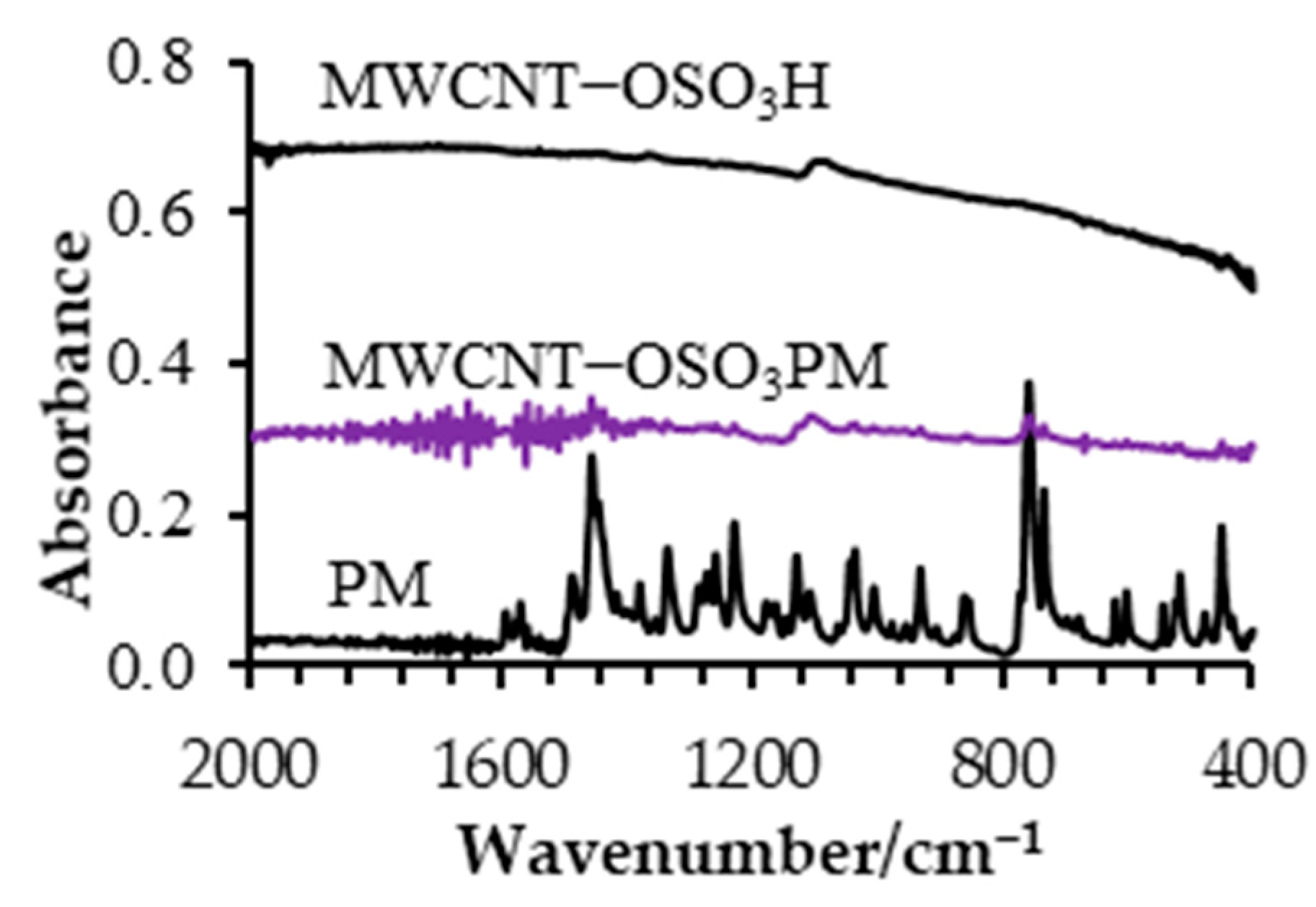
3.1. ATR-FTIR Characterization of MWCNT−OSO3PM
3.2. Response of the Sensor
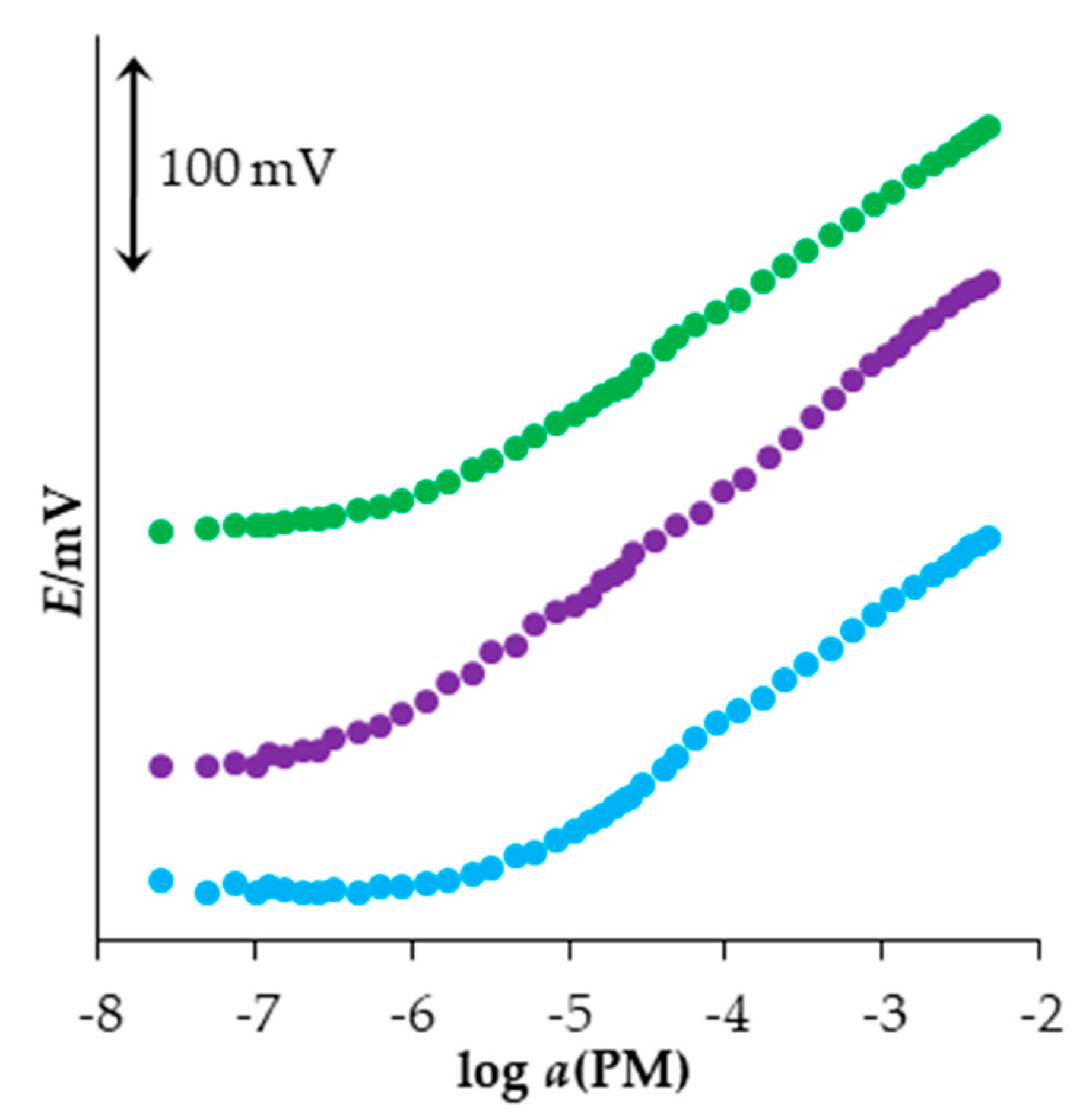
3.3. Selection of the Plasticizer
3.4. Optimization of the Sensor Material Content
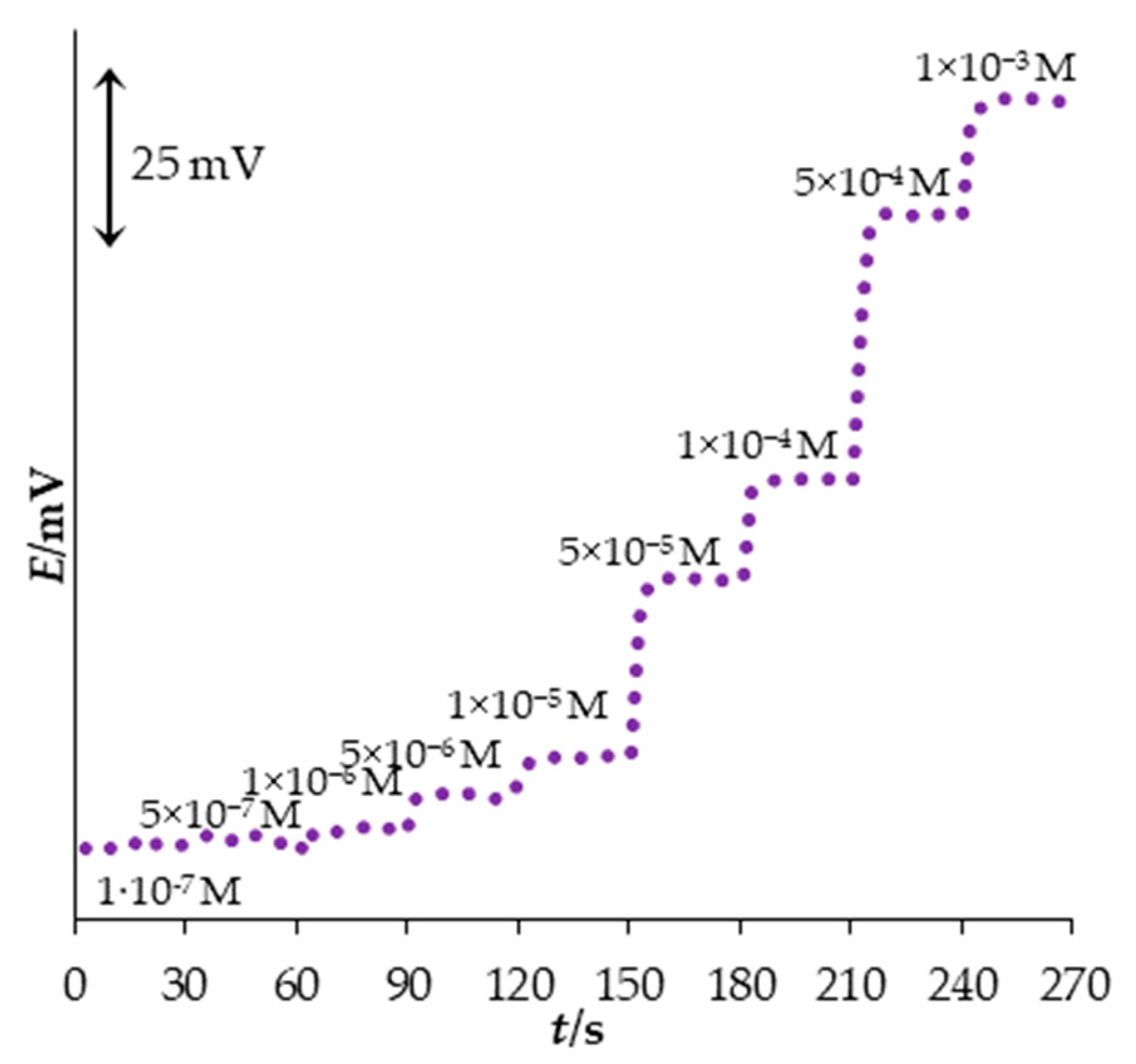
3.5. Dynamic Response
3.6. Signal Drift
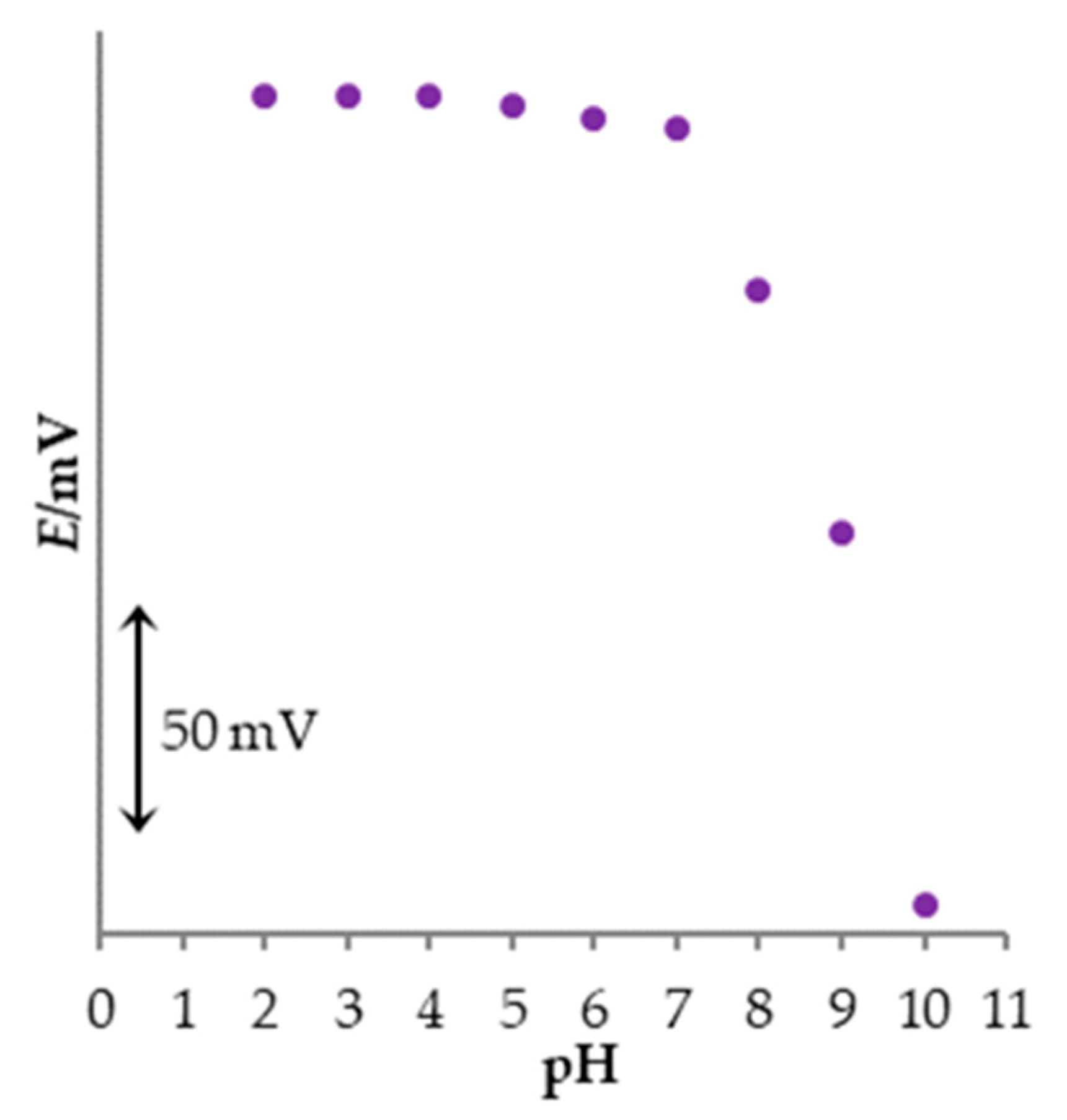
3.7. The Influence of the pH
3.8. The Selectivity
3.9. Determination of PM
3.10. The Lifetime
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sebastian, N.; Yu, W.C.; Hu, Y.C.; Balram, D.; Yu, Y.H. Morphological Evolution of Nanosheets-Stacked Spherical ZnO for Preparation of GO-Zn/ZnO Ternary Nanocomposite: A Novel Electrochemical Platform for Nanomolar Detection of Antihistamine Promethazine Hydrochloride. J. Alloys Compd. 2022, 890, 161768. [Google Scholar] [CrossRef]
- Muthukutty, B.; Vivekanandan, A.K.; Chen, S.M.; Sivakumar, M.; Chen, S.H. Designing Hybrid Barium Tungstate on Functionalized Carbon Black as Electrode Modifier for Low Potential Detection of Antihistamine Drug Promethazine Hydrochloride. Compos. Part B Eng. 2021, 215, 108789. [Google Scholar] [CrossRef]
- Cantisani, C.; Ricci, S.; Grieco, T.; Paolino, G.; Faina, V.; Silvestri, E.; Calvieri, S. Topical Promethazine Side Effects: Our Experience and Review of the Literature. BioMed Res. Int. 2013, 2013, 151509. [Google Scholar] [CrossRef]
- Liu, D.Y.; Liu, J.C.; Liang, S.; Meng, X.H.; Greenbaum, J.; Xiao, H.M.; Tan, L.J.; Deng, H.W. Drug Repurposing for COVID-19 Treatment by Integrating Network Pharmacology and Transcriptomics. Pharmaceutics 2021, 13, 545. [Google Scholar] [CrossRef]
