Analysis of Meditation vs. Sensory Engaged Brain States Using Shannon Entropy and Pearson’s First Skewness Coefficient Extracted from EEG Data
Abstract
1. Introduction
2. Materials
- Meditation (MED): Participants were asked to engage in the meditation of their choice for 7 min with their eyes closed. Alternatively, if people were unfamiliar with meditating, they were asked to relax with their eyes closed. After the proper preparation, each participant pressed the space bar key on the keyboard to signal the beginning of the meditation period, which continued until the preprogramed end signal informed them of the end of the session.
- Video (VDO): In this final modality, the participants were presented with a video containing a sequence of ambiguous images with the song ‘Imagine’ by John Lennon, playing throughout the video. The ambiguous images aimed to evoke mental responses similar to the well-known Necker cube. The ambiguous images used in this task were designed by Oleg Shupliak, and they can be found in [39]. The duration of this experiment was 1 min and 50 s for each participant. There was no task the participants were asked to perform besides watching the video and listening to the song. After reading the instructions, the participants pressed the space bar key on the keyboard to signal the beginning of the video-watching period, until the video finished playing.
3. Methods
3.1. Preprocessing
- 8 ms for anti-alias filter, which essentially accounts for the required time of the amplifier to do the conversion;
- 14 ms for the screen refresh rate, adding to a total of a 22 ms shift to match the recorded event markers with the actual event time.
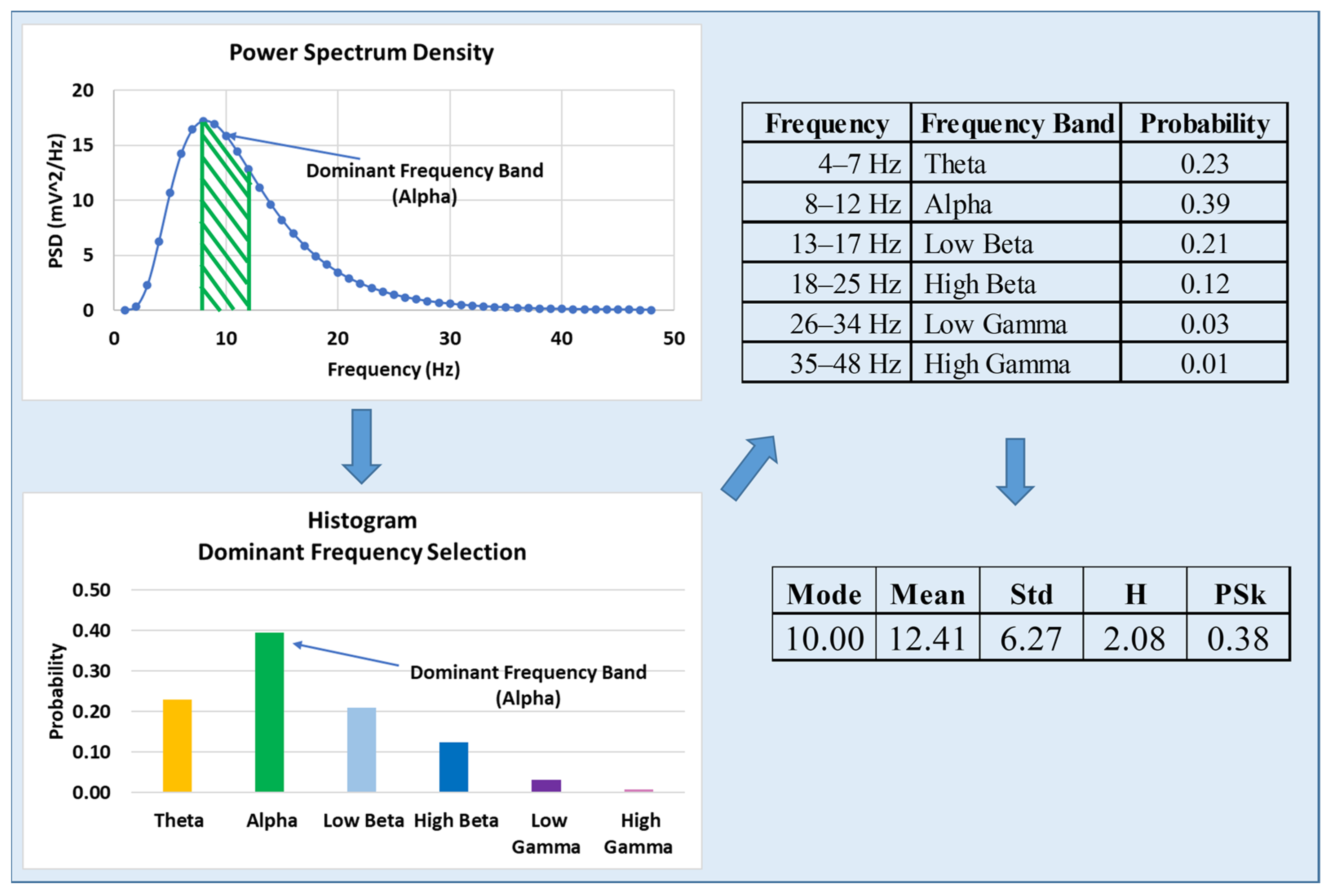
3.2. Entropy and Information Theoretical Indices
- 3.
- Entropy measure (H), as introduced by Shannon [46], providing us with the degree of randomness in an EEG signal.
- 4.
- PSk as a measure of information derived from the frequency distribution structure represented by the degree of asymmetry, which was derived by [47] and discussed by [48]. The version of the PSk (1st order skewness coefficient) described and formulated in [49] in terms of the mean, standard deviation and mode (dominant frequency or frequency band) is used for this study.
