Diffuse Correlation Spectroscopy: A Review of Recent Advances in Parallelisation and Depth Discrimination Techniques
Abstract
1. Introduction
2. Motivation
‘CBF is an important biomarker of brain health and function. It is a critical physiological parameter linking metabolic demand to oxygen supply, oxygen supply to metabolic consumption, and metabolic consumption to byproduct clearance.’
- a more accurate assessment of CA may be inferred from direct measurements of local CBF [24], and evaluation of its correlation with MAP, together with evaluation of clinical neurological signs. There should be no correlation between CBF and MAP when CA is operating effectively.
‘The ideal BF measurement should provide quantitative information about macro- and microvasculature with millisecond temporal resolution. The measurements should be carried out continuously, noninvasively, and without risk to subjects. Furthermore, ideal measurements would not be limited to the tissue surface, i.e., it is desirable to probe BF in deep tissues. Unfortunately, no such ideal modality exists’.
3. Laser Speckle
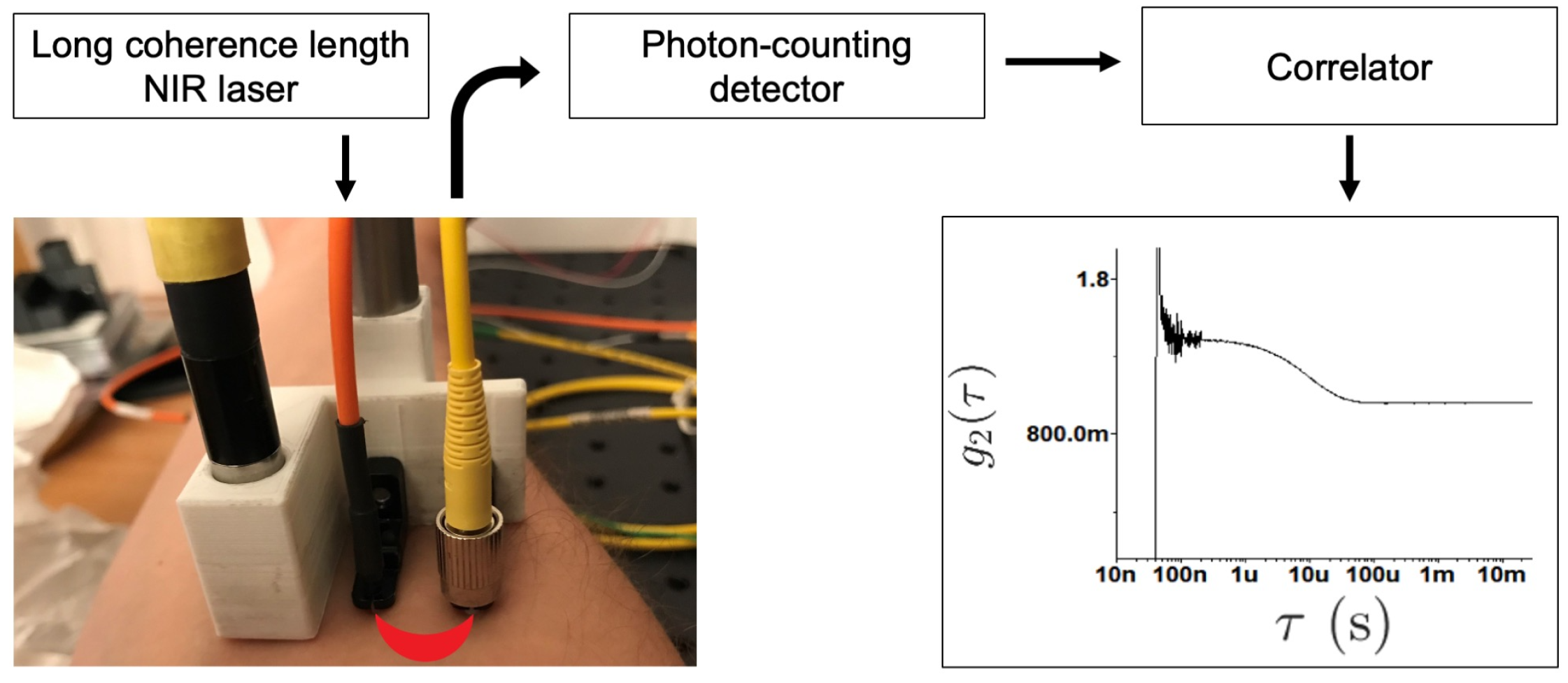
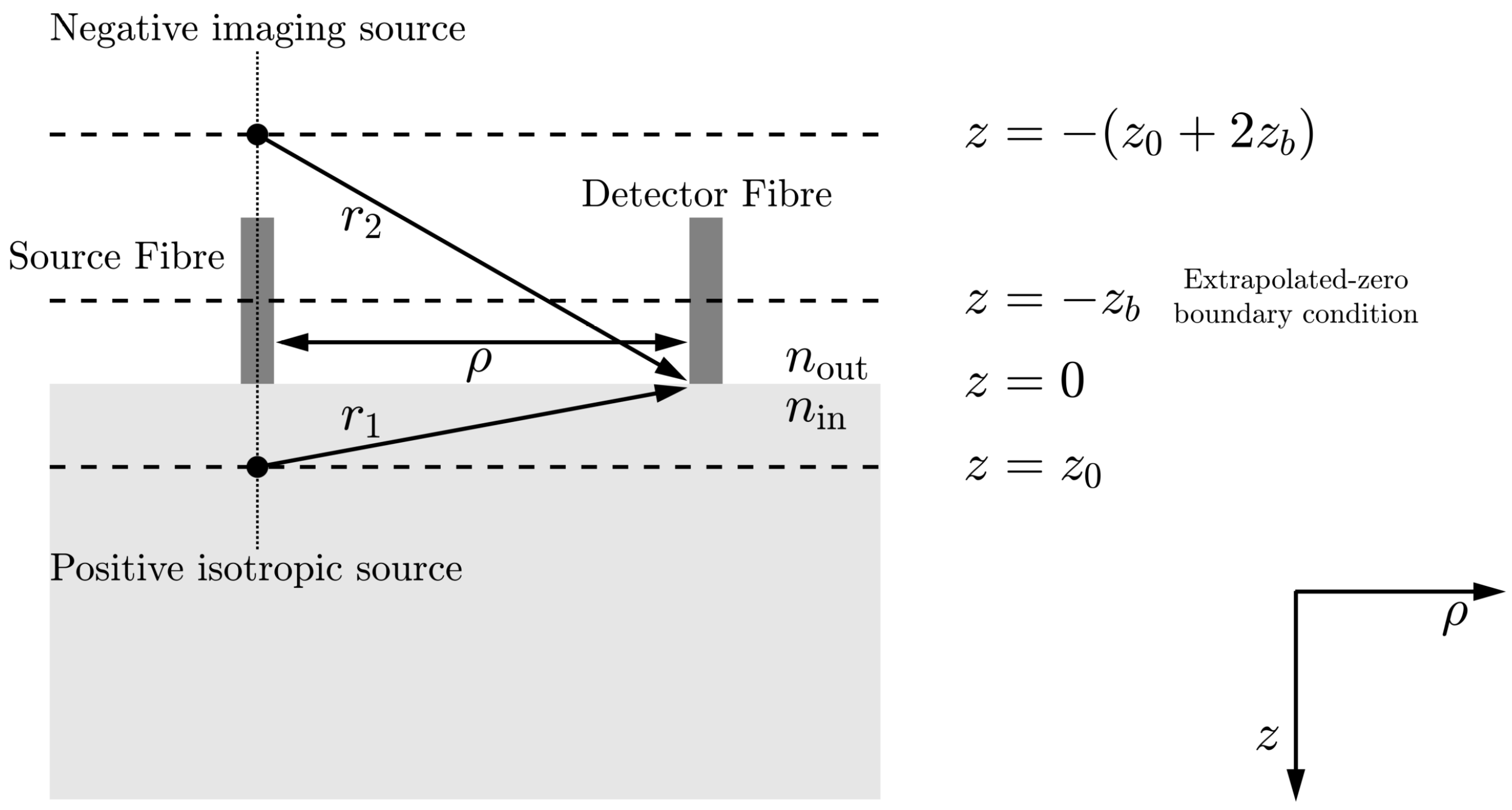
4. Diffuse Correlation Spectroscopy
- is the optical source intensity;
- D is the optical diffusion coefficient, , where is the absorption coefficient and is the reduced scattering coefficient;
- is the decay constant, where is the wavenumber of the incident light field, and n is the refractive index of tissue;
- is the depth into the medium at which the collimated source is approximated as a positive isotropic source;
- is the distance between the optical source and detector;
- is the effective reflection coefficient and accounts for the reflective index mismatch between air () and tissue (), where /;
- , is the position at which there should be a signal size of zero to fulfil the extrapolated boundary condition [49];
- is the distance between the detector and an approximated positive isotropic imaging source;
- is the distance between the detector and an approximated negative isotropic imaging source located at position .
4.1. Key Limitations
4.2. Multispeckle Approaches
4.3. Long-Wavelength Approaches
4.4. Interferometric Approaches
4.5. Depth Discrimination Techniques
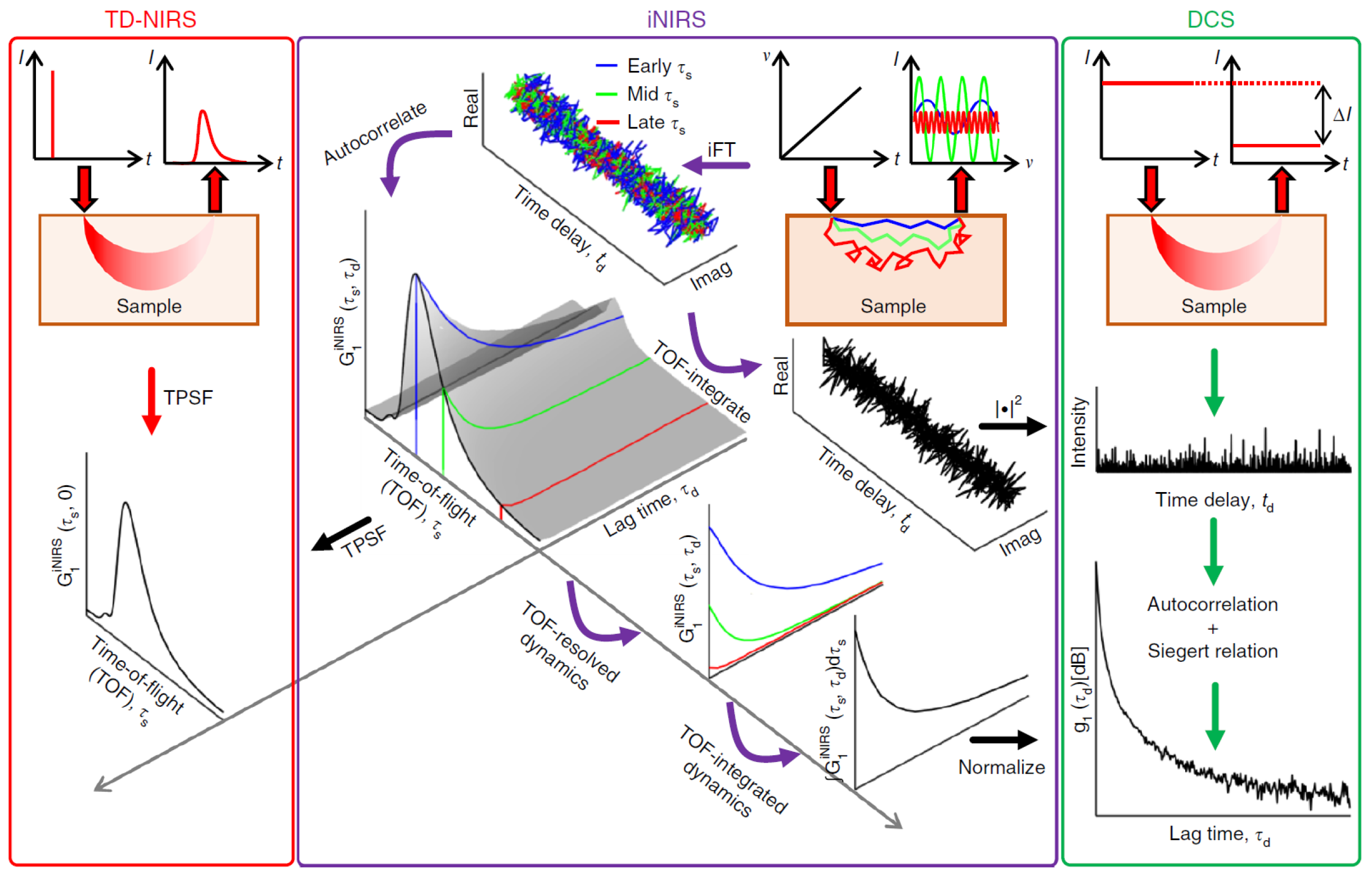
4.6. Time-Domain Approaches
4.7. Other Approaches
5. Interferometric Near-Infrared Spectroscopy
6. Summary and Outlook
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | two-dimensional |
| 3D | three-dimensional |
| ABP | arterial blood pressure |
| AOM-DCS | acousto-optic modulated diffuse correlation spectroscopy |
| AOT | acousto-optic tomography |
| APD | avalanche photodiode |
| ARF | acoustic radiation force |
| ASL-MRI | arterial spin labelling magnetic resonance imaging |
| BF | blood flow |
| BFI | blood flow index |
| CA | cerebral autoregulation |
| CBF | cerebral blood flow |
| CBV | cerebral blood volume |
| CMOS | complementary metal-oxide-semiconductor |
| CMRO2 | cerebral metabolic rate of oxygen extraction |
| CPP | cerebral perfusion pressure |
| CSF | cerebrospinal fluid |
| CT | computed tomography |
| CVR | cerebrovascular resistance |
| CW NIRS | continuous wave parallel interferometric near infrared spectroscopy |
| CW-DCS | continuous wave diffuse correlation spectroscopy |
| DCS | diffuse correlation spectroscopy |
| DCT | diffuse correlation tomography |
| DP | diastolic pressure |
| DWS | diffusing wave spectroscopy |