- Kumar, D.G.S.; Vadgaonkar, D.A.; Purunaik, D.S.; Shelatkar, R.; Vaidya, V.G.; Ganu, D.G.; Vadgaonkar, D.A.; Joshi, S. Efficacy and Safety of Aspirin, Promethazine, and Micronutrients for Rapid Clinical Recovery in Mild to Moderate COVID-19 Patients: A Randomized Controlled Clinical Trial. Cureus 2022, 14, e25467. [Google Scholar] [CrossRef]
- Cohen, M.R. Frequency and Severity of Promethazine Adverse Event Reports Obligated the ISMP Alert. Jt. Comm. J. Qual. Patient Saf. 2010, 36, 143–144. [Google Scholar] [CrossRef]
- FDA Issues Label Change for Promethazine HCl. AAP News 2006, 27, 2006206. [CrossRef]
- U.S. Food and Drug Administration Promethazine HCl (Marketed as Phenergan) Information|FDA. Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/promethazine-hcl-marketed-phenergan-information (accessed on 28 September 2022).
- Lynch, K.L.; Shapiro, B.J.; Coffa, D.; Novak, S.P.; Kral, A.H. Promethazine Use among Chronic Pain Patients. Drug Alcohol Depend. 2015, 150, 92–97. [Google Scholar] [CrossRef]
- Lantam, A.; Limbut, W.; Thiagchanya, A.; Phonchai, A. A Portable Optical Colorimetric Sensor for the Determination of Promethazine in Lean Cocktail and Pharmaceutical Doses. Microchem. J. 2020, 159, 105519. [Google Scholar] [CrossRef]
- Ogawa, T.; Kondo, F.; Iwai, M.; Matsuo, T.; Kubo, K.; Seno, H. Novel Extraction Method Using an ISOLUTE PLD+ Protein and Phospholipid Removal Column for Liquid Chromatography-Tandem Mass Spectrometry Analysis of 20 Psychoactive Drugs in Postmortem Whole Blood Samples. Forensic Sci. Int. 2022, 331, 111130. [Google Scholar] [CrossRef]
