Ultra-High Sensitivity Terahertz Microstructured Fiber Biosensor for Diabetes Mellitus and Coronary Heart Disease Marker Detection
Abstract
:1. Introduction
2. Design Approach and Theoretical Derivations
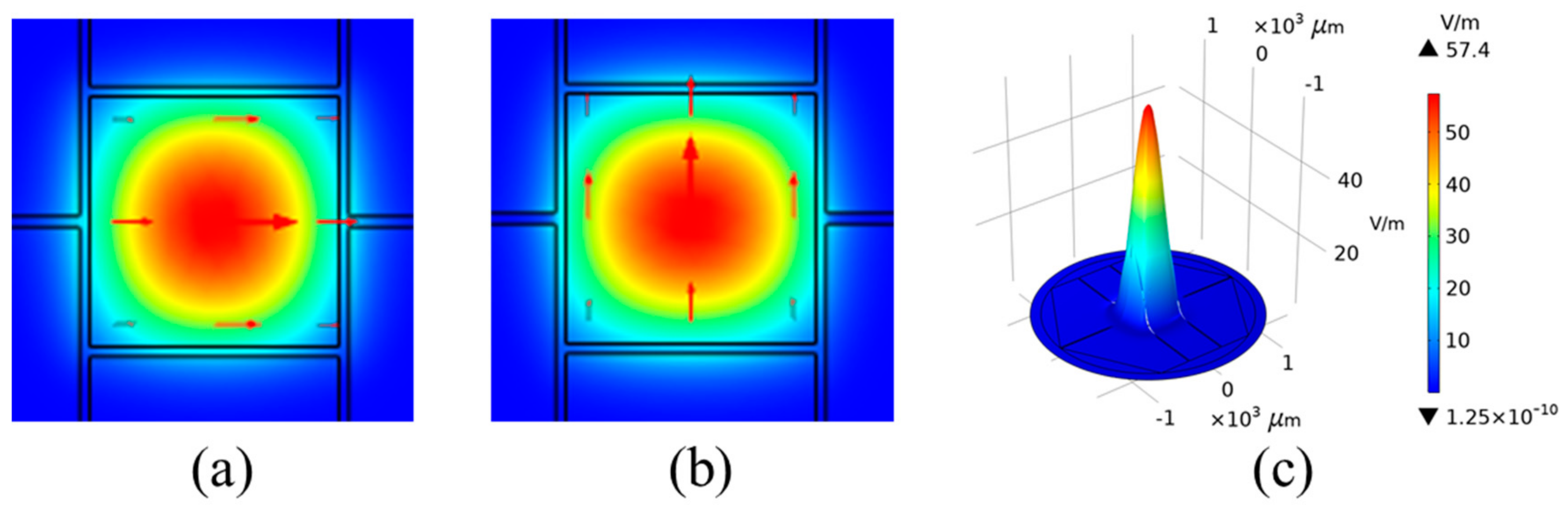
2.1. Design and Configurations of MSF
2.2. Theoretical Derivations
3. Modelling Results and Performance Analysis
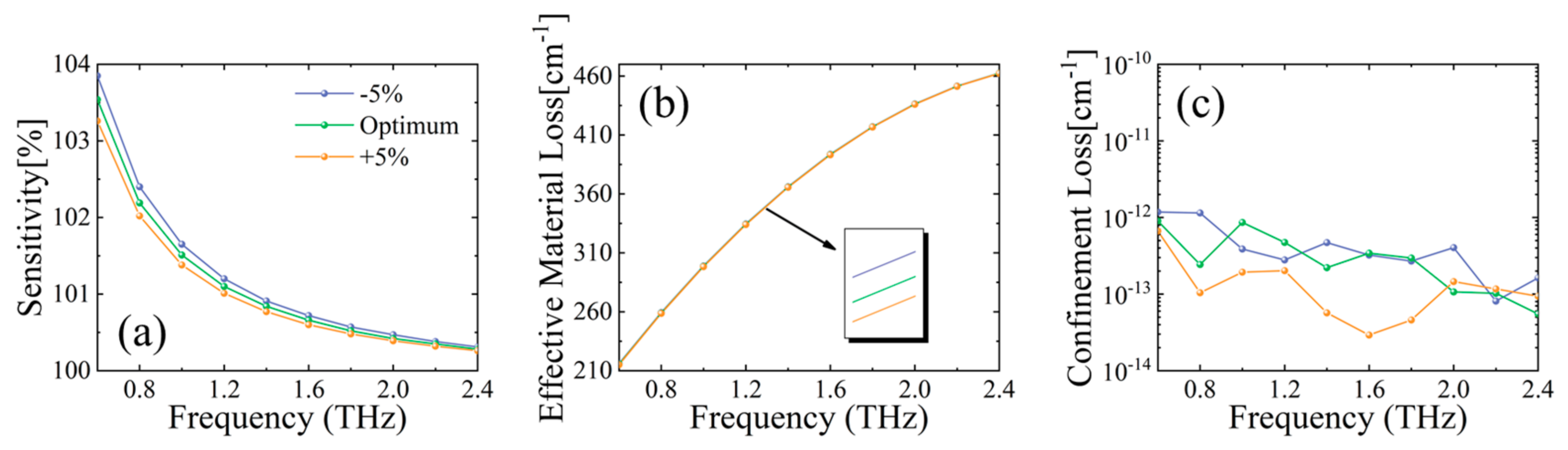
3.1. Optimization of MSF Constructing Parameters
3.1.1. Effect of Core Side Length l on MSF Sensor Performance
3.1.2. Effect of Vertical Support Arm Thickness d on MSF Sensor Performance
3.1.3. Effect of Horizontal Support Arm Thickness p on MSF Sensor Performance
3.2. Exploring a High-Sensitivity MSF Sensor for Detecting DM and CHD
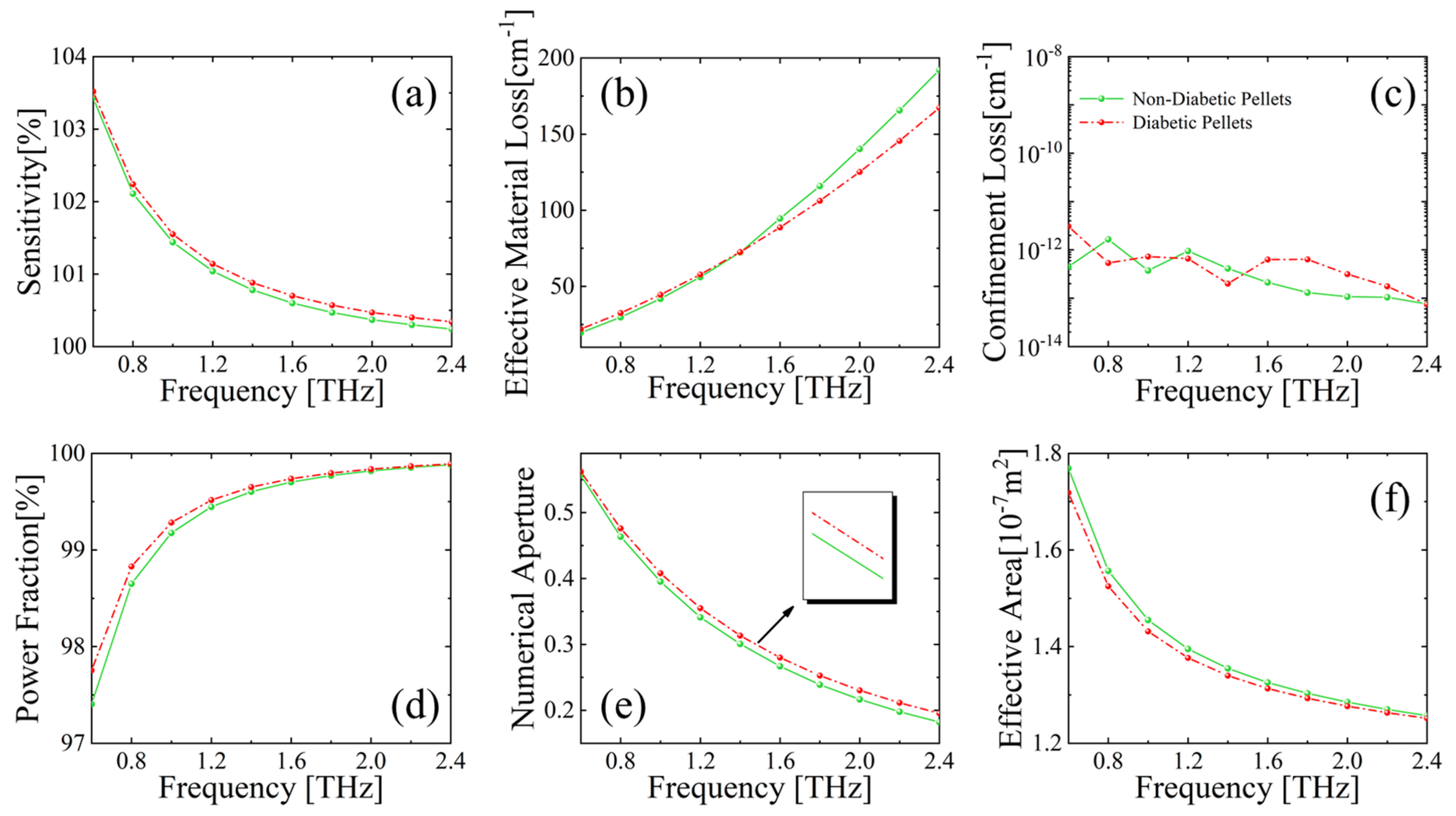
3.2.1. Detection of DM Markers by the MSF Sensor
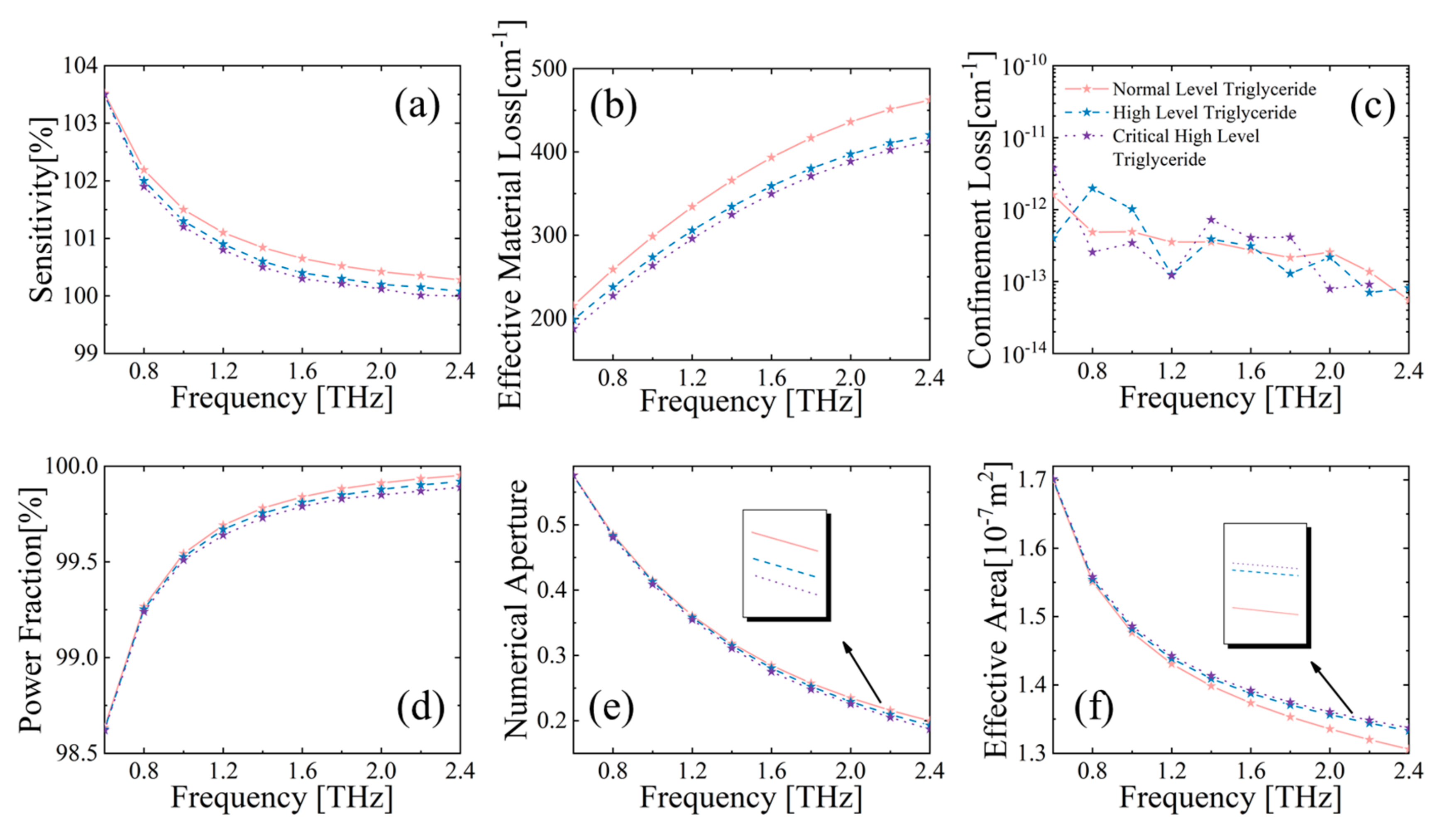
3.2.2. Detection of CHD Markers by the MSF Sensor
4. Feasibility of Preparation for the Designed MSF Biosensor
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Tan, Y.; Shi, Y.; Wang, X.; Liao, Z.; Wei, P. Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front Endocrinol 2020, 11, 568. [Google Scholar] [CrossRef]
- Lykina, A.A.; Nazarov, M.M.; Konnikova, M.R.; Mustafin, I.A.; Vaks, V.L.; Anfertev, V.A. Terahertz spectroscopy of diabetic and non-diabetic human blood plasma pellets. J. Biomed. Opt. 2021, 26, 43006. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.M.M.; Chua, Z.J.Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina 2019, 55, 546. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Han, J.; Li, X.; Chen, X.; Qiu, T.; Chen, H. Quantification of triglyceride levels in fresh human blood by terahertz time-domain spectroscopy. Sci. Rep. 2021, 11, 13209. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Kolovou, G.; Kolovou, V.; Koutelou, M.; Mavrogeni, S. Atherosclerotic and Non-Atherosclerotic Coronary Heart Disease in Women. Curr. Med. Chem. 2015, 22, 3555–3564. [Google Scholar] [CrossRef]