| FD-DCS | Fourier domain diffuse correlation spectroscopy |
| FD-NIRS | frequency-domain near-infrared spectroscopy |
| FPGA | field-programmable gate array |
| ICP | intracranial pressure |
| iDCS | interferometric diffuse correlation spectroscopy |
| iDWS | interferometric diffusing wave spectroscopy |
| InGaAs | indium gallium arsenide |
| iNIRS | interferometric near-infrared spectroscopy |
| InP | indium phosphide |
| IRF | instrument response function |
| iSVS | interferometric speckle visibility spectroscopy |
| LW-DCS | long wavelength diffuse correlation spectroscopy |
| LW-iDCS | long wavelength interferometric diffuse correlation spectroscopy |
| MAP | mean arterial pressure |
| MiDWS | multi-exposure interferometric diffusing wave spectroscopy |
| MRI | magnetic resonance imaging |
| NIO | number of independent observables |
| NIR | near-infrared |
| NIRS | near-infrared spectroscopy |
| PDE | photon detection efficiency |
| PMT | photon multiplying tube |
| PSD | power spectral density |
| RBC | red blood cell |
| SAP | systemic arterial pressure |
| SDS | source-detector separation |
| SNR | signal-to-noise ratio |
| SNSPD | superconducting nanowire single-photon detection |
| SP | systolic pressure |
| SPAD | single photon avalanche detector |
| TD-DCS | time-domain diffuse correlation spectroscopy |
| TD-NIRS | time-domain near-infrared spectroscopy |
| TOF | time of flight |
| TPSF | temporal point spread function |
| UTL | ultrasound tagging of light |
References
- Wang, D.; Parthasarathy, A.B.; Baker, W.B.; Gannon, K.; Kavuri, V.; Ko, T.; Schenkel, S.; Li, Z.; Li, Z.; Mullen, M.T.; et al. Fast blood flow monitoring in deep tissues with real-time software correlators. Biomed. Opt. Express 2016, 7, 776–797. [Google Scholar] [CrossRef]
- Selb, J.; Wu, K.C.; Sutin, J.; Lin, P.Y.I.; Farzam, P.; Bechek, S.; Shenoy, A.; Patel, A.B.; Boas, D.A.; Franceschini, M.A.; et al. Prolonged monitoring of cerebral blood flow and autoregulation with diffuse correlation spectroscopy in neurocritical care patients. Neurophotonics 2018, 5, 045005. [Google Scholar] [CrossRef] [PubMed]
- Sie, E.J.; Chen, H.; Saung, E.F.; Catoen, R.; Tiecke, T.; Chevillet, M.A.; Marsili, F. High-sensitivity multispeckle diffuse correlation spectroscopy. Neurophotonics 2020, 7, 035010. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Sie, E.J.; Naufel, S.; Boas, D.A.; Marsili, F. Measuring neuronal activity with diffuse correlation spectroscopy: A theoretical investigation. Neurophotonics 2021, 8, 035004. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhao, M.; Kholiqov, O.; Srinivasan, V. Multi-exposure interferometric diffusing wave spectroscopy. Opt. Lett. 2021, 46, 4498–4501. [Google Scholar] [CrossRef]
- Zhou, W.; Kholiqov, O.; Zhu, J.; Zhao, M.; Zimmermann, L.L.; Martin, R.M.; Lyeth, B.G.; Srinivasan, V.J. Functional interferometric diffusing wave spectroscopy of the human brain. Sci. Adv. 2021, 7, eabe0150. [Google Scholar] [CrossRef]
- Boas, D.A.; Yodh, A.G. Spatially varying dynamical properties of turbid media probed with diffusing temporal light correlation. J. Opt. Soc. Am. A 1997, 14, 192–215. [Google Scholar] [CrossRef]
- Carp, S.A.; Robinson, M.B.; Franceschini, M.A. Diffuse correlation spectroscopy: Current status and future outlook. Neurophotonics 2023, 10, 013509. [Google Scholar] [CrossRef]
- Murali, K.; Varma, H.M. Multi-speckle diffuse correlation spectroscopy to measure cerebral blood flow. Biomed. Opt. Express 2020, 11, 6699–6709. [Google Scholar] [CrossRef]