- Tanaka, E.; Nakamura, T.; Terada, M.; Shinozuka, T.; Hashimoto, C.; Kurihara, K.; Honda, K. Simple and Simultaneous Determination for 12 Phenothiazines in Human Serum by Reversed-Phase High-Performance Liquid Chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 854, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Hu, B. Hollow Fiber-Liquid Phase Microextraction Combined with Gas Chromatography for the Determination of Phenothiazine Drugs in Urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 1599–1604. [Google Scholar] [CrossRef]
- Raja, D.A.; Shah, M.R.; Malik, M.I. Polyethyleneimine Stabilized Silver Nanoparticles as an Efficient and Selective Colorimetric Assay for Promethazine. Anal. Chim. Acta 2022, 1223, 340216. [Google Scholar] [CrossRef]
- Saif, M.J.; Anwar, J. A New Spectrophotometric Method for the Determination of Promethazine-HCl from Pure and Pharmaceutical Preparations. Talanta 2005, 67, 869–872. [Google Scholar] [CrossRef]
- Calatayud, J.M.; Sarrion, S.N.; Sampedro, A.S.; Benito, C.G. Determination of Promethazine Hydrochloride with Bromophenol Blue by a Turbidimetric Method and Flow Injection Analysis. Microchem. J. 1992, 45, 129–136. [Google Scholar] [CrossRef]
- Lara, F.J.; García-Campaña, A.M.; Alés-Barrero, F.; Bosque-Sendra, J.M. Determination of Thiazinamium, Promazine and Promethazine in Pharmaceutical Formulations Using a CZE Method. Anal. Chim. Acta 2005, 535, 101–108. [Google Scholar] [CrossRef]
- Sultan, S.M.; Hassan, Y.A.M.; Abulkibash, A.M. Chemiluminescence Assay of Promethazine Hydrochloride Using Acidic Permanganate Employing Flow Injection Mode Operated with Syringe and Peristaltic Pumps. Talanta 2003, 59, 1073–1080. [Google Scholar] [CrossRef]
- Badawy, S.S.; Said, S.A.E.S. El Promethazine-Tetraphenyl Boron(III) Modified Carbon Paste Electrode for the Determination of Promethazine Hydrochloride. Am. J. Anal. Chem. 2013, 04, 258–264. [Google Scholar] [CrossRef]