- Achenbach, S.; Daniel, W.G. Noninvasive coronary angiography-an acceptable alternative. N. Engl. J. Med. 2001, 345, 1909–1910. [Google Scholar] [CrossRef]
- Elnabawi, Y.A.; Dey, A.K.; Mehta, N.N. Emerging Applications of Coronary CT Angiography in Coronary Heart Disease: Getting Better with Time. Eur. Heart J. 2018, 39, 3682–3684. [Google Scholar] [CrossRef]
- Habib, A.; Anower, S.; Abdulrazak, L.F.; Reza, S. Hollow core photonic crystal fiber for chemical identification in terahertz regime. Opt. Fiber Technol. 2019, 52, 101933. [Google Scholar] [CrossRef]
- Wang, J.; Lindley-Hatcher, H.; Chen, X.; Pickwell-MacPherson, E. THz Sensing of Human Skin: A Review of Skin Modeling Approaches. Sensors 2021, 21, 3624. [Google Scholar] [CrossRef]
- Qiu, S.; Yuan, J.; Duan, S.; Zhou, X.; Mei, C.; Qu, Y. High sensitivity temperature sensor based on a helically twisted photonic crystal fiber. Results Phys. 2021, 29, 104767. [Google Scholar] [CrossRef]
- An, W.; Li, C.; Wang, D.; Chen, W.; Guo, S.; Gao, S.; Zhang, C. Flat Photonic Crystal Fiber Plasmonic Sensor for Simultaneous Measurement of Temperature and Refractive Index with High Sensitivity. Sensors 2022, 22, 9028. [Google Scholar] [CrossRef]
- Yu, R.; Chen, Y.; Shui, L.; Xiao, L. Hollow-Core Photonic Crystal Fiber Gas Sensing. Sensors 2020, 20, 2996. [Google Scholar] [CrossRef]
- BBulbul, A.M.; Rahaman, H.; Biswas, S.; Hossain, R.; Nahid, A. Design and numerical analysis of a PCF-based bio-sensor for breast cancer cell detection in the THz regime. Sens. Bio-Sens. Res. 2020, 30, 100388. [Google Scholar] [CrossRef]