- Liu, W.; Qian, R.; Xu, S.; Chandra Konda, P.; Jönsson, J.; Harfouche, M.; Borycki, D.; Cooke, C.; Berrocal, E.; Dai, Q.; et al. Fast and sensitive diffuse correlation spectroscopy with highly parallelized single photon detection. APL Photonics 2021, 6, 026106. [Google Scholar] [CrossRef]
- Wayne, M.A.; Sie, E.J.; Ulku, A.C.; Mos, P.; Ardelean, A.; Marsili, F.; Bruschini, C.; Charbon, E. Massively parallel, real-time multispeckle diffuse correlation spectroscopy using a 500 × 500 SPAD camera. Biomed. Opt. Express 2023, 14, 703–713. [Google Scholar] [CrossRef]
- Sutin, J.; Zimmerman, B.; Tyulmankov, D.; Tamborini, D.; Wu, K.C.; Selb, J.; Gulinatti, A.; Rech, I.; Tosi, A.; Boas, D.A.; et al. Time-domain diffuse correlation spectroscopy. Optica 2016, 3, 1006–1013. [Google Scholar] [CrossRef]
- Carp, S.A.; Tamborini, D.; Mazumder, D.; Wu, K.C.T.; Robinson, M.R.; Stephens, K.A.; Shatrovoy, O.; Lue, N.; Ozana, N.; Blackwell, M.H.; et al. Diffuse correlation spectroscopy measurements of blood flow using 1064 nm light. J. Biomed. Opt. 2020, 25, 097003. [Google Scholar] [CrossRef]
- Ozana, N.; Zavriyev, A.I.; Mazumder, D.; Robinson, M.; Kaya, K.; Blackwell, M.; Carp, S.A.; Franceschini, M.A. Superconducting nanowire single-photon sensing of cerebral blood flow. Neurophotonics 2021, 8, 035006. [Google Scholar] [CrossRef]
- Robinson, M.B.; Renna, M.; Ozana, N.; Martin, A.N.; Otic, N.; Carp, S.A.; Franceschini, M.A. Portable, high speed blood flow measurements enabled by long wavelength, interferometric diffuse correlation spectroscopy (LW-iDCS). Sci. Rep. 2023, 13, 8803. [Google Scholar] [CrossRef]
- Zhou, W.; Kholiqov, O.; Chong, S.P.; Srinivasan, V.J. Highly parallel, interferometric diffusing wave spectroscopy for monitoring cerebral blood flow dynamics. Optica 2018, 5, 518–527. [Google Scholar] [CrossRef]
- Robinson, M.B.; Boas, D.A.; Sakadzic, S.; Franceschini, M.A.; Carp, S.A. Interferometric diffuse correlation spectroscopy improves measurements at long source–detector separation and low photon count rate. J. Biomed. Opt. 2020, 25, 097004. [Google Scholar] [CrossRef]
- James, E.; Powell, S. Diffuse correlation spectroscopy in the Fourier domain with holographic camera-based detection. In Proceedings of the Dynamics and Fluctuations in Biomedical Photonics XVII, San Francisco, CA, USA, 21 February 2020; Tuchin, V.V., Leahy, M.J., Wang, R.K., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2020; Volume 11239, p. 112390H. [Google Scholar] [CrossRef]
- Xu, J.; Jahromi, A.K.; Brake, J.; Robinson, J.E.; Yang, C. Interferometric speckle visibility spectroscopy (ISVS) for human cerebral blood flow monitoring. APL Photonics 2020, 5, 126102. [Google Scholar] [CrossRef]
- Robinson, M.B.; Carp, S.A.; Peruch, A.; Boas, D.A.; Franceschini, M.A.; Sakadžić, S. Characterization of continuous wave ultrasound for acousto-optic modulated diffuse correlation spectroscopy (AOM-DCS). Biomed. Opt. Express 2020, 11, 3071–3090. [Google Scholar] [CrossRef]
- Yu, G.; Durduran, T.; Zhou, C.; Cheng, R.; Yodh, A.G. Handbook of Biomedical Optics, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Tsalach, A.; Schiffer, Z.; Ratner, E.; Breskin, I.; Zeitak, R.; Shechter, R.; Balberg, M. Depth selective acousto-optic flow measurement. Biomed. Opt. Express 2015, 6, 4871–4886. [Google Scholar] [CrossRef]
- Lang, E.W.; Mudaliar, Y.; Lagopoulos, J.; Dorsch, N.; Yam, A.; Griffith, J.; Mulvey, J. A Review of Cerebral Autoregulation: Assessment and Measurements. Aust. Anaesth. 2005, 2005, 161–172. [Google Scholar]