- Hassan, A.K.; Saad, B.; Ghani, S.A.; Adnan, R.; Rahim, A.A.; Ahmad, N.; Mokhtar, M.; Ameen, S.T.; Al-Araji, S.M. Ionophore-Based Potentiometric Sensors for the Flow-Injection Determination of Promethazine Hydrochloride in Pharmaceutical Formulations and Human Urine. Sensors 2011, 11, 1028–1042. [Google Scholar] [CrossRef] [PubMed]
- Alagumalai, K.; Balamurugan, M.; Chen, S.M.; Selvaganapathy, M. One-Pot Engineering of Novel Cashew like Cobalt Tungstate; Dynamic Electrocatalyst for the Selective Detection of Promethazine Hydrochloride. Microchem. J. 2020, 159, 105381. [Google Scholar] [CrossRef]
- Alagumalai, K.; Shanmugam, R.; Chen, T.W.; Chen, S.M.; Balamurugan, M.; Choi, S.S.; Ali, M.A.; Al-Mohaimeed, A.M.; Fan, C.H. The Electrochemical Evaluation of Antipsychotic Drug (Promethazine) in Biological and Environmental Samples through Samarium Cobalt Oxide Nanoparticles. Mater. Today Chem. 2022, 25, 100961. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Vesimohammadi, B.; Riahi, S.; Norouzi, P. Promethazine Potentiometric Membrane Sensor for Promethazine Hydrochloride Pharmaceutical Analysis; Computational Study. Int. J. Electrochem. Sci. 2009, 4, 740–754. [Google Scholar]
- Mahmoud, N.F.; Fouad, O.A.; Ali, A.E.; Mohamed, G.G. Potentiometric Determination of the Al(III) Ion in Polluted Water and Pharmaceutical Samples by a Novel Mesoporous Copper Metal-Organic Framework-Modified Carbon Paste Electrode. Ind. Eng. Chem. Res. 2021, 60, 2374–2387. [Google Scholar] [CrossRef]
- Dandić, A.; Novak, I.; Jozanović, M.; Pukleš, I.; Széchenyi, A.; Budetić, M.; Samardžić, M. A New, MWCNT-Based, Solid-State Thiabendazole-Selective Sensor. Sensors 2022, 22, 3785. [Google Scholar] [CrossRef]