- Gandhi, M.S.A.; Zhao, Y.; Fu, H.Y.; Li, Q. A Highly Versatile Porous Core Photonic Quasicrystal Fiber Based Refractive Index Terahertz Sensor. Sensors 2022, 22, 3469. [Google Scholar] [CrossRef]
- Islam, S.; Sultana, J.; Ahmed, K.; Islam, M.; Dinovitser, A. A novel approach for spectroscopic chemical identification using photonic crystal fiber in the terahertz regime. IEEE Sens. J. 2017, 18, 575–582. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, L.; Shi, Y.; Liu, S.; Dong, Y. A Highly Birefringent Photonic Crystal Fiber for Terahertz Spectroscopic Chemical Sensing. Sensors 2021, 21, 1799. [Google Scholar] [CrossRef]
- Mahmoud, M.A.E.; Habib, H.; Anower, S.; Rashed, A.N.Z. Hollow Core Photonic Crystal Fiber (PCF)–Based Optical Sensor for Blood Component Detection in Terahertz Spectrum. Braz. J. Phys. 2021, 51, 1017–1025. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, P.; Jindal, P. Decagonal solid core PCF based refractive index sensor for blood cells detection in terahertz regime. Opt. Quantum Electron. 2021, 53, 165. [Google Scholar] [CrossRef]
- Talataisong, W.; Gorecki, J.; Van Putten, L.D.; Ismaeel, R.; Williamson, J. Hollow-core antiresonant terahertz fiber-based TOPAS extruded from a 3D printer using a metal 3D printed nozzle. Photonics Res. 2021, 9, 1513–1521. [Google Scholar] [CrossRef]
- Im, J.; Goo, T.; Kim, J.; Choi, S.; Hong, S.J.; Bahk, Y.M. Detection of Microplastic in Salts Using Terahertz Time-Domain Spectroscopy. Sensors 2021, 21, 3161. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Harris, Z.B.; Virk, A.; Abazari, A.; Varadaraj, K.; Honkanen, R.; Arbab, M.H. Assessing Corneal Endothelial Damage Using Terahertz Time-Domain Spectroscopy and Support Vector Machines. Sensors 2022, 22, 9071. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Skorobogatiy, M. Surface Plasmon Resonance-like integrated sensor at terahertz frequencies for gaseous analytes. Opt. Express 2008, 16, 20206–20214. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; He, M.; Tian, Z.; Li, J.; Liu, J. Terahertz surface plasmon sensor for distinguishing gasolines. Appl. Opt. 2013, 52, 5695–5700. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, J.; Han, Z.; Maeda, H.; Ma, Y. Bragg-Mirror-Assisted High-Contrast Plasmonic Interferometers: Concept and Potential in Terahertz Sensing. Nanomaterials 2020, 10, 1385. [Google Scholar] [CrossRef]
- Ma, Y.; Nguyen-Huu, N.; Zhou, J.; Maeda, H.; Wu, Q.; Eldlio, M.; Pistora, J.; Cada, M. Mach–Zehnder Interferometer-Based Integrated Terahertz Temperature Sensor. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 4601607. [Google Scholar] [CrossRef]
- Islam, S.; Sultana, J.; Faisal, M.; Islam, M.R.; Dinovitser, A. A modified hexagonal photonic crystal fiber for terahertz applications. Opt. Mater. 2018, 79, 336–339. [Google Scholar] [CrossRef]
- Leon, J.B.M.; Abedin, S.; Kabir, A. A photonic crystal fiber for liquid sensing application with high sensitivity, birefringence and low confinement loss. Sens. Int. 2021, 2, 100061. [Google Scholar] [CrossRef]
- Podder, E.; Hossain, B.; Rahaman, E.; Bulbul, A.A.; Ahmed, K. Design and optimization of terahertz blood components sensor using photonic crystal fiber. Sens. Bio-Sens. Res. 2020, 30, 100386. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, G.; Yu, Z.; Wang, Q.; Jiang, X. Spider web-shaped photonic crystal fiber for THz wave propagation. Results Opt. 2021, 5, 100137. [Google Scholar] [CrossRef]