- Durduran, T.; Yodh, A.G. Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement. NeuroImage 2014, 85, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Buckley, E.M.; Parthasarathy, A.B.; Grant, P.E.; Yodh, A.G.; Franceschini, M.A. Diffuse correlation spectroscopy for measurement of cerebral blood flow: Future prospects. Neurophotonics 2014, 1, 011009. [Google Scholar] [CrossRef]
- Fantini, S.; Sassaroli, A.; Tgavalekos, K.T.; Kornbluth, J. Cerebral blood flow and autoregulation: Current measurement techniques and prospects for noninvasive optical methods. Neurophotonics 2016, 3, 031411. [Google Scholar] [CrossRef]
- Smith, M. Monitoring intracranial pressure in traumatic brain injury. Anesth. Analg. 2008, 106, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Steiner, L.A.; Andrews, P.J. Monitoring the injured brain: ICP and CBF. Br. J. Anaesth. 2006, 97, 26–38. [Google Scholar] [CrossRef]
- Durduran, T.; Choe, R.; Baker, W.B.; Yodh, A.G. Diffuse optics for tissue monitoring and tomography. Rep. Prog. Phys. 2010, 73, 076701. [Google Scholar] [CrossRef]
- Tavakoli, S.; Peitz, G.; Ares, W.; Hafeez, S.; Grandhi, R. Complications of invasive intracranial pressure monitoring devices in neurocritical care. Neurosurg. Focus 2017, 43, E6. [Google Scholar] [CrossRef]
- Müller, S.J.; Henkes, E.; Gounis, M.J.; Felber, S.; Ganslandt, O.; Henkes, H. Non-Invasive Intracranial Pressure Monitoring. J. Clin. Med. 2023, 12, 2209. [Google Scholar] [CrossRef]
- James, E. Holographic Fourier Domain Diffuse Correlation Spectroscopy. Ph.D. Thesis, University College London, London, UK, 2022. [Google Scholar]
- DeSantis, C.E.; Bray, F.; Ferlay, J.; Lortet-Tieulent, J.; Anderson, B.O.; Jemal, A. International Variation in Female Breast Cancer Incidence and Mortality Rates. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1495–1506. [Google Scholar] [CrossRef]
- Durduran, T.; Choe, R.; Yu, G.; Zhou, C.; Tchou, J.C.; Czerniecki, B.J.; Yodh, A.G. Diffuse optical measurement of blood flow in breast tumors. Opt. Lett. 2005, 30, 2915–2917. [Google Scholar] [CrossRef]
- Zhou, C.; Choe, R.; Shah, N.; Durduran, T.; Yu, G.; Durkin, A.; Hsiang, D.; Mehta, R.; Butler, J.; Cerussi, A.; et al. Diffuse optical monitoring of blood flow and oxygenation in human breast cancer during early stages of neoadjuvant chemotherapy. J. Biomed. Opt. 2007, 12, 051903. [Google Scholar] [CrossRef] [PubMed]
- Sunar, U.; Quon, H.; Durduran, T.; Zhang, J.; Du, J.; Zhou, C.; Yu, G.; Choe, R.; Kilger, A.; Lustig, R.; et al. Noninvasive diffuse optical measurement of blood flow and blood oxygenation for monitoring radiation therapy in patients with head and neck tumors: A pilot study. J. Biomed. Opt. 2006, 11, 064021. [Google Scholar] [CrossRef]
- Yu, G.; Durduran, T.; Zhou, C.; Zhu, T.C.; Finlay, J.C.; Busch, T.M.; Malkowicz, S.B.; Hahn, S.M.; Yodh, A.G. Real-time In Situ Monitoring of Human Prostate Photodynamic Therapy with Diffuse Light. Photochem. Photobiol. 2006, 82, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Durduran, T.; Zhou, C.; Wang, H.W.; Putt, M.E.; Saunders, H.M.; Sehgal, C.M.; Glatstein, E.; Yodh, A.G.; Busch, T.M. Noninvasive Monitoring of Murine Tumor Blood Flow During and After Photodynamic Therapy Provides Early Assessment of Therapeutic Efficacy. Clin. Cancer Res. 2005, 11, 3543–3552. [Google Scholar] [CrossRef]