- Lima, J.L.F.C.; Montenegro, M.C.B.S.M.; Sales, M.G. Ion-Selective Electrodes for Promethazine Determinations in Pharmaceutical Preparations and Application to Flow Injection Analysis. J. Pharm. Sci. 1997, 86, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Al-Saidi, K.H.; Ahmed, Z.W. Construction of Promethazine Hydrochloride Selective Electrodes in a PVC Matrix Membrane. J. Al-Nahrain Univ. Sci. 2011, 14, 11–17. [Google Scholar] [CrossRef]
- Sarma, B.K.; Rani, S. An Electrochemical Characteristics of Promethazine HCl Using Ion Selective Electrodes. Int. J. Curr. Res. 2017, 9, 60523–60525. [Google Scholar]
- Nassory, N.S.; Maki, S.; Al-Phalahy, B.A. Preparation and Potentiometric Study of Promethazine Hydrochloride Selective Electrodes and Their Use in Determining Some Drugs. Turkish J. Chem. 2008, 32, 539–548. [Google Scholar]
- Zahran, E.M.; New, A.; Gavalas, V.; Bachas, L.G. Polymeric Plasticizer Extends the Lifetime of PVC-Membrane Ion-Selective Electrodes. Analyst 2014, 139, 757–763. [Google Scholar] [CrossRef]
- Hajduković, M.; Samardžić, M.; Galović, O.; Széchenyi, A.; Sak-Bosnar, M. A Functionalized Nanomaterial Based, New, Solid State Cationic-Surfactant-Selective Sensor with Fast Response and Low Noise. Sens. Actuators B Chem. 2017, 251, 795–803. [Google Scholar] [CrossRef]
- Buck, R.P.; Lindner, E. Recommendations for Nomenclature of Ion-Selective Electrodes (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Manzur, M.E.; Brandán, S.A. S(-) and R(+) Species Derived from Antihistaminic Promethazine Agent: Structural and Vibrational Studies. Heliyon 2019, 5, e02322. [Google Scholar] [CrossRef]