- Islam, M.R.; Iftekher, A.N.M.; Mou, F.A.; Rahman, M.; Bhuiyan, M.I.H. Design of a Topas-based ultrahigh-sensitive PCF biosensor for blood component detection. Appl. Phys. A 2021, 127, 109. [Google Scholar] [CrossRef]
- Woyessa, G.; Fasano, A.; Markos, C.; Stefani, A.; Rasmussen, H.K. Zeonex microstructured polymer optical fiber: Fabrication friendly fibers for high temperature and humidity insensitive Bragg grating sensing. Opt. Mater. Express 2017, 7, 286–295. [Google Scholar] [CrossRef]
- Anthony, J.; Leonhardt, R.; Argyros, A. Characterization of a microstructured Zeonex terahertz fiber. JOSA B 2011, 28, 1013–1018. [Google Scholar] [CrossRef]
- Phanchat, N.; Talataisong, W.; Klokkou, N.; Chitaree, R.; Apostolopoulos, V.; Beresna, M.; Brambilla, G. Extruded TOPAS hollow-core anti-resonant fiber optimized for THz guidance at 0.9THz. Opt. Express 2022, 30, 13059–13069. [Google Scholar] [CrossRef]
- Talataisong, W.; Gorecki, J.; Ismaeel, R.; Beresna, M.; Schwendemann, D.; Apostolopoulos, V.; Brambilla, G. Singlemoded THz guidance in bendable TOPAS suspended-core fiber directly drawn from a 3D printer. Sci. Rep. 2020, 10, 11045. [Google Scholar] [CrossRef]
- Wu, B.; Lu, Y.; Hao, C.; Duan, L. Hollow-Core Photonic Crystal Fiber Based on C2H2 and NH3 Gas Sensor. Appl. Mech. Mater. 2013, 411, 1577–1580. [Google Scholar] [CrossRef]
- Arif, F.H.; Biddut, J.H. A new structure of photonic crystal fiber with high sensitivity, high nonlinearity, high birefringence and low confinement loss for liquid analyte sensing applications. Sens. Bio-Sens. Res. 2017, 12, 8–14. [Google Scholar] [CrossRef]
- Cordeiro, M.B.C.; Franco, M.A.R.; Chesini, G. Microstructured-core optical fibre for evanescent sensing applications. Opt. Express 2006, 14, 13056–13066. [Google Scholar] [CrossRef]