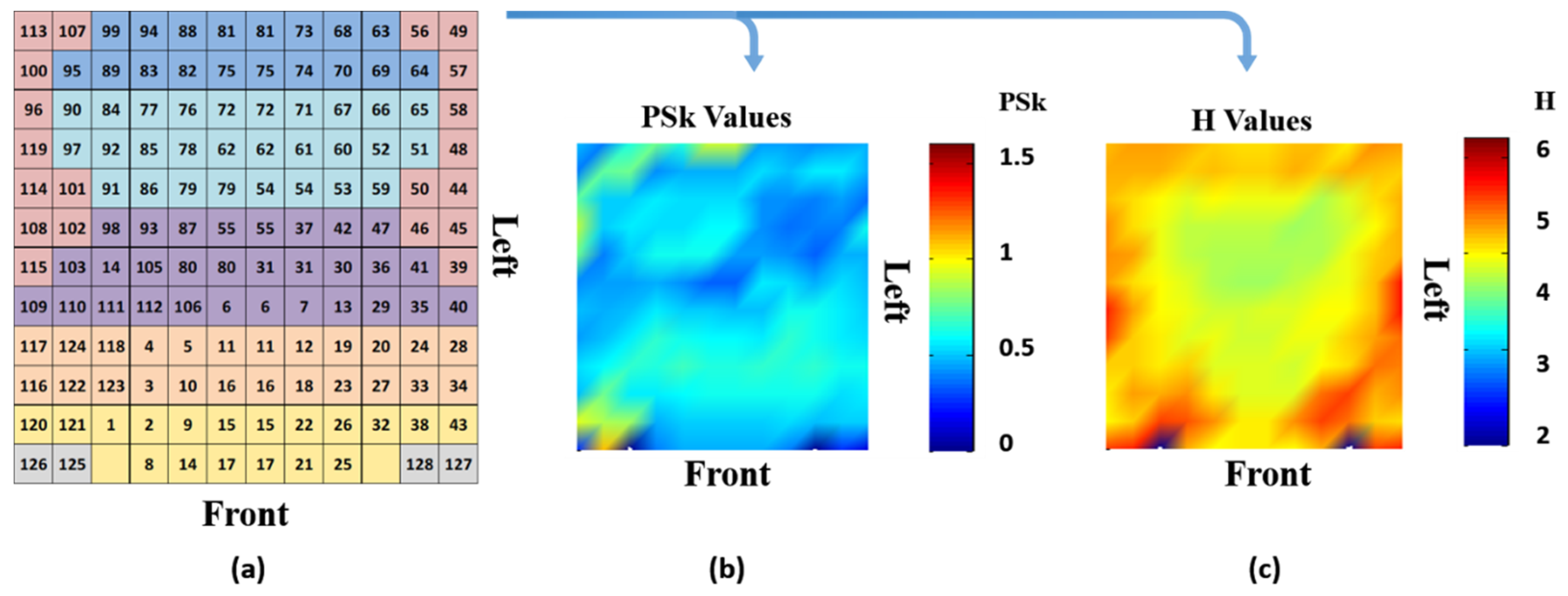
3.3. Computation of the H and PSk Indices
3.4. Analysis of Multi-Variate EEG Data
4. Results
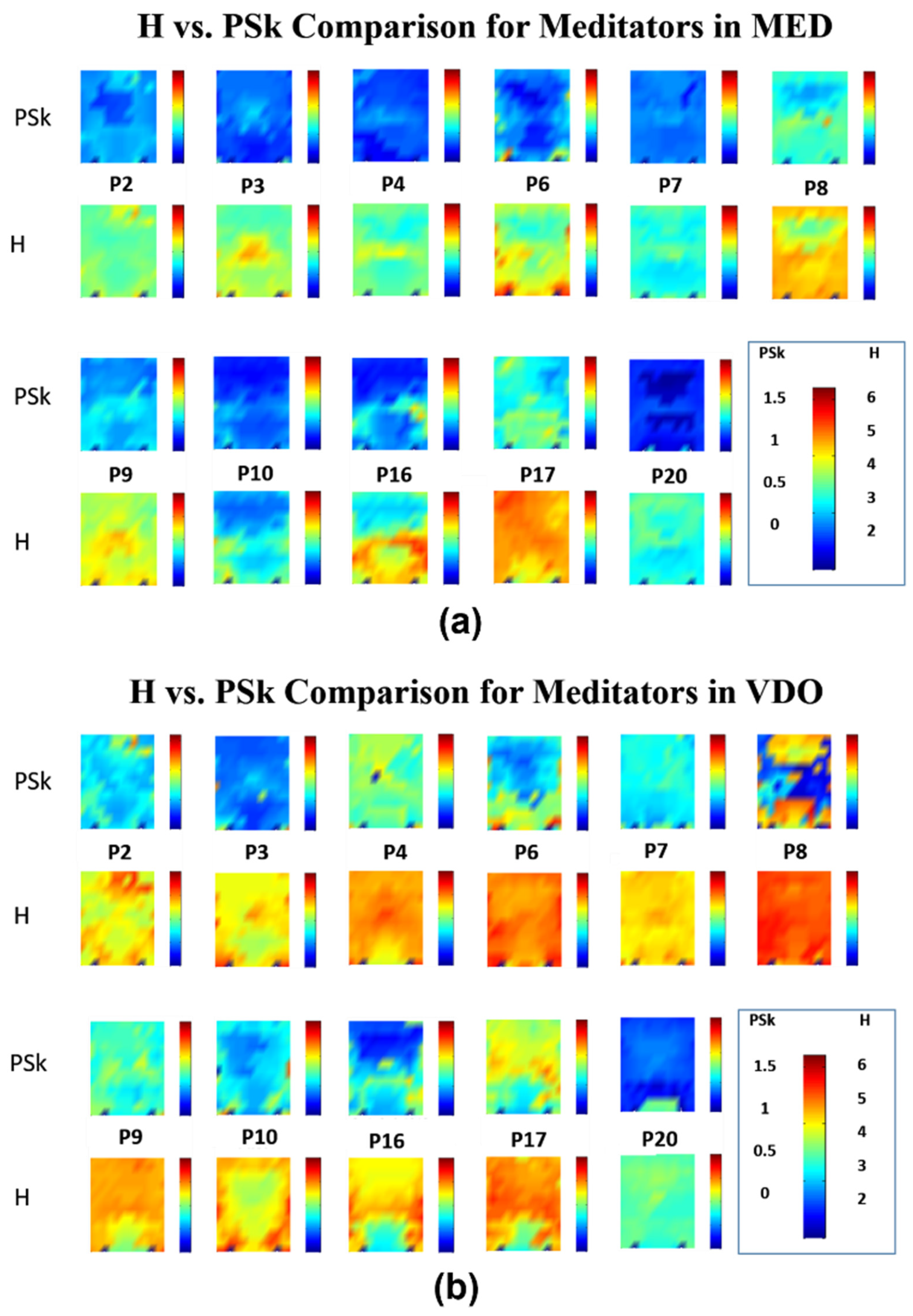
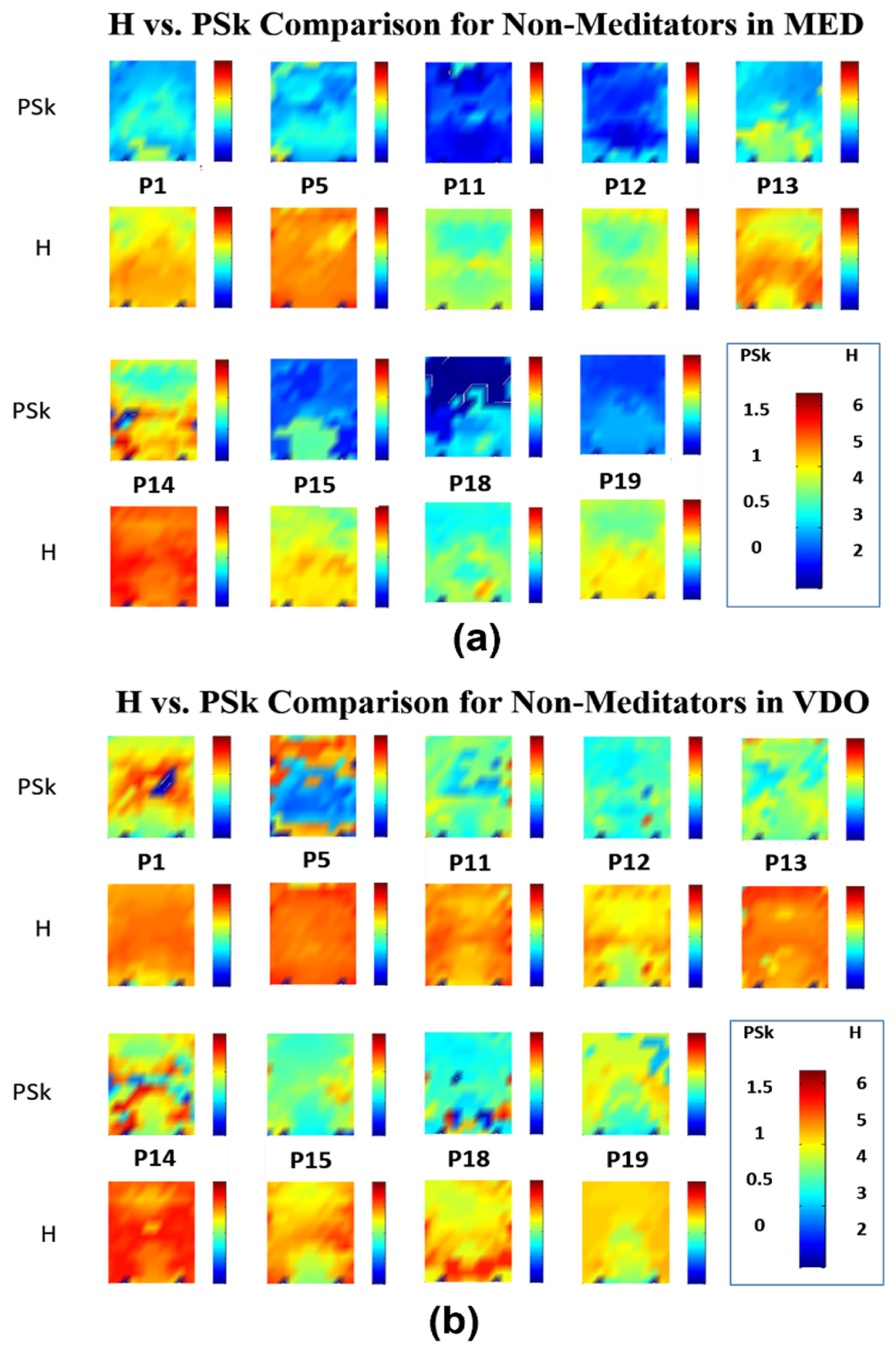
4.1. Qualitative Analysis of the EEG Data
4.2. Detailed Quantitative Analysis of Brain Dynamics
5. Discussion
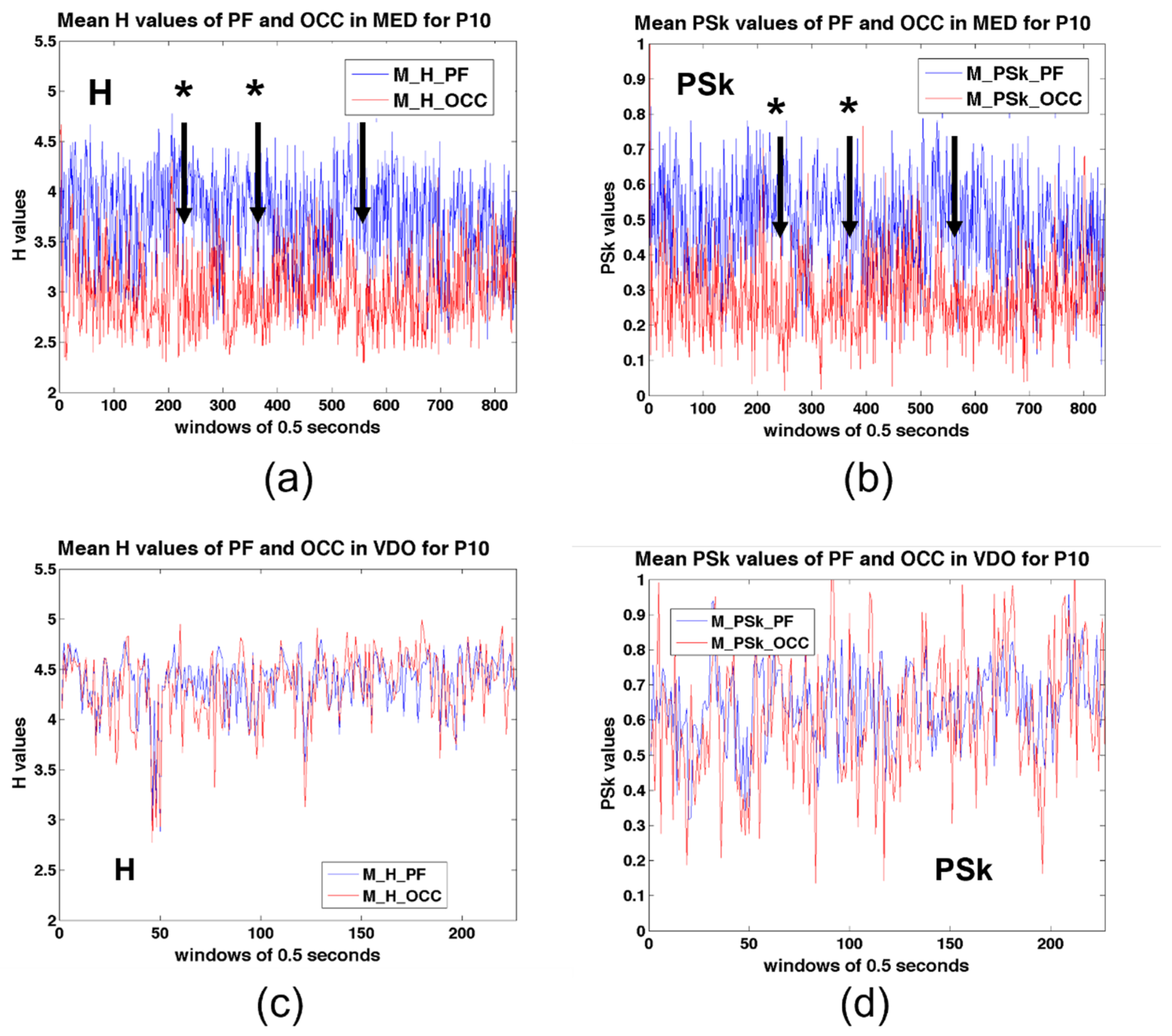
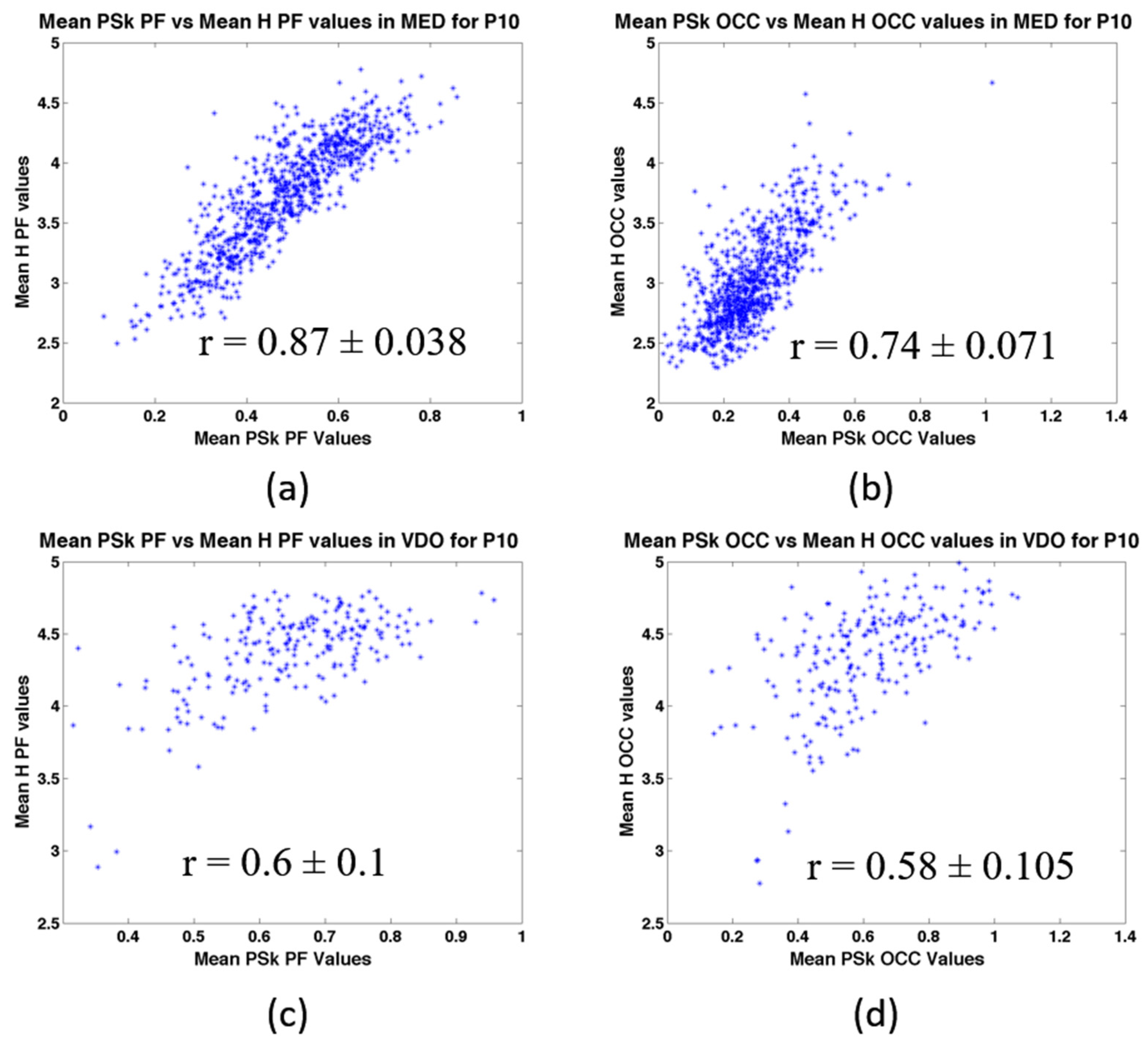
5.1. Discrimination across Modalities and Brain Regions for a Representative Participant (P10)
5.2. Discrimination Results in Populations of Meditators and Non-Meditator Participants
6. Conclusions
- We conjecture that more relaxed states showing alpha dominance, accompanied with lower values of H and PSk, are achieved by: (1) masterful meditators, (2) people who practice relaxation techniques and (3) people who are naturally more relaxed (less stressed), who might be able to mitigate environmental signals and demands when existing in such relaxed emotional and coherent mental states. This we can derive from the data associated with the modality VDO when contrasted with the one of MED for both groups: Meditator and Non-Meditator. It is relevant to note that the Meditator group showed lower values for H than the Non-Meditator group, which indicates that meditative states are more likely different than relaxed states when participants have their eyes closed.