- Hu, J.; Stein, A.; Bühlmann, P. Rational Design of All-Solid-State Ion-Selective Electrodes and Reference Electrodes. TrAC—Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Crespo, G.A.; Macho, S.; Bobacka, J.; Rius, F.X. Transduction Mechanism of Carbon Nanotubes in Solid-Contact Ion-Selective Electrodes. Anal. Chem. 2009, 81, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.; Crespo, G.; Grygolowicz-Pawlak, E.; Mistlberger, G.; Pawlak, M.; Xie, X. Advancing Membrane Electrodes and Optical Ion Sensors. Chimia 2011, 65, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.M.; Hua, Y.; Li, Y.; Flood, A.H.; Bachas, L.G. Triazolophanes: A New Class of Halide-Selective Lonophores for Potentiometrie Sensors. Anal. Chem. 2010, 82, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 0-8493-7248-8. [Google Scholar]
- Umezawa, Y.; Bühlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric Selectivity Coefficients of Ion-Selective Electrodes Part I. Inorganic Cations (Technical Report). Pure Appl. Chem. 2000, 72, 1851–2082. [Google Scholar] [CrossRef]
- Maccà, C. Response Time of Ion-Selective Electrodes: Current Usage versus IUPAC Recommendations. Anal. Chim. Acta 2004, 512, 183–190. [Google Scholar] [CrossRef]
- Rasha, S.; Heyam, S.A.; Babiker, M.A.E.; Mai, A.A. Antioxidant Assessment on Promethazine HCl Decomposition Using RP-HPLC Assay Method. Afr. J. Biotechnol. 2016, 15, 2272–2281. [Google Scholar] [CrossRef]
- Eggins, B.R. Chemical Sensors and Biosensors; John Wiley & Sons: Hoboken, NJ, USA, 2002; ISBN 9780471899136. [Google Scholar]
| Ra | δd | δp | δh | logP | logS | |
|---|---|---|---|---|---|---|
| PM | - | 19.52 | 6.64 | 5.33 | 4.27 | −1.99 |