- Rahaman, E.; Jibon, R.H.; Mondal, H.S.; Hossain, B.; Bulbul, A.A. Design and optimization of a PCF-based chemical sensor in THz regime. Sens. Bio-Sens. Res. 2021, 32, 100422. [Google Scholar] [CrossRef]
- Habib, A.; Rashed, A.N.Z.; El-Hageen, H.M.; Alatwi, A.M. Extremely Sensitive Photonic Crystal Fiber–Based Cancer Cell Detector in the Terahertz Regime. Plasmonics 2021, 16, 1297–1306. [Google Scholar] [CrossRef]
- Markelz, A.G.; Roitberg, A.; Heilweil, E.J. Pulsed terahertz spectroscopy of DNA, bovine serum albumin and collagen between 0.1 and 2.0 THz. Chem. Phys. Lett. 2000, 320, 42–48. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Ma, S.; Wu, X. Quantify Glucose Level in Freshly Diabetic’s Blood by Terahertz Time-Domain Spectroscopy. J. Infrared Millim. Terahertz Waves 2018, 39, 399–408. [Google Scholar] [CrossRef]
- BSuhaimi, N.A.N.; Yakasai, I.K.; Abas, E.; Kaijage, B.; Begum, F. Modelling and simulation of novel liquid-infiltrated PCF biosensor in Terahertz frequencies. IET Optoelectron. 2020, 14, 411–416. [Google Scholar] [CrossRef]
- Islam, M.R.; Kabir, F.; Talha, K.A.; Islam, S. A novel hollow core terahertz refractometric sensor. Sens. Bio-Sens. Res. 2019, 25, 100295. [Google Scholar] [CrossRef]
- Bulbul, A.A.-M.; Rashed, A.N.Z.; El-Hageen, H.M.; Alatwi, A.M. Design and numerical analysis of an extremely sensitive PCF-based sensor for detecting kerosene adulteration in petrol and diesel. Alex. Eng. J. 2021, 60, 5419–5430. [Google Scholar] [CrossRef]
- Islam, M.R.; Naser, A.M.A.; Jaba, F.Z.; Anzum, F.; Iftekher, A.N.M.; Khan, M.R.H.; Nishat, M.M. Design of a hexagonal outlined porous cladding with vacant core photonic crystal fibre biosensor for cyanide detection at THz regime. IET Optoelectron. 2022, 16, 160–173. [Google Scholar] [CrossRef]




| Reference | Biological Materials | Sensitivity [%] | CL [cm−1] | EML [cm−1] |