- Cheatle, T.R.; Potter, L.A.; Cope, M.; Delpy, D.T.; Smith, P.D.C.; Scurr, J.H. Near-infrared spectroscopy in peripheral vascular disease. Br. J. Surg. 1991, 78, 405–408. [Google Scholar] [CrossRef]
- Gunther, J.; Andersson-Engels, S. Review of current methods of acousto-optical tomography for biomedical applications. Front. Optoelectron. 2017, 10, 211–238. [Google Scholar] [CrossRef]
- Parthasarathy, A.B.; Tom, W.J.; Gopal, A.; Zhang, X.; Dunn, A.K. Robust flow measurement with multi-exposure speckle imaging. Opt. Express 2008, 16, 1975–1989. [Google Scholar] [CrossRef]
- Xu, J.; Jahromi, A.K.; Yang, C. Diffusing wave spectroscopy: A unified treatment on temporal sampling and speckle ensemble methods. APL Photonics 2021, 6, 016105. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, W.; Srinivasan, V.J. Approaches to Improve Brain Specificity and Accuracy with Interferometric Diffusing Wave Spectroscopy. In Proceedings of the Biophotonics Congress, Fort Lauderdale, FL, USA, 7–10 April 2021; Optica Publishing Group: Washington, DC, USA, 2021; p. 1. [Google Scholar] [CrossRef]
- Lemieux, P.A.; Durian, D.J. Investigating non-Gaussian scattering processes by using nth-order intensity correlation functions. J. Opt. Soc. Am. A 1999, 16, 1651–1664. [Google Scholar] [CrossRef]
- Fercher, A.F.; Briers, J.D. Flow Visualization By Means Of Single-Exposure Speckle Photography. Opt. Commun. 1981, 37, 326–330. [Google Scholar] [CrossRef]
- Dong, L.; He, L.; Lin, Y.; Shang, Y.; Yu, G. Simultaneously Extracting Multiple Parameters via Fitting One Single Autocorrelation Function Curve in Diffuse Correlation Spectroscopy. IEEE Trans. Biomed. Eng. 2013, 60, 361–368. [Google Scholar] [CrossRef]
- Boas, D.A.; Sakadžic, S.; Selb, J.; Farzam, P.; Franceschini, M.A.; Carp, S.A. Establishing the diffuse correlation spectroscopy signal relationship with blood flow. Neurophotonics 2016, 3, 031412. [Google Scholar] [CrossRef]
- du Le, V.N.; Srinivasan, V.J. Beyond diffuse correlations: Deciphering random flow in time-of-flight resolved light dynamics. Opt. Express 2020, 28, 11191–11214. [Google Scholar] [CrossRef] [PubMed]
- Haskell, R.C.; Svaasand, L.O.; Tsay, T.T.; Feng, T.C.; Tromberg, B.J.; McAdams, M.S.; Tromberg, B.J. Boundary conditions for the diffusion equation in radiative transfer. J. Opt. Soc. Am. A 1994, 11, 2727–2741. [Google Scholar] [CrossRef]
- James, E.; Powell, S. Fourier domain diffuse correlation spectroscopy with heterodyne holographic detection. Biomed. Opt. Express 2020, 11, 6755–6779. [Google Scholar] [CrossRef]
- Elson, D.S.; Li, R.; Dunsby, C.; Eckersley, R.; Tang, M.X. Ultrasound-mediated optical tomography: A review of current methods. Interface Focus 2011, 1, 632–648. [Google Scholar] [CrossRef][Green Version]
- Rudin, M. Molecular Imaging: Basic Principles and Applications in Biomedical Research, 2nd ed.; Imperial College Press: London, UK, 2013. [Google Scholar] [CrossRef]
- Dietsche, G.; Ninck, M.; Ortolf, C.; Li, J.; Jaillon, F.; Gisler, T. Fiber-based multispeckle detection for time-resolved diffusing-wave spectroscopy: Characterization and application to blood flow detection in deep tissue. Appl. Opt. 2007, 46, 8506–8514. [Google Scholar] [CrossRef]
- He, L.; Lin, Y.; Shang, Y.; Shelton, B.J.; Yu, G. Using optical fibers with different modes to improve the signal-to-noise ratio of diffuse correlation spectroscopy flow-oximeter measurements. J. Biomed. Opt. 2013, 18, 037001. [Google Scholar] [CrossRef]
- BS EN 60825-1:2014; Safety of Laser Products. The British Standards Institution: London, UK, 2017.