| NPPE | 1.10 | 19.87 | 7.39 | 5.01 | 3.23 | −2.68 |
| DBP | 4.37 | 17.57 | 6.7 | 3.28 | 4.93 | −3.64 |
| o-NPOE | 5.18 | 17.71 | 10.21 | 4.21 | 4.95 | −4.32 |
| DOP | 6.15 | 16.91 | 5.15 | 2.37 | 8.79 | −5.75 |
| DS | 7.54 | 16.23 | 3.11 | 4.03 | 6.82 | −4.94 |
| Plasticizer | Content of the Sensor Material (%) | Slope (mV/Decade of Activity) | Standard Error | Correl. Coeff. (R2) | LOD (M) | Useful Conc. Range (M) |
|---|---|---|---|---|---|---|
| DOP | 2 | 51.0 ± 1.4 | 3.9 | 0.9947 | 1.7 × 10−6 | 2.4 × 10−6–5.0 × 10−3 |
| DS | 2 | 51.2 ± 1.3 | 3.7 | 0.9953 | 8.6 × 10−7 | 1.7 × 10−6–5.0 × 10−3 |
| o-NPOE | 2 | 52.1 ± 0.8 | 1.8 | 0.9987 | 1.7 × 10−6 | 4.6 × 10−6–5.0 × 10−3 |
| DBP | 2 | 53.8 ± 0.5 | 1.1 | 0.9995 | 1.2 × 10−6 | 3.3 × 10−6–5.0 × 10−3 |
| NPPE | 2 | 55.8 ± 0.6 | 1.5 | 0.9992 | 1.2 × 10−6 | 1.7 × 10−6–5.0 × 10−3 |
| Plasticizer | Content of the Sensor Material (%) | Slope (mV/Decade of Activity) | Standard Error | Correl. Coeff. (R2) | LOD (M) | Useful Conc. Range (M) |
|---|---|---|---|---|---|---|
| NPPE | 2 | 55.8 ± 0.6 | 1.5 | 0.9992 | 1.2 × 10−6 | 1.7 × 10−6–5.0 × 10−3 |
| NPPE | 4 | 59.4 ± 1.1 | 3.8 | 0.9973 | 1.5 × 10−7 | 6.2 × 10−7–5.0 × 10−3 |
| NPPE | 6 | 52.1 ± 1.1 | 3.5 | 0.9966 | 4.5 × 10−7 | 8.6 × 10−7–5.0 × 10−3 |
| Interference | |
|---|---|
| Ammonium | 8.16 × 10−4 |
| Sodium | 3.66 × 10−4 |
| Calcium | 6.99 × 10−5 |
| Magnesium | 2.21 × 10−4 |
| Ascorbic acid | 6.11 × 10−3 |
| Manganese | 6.84 × 10−5 |
| Potassium | 4.98 × 10−4 |
| Copper | 2.08 × 10−4 |
| Zinc | 6.53 × 10−5 |
| Lithium | 2.24 × 10−3 |
| Iron (III) | 1.20 × 10−5 |
| Cobalt | 2.36 × 10−4 |
| Naproxen | 1.28 × 10−3 |
| Glycine | 3.09 × 10−3 |
| Caffeine | 2.06 × 10−4 |