|---|---|---|---|---|
| [9] | Benzene | 89.00 | 1.15 × 10−9 | 0.0280 |
| This Work | 99.76 | 1.87 × 10−13 | 0.0040 | |
| [16] | Ethanol | 85.70 | 1.70 × 10−7 | - |
| This Work | 99.73 | 2.74 × 10−13 | 0.0042 | |
| [28] | Glucose | 47.59 | - | - |
| This Work | 99.71 | 3.84 × 10−13 | 0.0043 | |
| [28] | Human Mucosa | 47.31 | - | - |
| This Work | 99.68 | 1.94 × 10−13 | 0.0044 | |
| [29] | Red Blood Cells | 94.38 | 2.75 × 10−13 | - |
| This Work | 99.80 | 1.81 × 10−13 | 0.0036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, J.; Zhang, Y.; Guang, Z.; Miao, T.; Ali, Z.; Qiao, D.; Yao, Y.; Wu, K.; Zhou, L.; Meng, C.; et al. Ultra-High Sensitivity Terahertz Microstructured Fiber Biosensor for Diabetes Mellitus and Coronary Heart Disease Marker Detection. Sensors 2023, 23, 2020. https://doi.org/10.3390/s23042020
Xue J, Zhang Y, Guang Z, Miao T, Ali Z, Qiao D, Yao Y, Wu K, Zhou L, Meng C, et al. Ultra-High Sensitivity Terahertz Microstructured Fiber Biosensor for Diabetes Mellitus and Coronary Heart Disease Marker Detection. Sensors. 2023; 23(4):2020. https://doi.org/10.3390/s23042020
Chicago/Turabian StyleXue, Jia, Yani Zhang, Zhe Guang, Ting Miao, Zohaib Ali, Dun Qiao, Yiming Yao, Kexin Wu, Lei Zhou, Cheng Meng, and et al. 2023. "Ultra-High Sensitivity Terahertz Microstructured Fiber Biosensor for Diabetes Mellitus and Coronary Heart Disease Marker Detection" Sensors 23, no. 4: 2020. https://doi.org/10.3390/s23042020
APA StyleXue, J., Zhang, Y., Guang, Z., Miao, T., Ali, Z., Qiao, D., Yao, Y., Wu, K., Zhou, L., Meng, C., & Copner, N. (2023). Ultra-High Sensitivity Terahertz Microstructured Fiber Biosensor for Diabetes Mellitus and Coronary Heart Disease Marker Detection. Sensors, 23(4), 2020. https://doi.org/10.3390/s23042020