- Gisler, T.; Rüger, H.; Egelhaaf, S.U.; Tschumi, J.; Schurtenberger, P.; Rička, J. Mode-selective dynamic light scattering: Theory versus experimental realization. Appl. Opt. 2009, 34, 3546–3553. [Google Scholar] [CrossRef]
- Irwin, D.; Dong, L.; Shang, Y.; Cheng, R.; Kudrimoti, M.; Stevens, S.D.; Yu, G. Influences of tissue absorption and scattering on diffuse correlation spectroscopy blood flow measurements. Biomed. Opt. Express 2011, 2, 1969–1985. [Google Scholar] [CrossRef]
- Zhou, C.; Yu, G.; Furuya, D.; Greenberg, J.H.; Yodh, A.G.; Durduran, T. Diffuse optical correlation tomography of cerebral blood flow during cortical spreading depression in rat brain. Opt. Express 2006, 14, 1125–1144. [Google Scholar] [CrossRef]
- Carp, S.A. Novel Approaches for Increased Sensitivity to Cerebral Blood Flow Using Diffuse Correlation Spectroscopy. In Proceedings of the Biophotonics Congress: Biomedical Optics 2020 (Translational, Microscopy, OCT, OTS, BRAIN), Washington, DC, USA, 20–23 April 2020; Optica Publishing Group: Washington, DC, USA, 2020; p. BTh3C.1. [Google Scholar] [CrossRef]
- Johansson, J.D.; Portaluppi, D.; Buttafava, M.; Villa, F. A multipixel diffuse correlation spectroscopy system based on a single photon avalanche diode array. J. Biophotonics 2019, 12, e201900091. [Google Scholar] [CrossRef]
- Della Rocca, F.M.; Sie, E.J.; Catoen, R.; Marsili, F.; Henderson, R.K. Field programmable gate array compression for large array multispeckle diffuse correlation spectroscopy. J. Biomed. Opt. 2023, 28, 057001. [Google Scholar] [CrossRef]
- Renna, M.; Richardson, J.M.; Jordy, G.; Cheng, T.; Ringdahl, E.; Robinson, M.; Ozana, N.; Lue, N.; Aull, B.; Carp, S.; et al. A novel 32 × 32 InP/InGaAs SPAD array for multi-channel time-gated diffuse correlation spectroscopy. In Proceedings of the Advanced Photon Counting Techniques XVI, Orlando, FL, USA, 3 April–13 June 2022; Itzler, M.A., Bienfang, J.C., McIntosh, K.A., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2022; Volume PC12089, p. PC120890E. [Google Scholar] [CrossRef]
- Boas, D.A.; Dunn, A.K. Laser speckle contrast imaging in biomedical optics. J. Biomed. Opt. 2010, 15, 011109. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Du, Y.; Singh, G.; Ho, J.H.; Zhang, S.; Ebrahim Attia, A.B.; Li, X.; Olivo, M.C. Fast pulsatile blood flow measurement in deep tissue through a multimode detection fiber. J. Biomed. Opt. 2020, 25, 055003. [Google Scholar] [CrossRef]
- Murali, K.; Nandakumaran, A.K.; Durduran, T.; Varma, H.M. Recovery of the diffuse correlation spectroscopy data-type from speckle contrast measurements: Towards low-cost, deep-tissue blood flow measurements. Biomed. Opt. Express 2019, 10, 5395–5413. [Google Scholar] [CrossRef]
- Murali, K.; Nandakumaran, A.K.; Varma, H.M. On the equivalence of speckle contrast-based and diffuse correlation spectroscopy methods in measuring in vivo blood flow. Opt. Lett. 2020, 45, 3993–3996. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, M.; Berger, R.; Jordy, G.; Frechette, J.; Aull, B.; Duerr, E.K.; Robinson, M.; Tamborini, D.; Carp, S.A.; Franceschini, M.A. Novel detector solutions for diffuse correlation spectroscopy at 1064 nm (Conference Presentation). In Proceedings of the Dynamics and Fluctuations in Biomedical Photonics XVII, San Francisco, CA, USA, 1–6 February 2020; Tuchin, V.V., Leahy, M.J., Wang, R.K., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2020; Volume 11239, p. 112390E. [Google Scholar] [CrossRef]
- Robinson, M.B.; Renna, M.; Ozana, N.N.; Peruch, A.; Sakadžić, S.; Blackwell, M.L.; Richardson, J.M.; Aull, B.F.; Carp, S.A.; Franceschini, M.A. Diffuse Correlation Spectroscopy Beyond the Water Peak Enabled by Cross-Correlation of the Signals From InGaAs/InP Single Photon Detectors. IEEE Trans. Biomed. Eng. 2022, 69, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Ozana, N.; Lue, N.; Renna, M.; Robinson, M.B.; Martin, A.; Zavriyev, A.I.; Carr, B.; Mazumder, D.; Blackwell, M.H.; Franceschini, M.A.; et al. Functional Time Domain Diffuse Correlation Spectroscopy. Front. Neurosci. 2022, 16, 1123. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, W.; Aparanji, S.; Mazumder, D.; Srinivasan, V.J. Interferometric diffusing wave spectroscopy imaging with an electronically variable time-of-flight filter. Optica 2023, 10, 42–52. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, W.; Aparanji, S.; Mazumder, D.; Srinivasan, V. Interferometric diffusing wave spectroscopy imaging with an electronically variable time-of-flight filter. In Proceedings of the Optical Tomography and Spectroscopy of Tissue XV, San Francisco, CA, USA, 28 January–3 February 2023; Fantini, S., Taroni, P., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2023; Volume PC12376, p. PC123760F. [Google Scholar] [CrossRef]