- We conjecture that meditators may carry these relaxed states into other activities, possibly due to the lasting psychophysiological effects derived from meditative practices [28,57,58,59] translating and continuing into other areas of life. This will require further investigation and studies with a larger sample size.
- When comparing the overall mean values for each modality, MED displays the smallest values of H and PSk, and VDO displays the largest for both groups. The overall distribution and values, however, are significantly different. These findings indicate that there is a distinct difference between meditators and non-meditators in brain dynamics, and that H and PSk taken together are a useful element to analyze and differentiate various cognitive states. Statistical hypothesis tests indicate that the H index is useful to discriminate between Meditator and Non-Meditator participants during MED over both the PF and OCC areas (p = 0.03), while the PSk index is useful to discriminate Meditators from Non-Meditators based on the PF areas for both MED and VDO (p = 0.05).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newberg, A. The neuroscientific study of spiritual practices. Front. Psychol. 2014, 5, 215. [Google Scholar] [CrossRef] [PubMed]
- Faber, E. The Neural Correlates of Two Forms of Spiritual Love: An EEG Study. 27 March 2016. Available online: https://www.biorxiv.org/content/10.1101/045898v1.full (accessed on 29 November 2022).
- Gao, J.; Leung, H.; Wu, B.; Skouras, S. The neurophysiological correlates of religious chanting. Sci. Rep. 2019, 9, 4262. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G. Rhythms of the Brain; Oxford University Press: New York, NY, USA, 2006; ISBN 978-01-9530-106-9. [Google Scholar]
- Davis, J.J.J.; Schübeler, F.; Ji, S.; Kozma, R. Discrimination Between Brain Cognitive States Using Shannon Entropy and Skewness Information Measure. In Proceedings of the IEEE International Conference on Systems, Man, and Cybernetics (SMC 2020), Toronto, ON, Canada, 11–14 October 2020; pp. 4026–4031. [Google Scholar]
- Schoenberg, P.; Vago, D. Mapping meditative states and stages with electrophysiology: Concepts, classifications, and methods. Curr. Opin. Psychol. 2019, 28, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Jachs, B. The Neurophenomenology of Meditative States: Introducing Temporal Experience Tracing to Capture Subjective Experience States and their Neural Correlates. Ph.D. Thesis, University of Cambridge, Cambridge, UK, May 2021. [Google Scholar]
- Lutz, A.; Greischar, L.; Rawlings, N.; Ricard, M.; Davidson, R. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. PNAS 2004, 101, 16369–16373. [Google Scholar] [CrossRef] [PubMed]
- Kasamatsu, A.; Hirai, T. An electroencephalographic study on the zen meditation (zazen). Folia Psychiat. Neurol. Jpn. 1996, 20, 315–336. [Google Scholar] [CrossRef]
- Austin, J.H. Zen and the Brain: Toward an Understanding of Meditation and Consciousness; The MIT Press: Cambridge, MA, USA, 1998; ISBN 978-0-262-51109-4. [Google Scholar]
- Davis, J.J.J.; Kozma, R. Visualization of Human Cognitive States Monitored by High-density EEG Arrays. In: INNS 3rd Conf. on Big Data and Deep Learning, April 17-19, 2018, Bali, Indonesia. Procedia Comput. Sci. 2018, 144, 219–231. [Google Scholar] [CrossRef]
- Davis, J.J.J.; Kozma, R.; Freeman, W.J. The Art of Encephalography to Understand and Discriminate Higher Cognitive Functions Visualizing Big Data on Brain Imaging using Brain Dynamics Movies. Procedia Comput. Sci. 2015, 53, 56–63. [Google Scholar] [CrossRef]
- Zafar, R.; Dass, S.C.; Malik, A.S. Electroencephalogram-based decoding cognitive states using convolutional neural network and likelihood ratio based score fusion. PLoS ONE 2017, 12, e0178410. [Google Scholar] [CrossRef]