| Sample | Pure PM Solution | Atosil Drops Solution | Promethazin-Neuraxpharm Drops Solution | |||
|---|---|---|---|---|---|---|
| Method | Titration Method | Gran Method | Titration Method | Gran Method | Titration Method | Gran Method |
| Claimed value (M) | 4.00 × 10−3 | 1.00 × 10−4 | 4.00 × 10−3 | 1.00 × 10−4 | 4.00 × 10−3 | 1.00 × 10−4 |
| PM found (M) ± RSD (%) | 4.09 × 10−3 ± 0.3 | 1.07 × 10−4 ± 4.7 | 4.02 × 10−3 ± 0.2 | 1.09 × 10−4 ± 5.1 | 4.13 × 10−3 ± 0.2 | 1.03 × 10−4 ± 6.1 |
| Recovery (%) | 102.3 | 107.0 | 100.5 | 109.0 | 103.3 | 103.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samardžić, M.; Peršić, M.; Széchenyi, A.; Jozanović, M.; Pukleš, I.; Budetić, M. Development of the New Sensor Based on Functionalized Carbon Nanomaterials for Promethazine Hydrochloride Determination. Sensors 2023, 23, 2641. https://doi.org/10.3390/s23052641
Samardžić M, Peršić M, Széchenyi A, Jozanović M, Pukleš I, Budetić M. Development of the New Sensor Based on Functionalized Carbon Nanomaterials for Promethazine Hydrochloride Determination. Sensors. 2023; 23(5):2641. https://doi.org/10.3390/s23052641
Chicago/Turabian StyleSamardžić, Mirela, Mateja Peršić, Aleksandar Széchenyi, Marija Jozanović, Iva Pukleš, and Mateja Budetić. 2023. "Development of the New Sensor Based on Functionalized Carbon Nanomaterials for Promethazine Hydrochloride Determination" Sensors 23, no. 5: 2641. https://doi.org/10.3390/s23052641
APA StyleSamardžić, M., Peršić, M., Széchenyi, A., Jozanović, M., Pukleš, I., & Budetić, M. (2023). Development of the New Sensor Based on Functionalized Carbon Nanomaterials for Promethazine Hydrochloride Determination. Sensors, 23(5), 2641. https://doi.org/10.3390/s23052641