- Schnars, U.; Jüptner, W.P.O. Digital recording and numerical reconstruction of holograms. Meas. Sci. Technol. 2002, 13, R85–R101. [Google Scholar] [CrossRef]
- Verpillat, F.; Joud, F.; Atlan, M.; Gross, M. Digital Holography at Shot Noise Level. J. Disp. Technol. 2010, 6, 455–464. [Google Scholar] [CrossRef]
- Gross, M.; Atlan, M.; Absil, E. Noise and aliases in off-axis and phase-shifting holography. Appl. Opt. 2008, 47, 1757–1766. [Google Scholar] [CrossRef]
- Gunther, J.; Walther, A.; Rippe, L.; Kröll, S.; Andersson-Engels, S. Deep tissue imaging with acousto-optical tomography and spectral hole burning with slow light effect: A theoretical study. J. Biomed. Opt. 2018, 23, 071209. [Google Scholar] [CrossRef]
- Gross, M. Selection of the tagged photons by off axis heterodyne holography in ultrasound-modulated optical tomography. Appl. Opt. 2017, 56, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Goy, P.; Al-Koussa, M. Shot-noise detection of ultrasound-tagged photons in ultrasound-modulated optical imaging. Opt. Lett. 2003, 28, 2482–2484. [Google Scholar] [CrossRef]
- Hussain, A.; Steenbergen, W.; Vellekoop, I.M. Imaging blood flow inside highly scattering media using ultrasound modulated optical tomography. J. Biophotonics 2017, 11, e201700013. [Google Scholar] [CrossRef] [PubMed]
- Magnain, C.; Castel, A.; Boucneau, T.; Simonutti, M.; Ferezou, I.; Rancillac, A.; Vitalis, T.; Sahel, J.A.; Paques, M.; Atlan, M. Holographic laser Doppler imaging of microvascular blood flow. J. Opt. Soc. Am. A 2014, 31, 2723–2735. [Google Scholar] [CrossRef]
- Brown, J.C. Optical correlations and spectra. Am. J. Phys. 1983, 51, 1008–1011. [Google Scholar] [CrossRef]
- Atlan, M.; Desbiolles, P.; Gross, M.; Coppey-Moisan, M. Parallel heterodyne detection of dynamic light-scattering spectra from gold nanoparticles diffusing in viscous fluids. Opt. Lett. 2010, 35, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J. Statistical Optics, 2nd ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- James, E.; Powell, S.; Munro, P. Performance optimisation of a holographic Fourier domain diffuse correlation spectroscopy instrument. Biomed. Opt. Express 2022, 13, 3836–3853. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, M.; Srinivasan, V.J. Interferometric diffuse optics: Recent advances and future outlook. Neurophotonics 2022, 10, 013502. [Google Scholar] [CrossRef]
- Verdecchia, K.; Diop, M.; Lee, A.; Morrison, L.B.; Lee, T.Y.; St. Lawrence, K. Assessment of a multi-layered diffuse correlation spectroscopy method for monitoring cerebral blood flow in adults. Biomed. Opt. Express 2016, 7, 3659–3674. [Google Scholar] [CrossRef]
- Forti, R.M.; Martins, G.G.; Baker, W.B.; Mesquita, R.C. Optimizing a two-layer method for hybrid diffuse correlation spectroscopy and frequency-domain diffuse optical spectroscopy cerebral measurements in adults. Neurophotonics 2023, 10, 025008. [Google Scholar] [CrossRef]
- Zhao, H.; Buckley, E.M. Influence of oversimplifying the head anatomy on cerebral blood flow measurements with diffuse correlation spectroscopy. Neurophotonics 2023, 10, 015010. [Google Scholar] [CrossRef]
- Paul, R.; Murali, K.; Varma, H.M. High-density diffuse correlation tomography with enhanced depth localization and minimal surface artefacts. Biomed. Opt. Express 2022, 13, 6081–6099. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.B.; Parthasarathy, A.B.; Ko, T.S.; Busch, D.R.; Abramson, K.; Tzeng, S.Y.; Mesquita, R.C.; Durduran, T.; Greenberg, J.H.; Kung, D.K.; et al. Pressure modulation algorithm to separate cerebral hemodynamic signals from extracerebral artifacts. Neurophotonics 2015, 2, 035004. [Google Scholar] [CrossRef]
- Mazumder, D.; Wu, M.M.; Ozana, N.; Tamborini, D.; Franceschini, M.A.; Carp, S.A. Optimization of time domain diffuse correlation spectroscopy parameters for measuring brain blood flow. Neurophotonics 2021, 8, 035005. [Google Scholar] [CrossRef]
- Samaei, S.; Colombo, L.; Borycki, D.; Pagliazzi, M.; Durduran, T.; Sawosz, P.; Wojtkiewicz, S.; Contini, D.; Torricelli, A.; Pifferi, A.; et al. Performance assessment of laser sources for time-domain diffuse correlation spectroscopy. Biomed. Opt. Express 2021, 12, 5351–5367. [Google Scholar] [CrossRef]