- Kasabov, N.; Capecci, E. Spiking neural network methodology for modelling, classification and understanding of EEG spatio-temporal data measuring cognitive processes. Inf. Sci. 2015, 294, 565–575. [Google Scholar] [CrossRef]
- Suhail, T.; Indiradevi, K.; Suhara, E.; Poovathinal, S.; Ayyappan, A. Distinguishing cognitive states using electroencephalography local activation and functional connectivity patterns. Biomed. Signal. Process. Control 2022, 77, 103742. [Google Scholar] [CrossRef]
- Quatieri, T.F.; Williamson, J.R.; Smalt, C.J.; Perricone, J.; Helfer, B.J.; Nolan, M.A.; Eddy, M.; Moran, J. Using EEG to Discriminate Cognitive Workload and Performance Based on Neural Activation and Connectivity; AD1033658; MIT Lincoln Laboratory Lexington United States: Lexington, MA, USA, 2016. [Google Scholar]
- Walter, N. Self-Organized Criticality as a Neurodynamical Correlate of Consciousness: A Neurophysiological Approach to Measure States of Consciousness Based on EEG-Complexity Features; Doctor Scientiarum Humanarum, Universität Regensburg: Regensburg, Germany, 2022. [Google Scholar]
- Dvorak, D.; Shang, A.; Abdel-Baki, S.; Suzuki, W.; Fenton, A. Cognitive behavior classification from scalp EEG signals. IEEE Trans. Neural. Syst. Rehabil. Eng. 2018, 26, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Doborjeh, M.; Doborjeh, Z.; Kasabov, N.; Barati, M.; Wang, G. Deep Learning of Explainable EEG Patterns as Dynamic Spatiotemporal Clusters and Rules in a Brain-Inspired Spiking Neural Network. Sensors 2021, 21, 4900. [Google Scholar] [CrossRef]
- Davis, J.J.J.; Kozma, R.; Lin, C.-T.; Freeman, W.J. Spatio-Temporal EEG Pattern Extraction Using High-Density Scalp Arrays. In Proceedings of the International Joint Conference on Neural Networks (IJCNN 2016), Vancouver, BC, Canada, 24–29 July 2016; pp. 889–896. [Google Scholar]
- Davis, J.J.J.; Lin, C.-T.; Gillett, G.; Kozma, R. An Integrative Approach to Analyze EEG Signal and Human Brain Dynamics in Different Cognitive States. J. Artif. Intell. 2017, 7, 287–299. [Google Scholar] [CrossRef]
- Walter, N.; Hinterberger, T. Determining states of consciousness in the electroencephalogram based on spectral, complexity, and criticality features. Neurosci. Conscious. 2022, 8, niac008. [Google Scholar] [CrossRef] [PubMed]
- Travis, F.; Wallace, R.K. Autonomic and EEG Patterns during Eyes-Closed Rest and Transcendental Meditation (TM) Practice: The Basis for a Neural Model of TM Practice. Conscious. Cogn. 1999, 8, 302–318. [Google Scholar] [CrossRef]
- Delmonte, M.M. Physiological responses during meditation and rest. AAPB 1984, 9, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Zangróniz, R.; Martínez-Rodrigo, A.; Pastor, J.; López, M.; Fernández-Caballero, A. Electrodermal Activity Sensor for Classification of Calm/Distress Condition. Sensors 2017, 17, 2324. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, D.; Zhang, P.; Zhang, Y.; Hou, Y.; Hu, B. Exploring EEG Features in Cross-Subject Emotion Recognition. Front. Neurosci. 2018, 12, 162. [Google Scholar] [CrossRef]
- Cossy, N.; Tzovara, A.; Simonin, A.; Rossetti, A.; De Lucia, M. Robust discrimination between EEG responses to categories of environmental. Front. Psychol. 2014, 5, 155. [Google Scholar] [CrossRef]
- Davis, J.J.J.; Kozma, R.; Schübeler, F. Stress Reduction, Relaxation, and Meditative States Using Psychophysiological Measurements Based on Biofeedback Systems via HRV and EEG. In Encyclopedia of Computer Graphics and Games; Lee, N., Ed.; Springer: Cham, Switzerland, 2006. [Google Scholar] [CrossRef]
- Kirk, I.J.; Spriggs, M.J.; Sumner, R.L. Human EEG and the mechanisms of memory: Investigating long-term potentiation (LTP) in sensory-evoked potentials. J. R. Soc. N. Z. 2021, 51, 24–40. [Google Scholar] [CrossRef]
- Shadli, S.M.; Ando, L.C.; McIntosh, J.; Lodhia, V.; Russell, B.R.; Kirk, I.J.; Glue, P.; McNaughton, N. Right frontal anxiolytic-sensitive eeg ‘theta’rhythm in the stop-signal task is a theory-based anxiety disorder biomarker. Sci. Rep. 2021, 11, 19746. [Google Scholar] [CrossRef]
- Zheng, Y.; Kirk, I.; Chen, T.; O’Hagan, M.; Waldie, K.E. Task-Modulated Oscillation Differences in Auditory and Spoken Chinese-English Bilingual Processing: An Electroencephalography Study. Front. Psychol. 2022, 13, 823700. [Google Scholar] [CrossRef]
- Huang, C.S.; Pal, N.R.; Chuang, C.H.; Lin, C.T. Identifying changes in EEG information transfer during drowsy driving by transfer entropy. Front. Hum. Neurosci. 2015, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Xie, J.; Yang, M.; Li, Z.; Li, Z.; Liao, D.; Xu, X.; Yang, X. A review of emotion recognition using physiological signals. Sensors 2018, 18, 2074. [Google Scholar] [CrossRef] [PubMed]
- Vivot, R.M.; Pallavicini, C.; Zamberlan, F.; Vigo, D.; Tagliazucchi, E. Meditation increases the entropy of brain oscillatory activity. Neuroscience 2020, 431, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevis, S.A.; Sankar, R. Applications of higher order statistics in electroencephalography signal processing: A comprehensive survey. IEEE Rev. Biomed. Eng. 2020, 13, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Freeman, W.J.; Holmes, M.D.; Burke, B.C.; Vanhatalo, S. Spatial spectra of scalp EEG and EMG from awake humans. Clin. Neurophysiol. 2003, 114, 1053–1068. [Google Scholar] [CrossRef]
- Freeman, W.J.; Quiroga, R.Q. Imaging Brain Function with EEG—Advanced Temporal and Spatial Analysis of Electroencephalographic Signals; Springer: New York, NY, USA, 2013; ISBN 978-14-614-498-36. [Google Scholar]
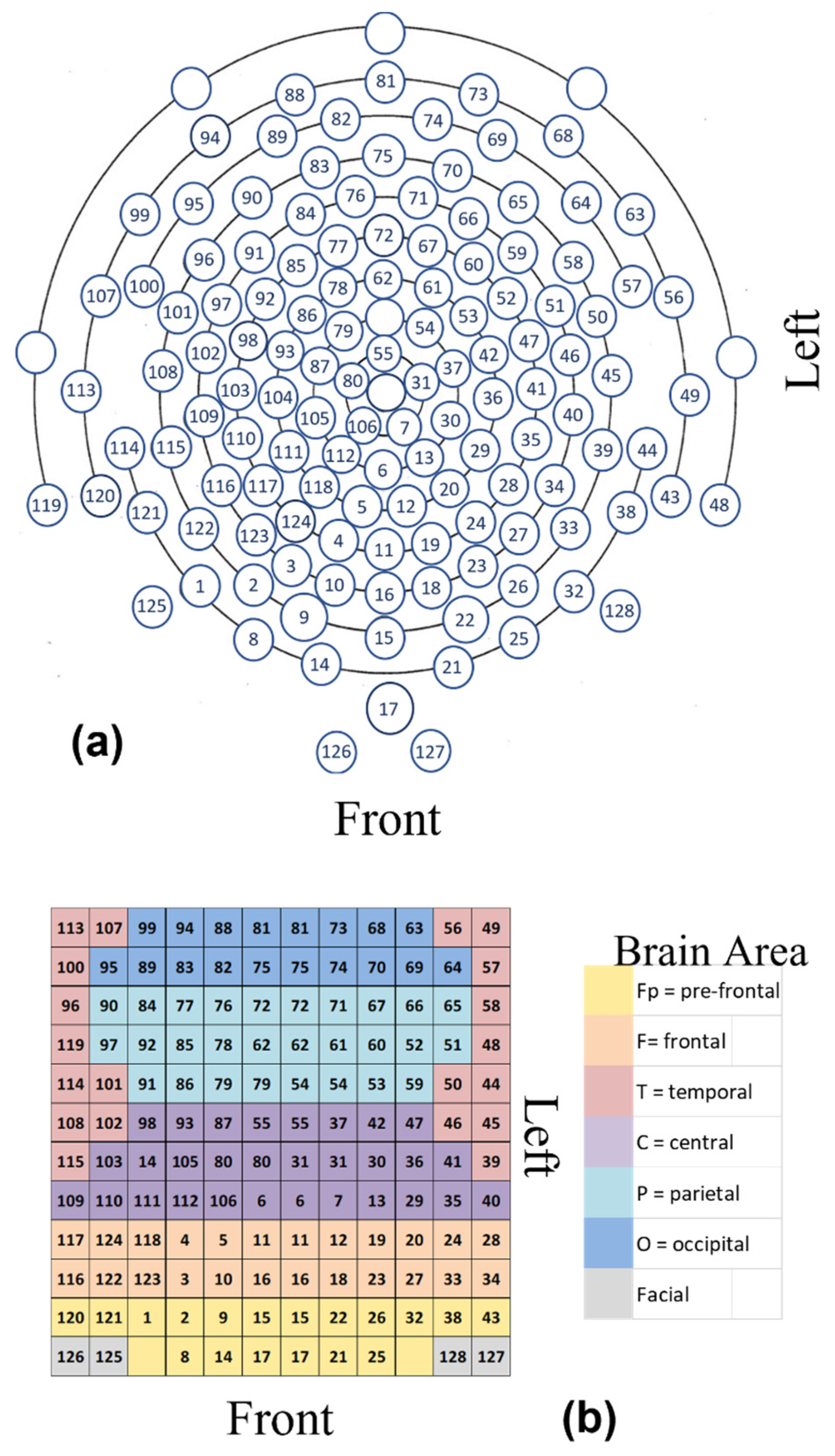
- Electrical Geodesics, Inc. Geodesic Sensor Net Technical Manual. 2007. Available online: https://philipsproductcontent.blob.core.windows.net/assets/20180705/6f388e7ade4d41e38ad5a91401755b6f.pdf (accessed on 30 November 2022).
- Shupliak, O. Hidden Images. From Oleg Shupliak—Official Website. Available online: https://shupliak.art/gallery/hidden-images (accessed on 11 January 2023).
- Telesca, L.; Lovallo, M. Analysis of time dynamics in wind records by means of multifractal detrended fluctuation analysis and Fisher-Shannon information plane. J. Stat. Mech. 2011, 2011, P07001. [Google Scholar] [CrossRef]
- Ji, S. The Planck-Shannon plot: A quantitative method for identifying ‘superstructures’ in cell biology and consciousness study. Cosm. Hist. 2020, 16, 142–164. [Google Scholar]
- Kozma, R.; Freeman, W.J. Cognitive Phase Transitions in the Cerebral Cortex—Enhancing the Neuron Doctrine by Modeling Neural Fields; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-24404-4. [Google Scholar]
- Kozma, R.; Freeman, W.J. Cinematic Operation of the Cerebral Cortex Interpreted via Critical Transitions in Self-Organized Dynamic Systems. Front. Syst. Neurosci. 2017, 11, 10. [Google Scholar] [CrossRef]
- Davis, J.J.J.; Gillett, G.; Kozma, R. Revisiting Brentano on Consciousness: Striking Correlations with Electrocorticogram Findings about the Action-Perception Cycle and the Emergence of Knowledge and Meaning. Mind Matter 2015, 13, 45–69. [Google Scholar]
- Kozma, R.; Davis, J.J.J.; Freeman, W.J. Synchronized Minima in ECoG Power at Frequencies Between Beta-Gamma Oscillations Disclose Cortical Singularities in Cognition. JNSNE 2012, 1, 13–23. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; The University of Illinois Press: Chicago, IL, USA, 1971; ISBN 978-02-5272-548-7. [Google Scholar]
- Pearson, K. Contributions to the Mathematical Theory of Evolution. II. Skew Variation in Homogeneous Material. Philos. Trans. R. Soc. 1895, 186, 343–414. [Google Scholar] [CrossRef]
- Groeneveld, R.A.; Meeden, G. Measuring Skewness and Kurtosis. Statistician 1984, 33, 391–399. [Google Scholar] [CrossRef]
- Weisstein, E.W. Pearson Mode Skewness. From MathWorld—A Wolfram Web Resource. Available online: https://mathworld.wolfram.com/PearsonModeSkewness.html (accessed on 30 November 2022).
- Rajaram, R.; Castellani, B.; Wilson, A.N. Advancing Shannon Entropy for Measuring Diversity in Systems. Complexity 2017, 2017, 8715605. [Google Scholar] [CrossRef]
- Berger, H. Über Das Elektrenkephalogramm Des Menschen. Arch. Psychiatr. Nervenkrankh. 1929, 87, 527–570. [Google Scholar] [CrossRef]
- Miraglia, F.; Vecchio, F.; Bramanti, P.; Rossini, P.M. EEG Characteristics in “Eyes-Open” versus “Eyes-Closed” Conditions: Small-World Network Architecture in Healthy Aging and Age-Related Brain Degeneration. Clin. Neurophysiol. 2016, 127, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J.; Magee, C.A.; Rushby, J.A. EEG Differences between Eyes-Closed and Eyes-Open Resting Conditions. Clin. Neurophysiol. 2007, 118, 2765–2773. [Google Scholar] [CrossRef]
- Ji, S. Planckian Information (Ip): A New Measure of Order in Atoms, Enzymes, Cells, Brains, Human Societies, and the Cosmos. In Unified Field Mechanics: Natural Science Beyond the Veil of Spacetime, Proceedings of the IX Symposium Honoring Noted French Mathematical Physicist Jean-Pierre Vigier, Baltimore, MD, USA, 16–19 November 2014; Amoroso, R.L., Kauffman, L.H., Rowlands, P., Eds.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2016; pp. 579–589. [Google Scholar]
- Law, A.M.; Kelton, W.D. Simulation Modeling & Analysis; McGraw-Hill, Inc.: New York, NY, USA, 1982; ISBN 0-07-036698-5. [Google Scholar]
- Freeman, W.J.; Zhai, J. Simulated power spectral density (PSD) of background electro-corticogram (ECoG). Cogn. Neurodyn. 2009, 3, 97–103. [Google Scholar] [CrossRef]
- Davis, J.J.J.; Schübeler, F.; Kozma, R. Psychophysiological Coherence in Community Dynamics—A Comparative Analysis between Meditation and Other Activities. OBM Integr. Complement. Med. 2019, 4, 015. [Google Scholar] [CrossRef]
- Elbers, J.; McCraty, R. HeartMath Approach to Self-Regulation and Psychosocial Well-Being. J. Psychol. Afr. 2020, 30, 69–79. [Google Scholar] [CrossRef]
- McCraty, R.; Atkinson, M.; Tomasino, D.; Bradley, R. The coherent heart heart-brain interactions, psychophysiological coherence, and the emergence of system-wide order. Integral Rev. 2009, 5, 10–115. [Google Scholar]
- Davis, J.J.J. The Brain of Melchizedek—A Cognitive Neuroscience Approach to Spirituality. Master’s Thesis, Otago University, Dunedin, New Zealand, 30 March 2009. [Google Scholar]
- Freeman, W.J. Nonlinear Brain Dynamics and Intention According to Aquinas. Mind Matter 2008, 6, 207–234. [Google Scholar]





| Group/Modality | MED | VDO |
|---|---|---|
| Meditator | 0.87 ± 0.033 | 0.99 ± 0.007 |
| Non-Meditator | 0.92 ± 0.043 | 0.98 ± 0.016 |
| Group/Modality | MED | VDO |
|---|---|---|
| Meditator | 0.72 ± 0.049 | 0.98 ± 0.016 |
| Non-Meditator | 0.76 ± 0.124 | 0.99 ± 0.021 |
| Test | p-Value | H0: μ1 = μ2 |
|---|---|---|
| Meditators vs. Non-Meditators for MED (H) | 0.033 | Reject |
| Meditators vs. Non-Meditators for MED (PSk) | 0.255 | Accept |
| Meditators vs. Non-Meditators for VDO (H) | 0.5 | Accept |
| Meditators vs. Non-Meditators for VDO (PSk) | 0.955 | Accept |
| Modality and Brain Region | Mean Value Correlation Coefficient (r) | Lower Bound | Upper Bound |
|---|---|---|---|
| MED-PF | 0.8649 | 0.832 | 0.908 |
| MED-OCC | 0.7355 | 0.669 | 0.811 |
| VDO-PF | 0.6011 | 0.5 | 0.7 |
| VDO-OCC | 0.5792 | 0.475 | 0.685 |
| Test | p-Value | H0: μ1 = μ2 |
|---|---|---|
| Meditators vs. Non-Meditators for MED (PF) | 0.0218 | Reject |
| Meditators vs. Non-Meditators for MED (OCC) | 0.0290 | Reject |
| Meditators vs. Non-Meditators for VDO (PF) | 0.0841 | Accept |
| Meditators vs. Non-Meditators for VDO (OCC) | 0.1718 | Accept |
| Test | p-Value | H0: μ1 = μ2 |
|---|---|---|
| Meditators vs. Non-Meditators for MED (PF) | 0.0437 | Reject |
| Meditators vs. Non-Meditators for MED (OCC) | 0.0936 | Accept |
| Meditators vs. Non-Meditators for VDO (PF) | 0.0112 | Reject |
| Meditators vs. Non-Meditators for VDO (OCC) | 0.8738 | Accept |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, J.J.J.; Kozma, R.; Schübeler, F. Analysis of Meditation vs. Sensory Engaged Brain States Using Shannon Entropy and Pearson’s First Skewness Coefficient Extracted from EEG Data. Sensors 2023, 23, 1293. https://doi.org/10.3390/s23031293
Davis JJJ, Kozma R, Schübeler F. Analysis of Meditation vs. Sensory Engaged Brain States Using Shannon Entropy and Pearson’s First Skewness Coefficient Extracted from EEG Data. Sensors. 2023; 23(3):1293. https://doi.org/10.3390/s23031293
Chicago/Turabian StyleDavis, Joshua J. J., Robert Kozma, and Florian Schübeler. 2023. "Analysis of Meditation vs. Sensory Engaged Brain States Using Shannon Entropy and Pearson’s First Skewness Coefficient Extracted from EEG Data" Sensors 23, no. 3: 1293. https://doi.org/10.3390/s23031293
APA StyleDavis, J. J. J., Kozma, R., & Schübeler, F. (2023). Analysis of Meditation vs. Sensory Engaged Brain States Using Shannon Entropy and Pearson’s First Skewness Coefficient Extracted from EEG Data. Sensors, 23(3), 1293. https://doi.org/10.3390/s23031293