- Poon, C.S.; Langri, D.S.; Rinehart, B.; Rambo, T.M.; Miller, A.J.; Foreman, B.; Sunar, U. First-in-clinical application of a time-gated diffuse correlation spectroscopy system at 1064 nm using superconducting nanowire single photon detectors in a neuro intensive care unit. Biomed. Opt. Express 2022, 13, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Renna, M.; Starkweather, Z.; Robinson, M.; Ozana, N.; Martin, A.; Carp, S.; Franceschini, M.A. Multistate time-multiplexed system for functional time-domain diffuse correlation spectroscopy with SNSPDs. In Proceedings of the Optical Tomography and Spectroscopy of Tissue XV, San Francisco, CA, USA, 28 January–3 February 2023; Fantini, S., Taroni, P., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2023; Volume PC12376, p. PC123760D. [Google Scholar] [CrossRef]
- Richardson, J.M.; Cheng, T.Y.; Renna, M.; Jordy, G.; Stamplis, M.S.; Vacca, A.M.M.; Bradshaw, M.; Ringdahl, E.; Aull, B.F.; Blackwell, M.H.; et al. A 256-channel SPAD detector for time-gated fNIRS and DCS. In Proceedings of the Advanced Photon Counting Techniques XVII, Orlando, FL, USA, 30 April–5 May 2023; Itzler, M.A., Bienfang, J.C., McIntosh, K.A., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 2023; Volume PC12512, p. PC1251208. [Google Scholar] [CrossRef]
- Chandran, R.S.; Devaraj, G.; Kanhirodan, R.; Roy, D.; Vasu, R.M. Detection and estimation of liquid flow through a pipe in a tissue-like object with ultrasound-assisted diffuse correlation spectroscopy. J. Opt. Soc. Am. A 2015, 32, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Resink, S.G.; Boccara, A.C.; Steenbergen, W. State-of-the art of acousto-optic sensing and imaging of turbid media. J. Biomed. Opt. 2012, 17, 040901. [Google Scholar] [CrossRef]
- Ling, H.; Gui, Z.; Hao, H.; Shang, Y. Enhancement of diffuse correlation spectroscopy tissue blood flow measurement by acoustic radiation force. Biomed. Opt. Express 2020, 11, 301–315. [Google Scholar] [CrossRef]
- Biswas, A.; Moka, S.; Muller, A.; Parthasarathy, A.B. Fast diffuse correlation spectroscopy with a low-cost, fiber-less embedded diode laser. Biomed. Opt. Express 2021, 12, 6686–6700. [Google Scholar] [CrossRef]
- Safi, A.M.; Moka, S.; Harrah, M.; Cini, S.; Parthasarathy, A.B. Quantitative Measurement of Static and Dynamic Tissue Optical Properties with Continuous Wave Pathlength Resolved Diffuse Correlation Spectroscopy. In Proceedings of the Biophotonics Congress 2021, Washington, DC, USA, 12–16 April 2021; Optica Publishing Group: Washington, DC, USA, 2021; p. BTh1B.6. [Google Scholar] [CrossRef]
- Poon, C.S.; Long, F.; Sunar, U. Deep learning model for ultrafast quantification of blood flow in diffuse correlation spectroscopy. Biomed. Opt. Express 2020, 11, 5557–5564. [Google Scholar] [CrossRef]
- Zhang, P.; Gui, Z.; Guo, G.; Shang, Y. Approaches to denoise the diffuse optical signals for tissue blood flow measurement. Biomed. Opt. Express 2018, 9, 6170–6185. [Google Scholar] [CrossRef]
- Xu, S.; Yang, X.; Konda, P.C.; Horstmeyer, R. Rapid imaging of deep-tissue motion with parallelized diffuse correlation spectroscopy. In Proceedings of the Biophotonics Congress 2021, Washington, DC, USA, 12–16 April 2021; Optica Publishing Group: Washington, DC, USA, 2021; p. BTh1B.3. [Google Scholar] [CrossRef]
- Borycki, D.; Kholiqov, O.; Chong, S.P.; Srinivasan, V.J. Interferometric Near-Infrared Spectroscopy (iNIRS) for determination of optical and dynamical properties of turbid media. Opt. Express 2016, 24, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Kholiqov, O.; Zhou, W.; Zhang, T.; Du Le, V.N.; Srinivasan, V.J. Time-of-flight resolved light field fluctuations reveal deep human tissue physiology. Nat. Commun. 2020, 11, 391. [Google Scholar] [CrossRef]
- Samaei, S.; Nowacka, K.; Gerega, A.; Pastuszak, Ż.; Borycki, D. Continuous-wave parallel interferometric near-infrared spectroscopy (CW πNIRS) with a fast two-dimensional camera. Biomed. Opt. Express 2022, 13, 5753–5774. [Google Scholar] [CrossRef]
- Ayaz, H.; Baker, W.B.; Blaney, G.; Boas, D.A.; Bortfeld, H.; Brady, K.; Brake, J.; Brigadoi, S.; Buckley, E.M.; Carp, S.A.; et al. Optical imaging and spectroscopy for the study of the human brain: Status report. Neurophotonics 2022, 9, S24001. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
James, E.; Munro, P.R.T. Diffuse Correlation Spectroscopy: A Review of Recent Advances in Parallelisation and Depth Discrimination Techniques. Sensors 2023, 23, 9338. https://doi.org/10.3390/s23239338
James E, Munro PRT. Diffuse Correlation Spectroscopy: A Review of Recent Advances in Parallelisation and Depth Discrimination Techniques. Sensors. 2023; 23(23):9338. https://doi.org/10.3390/s23239338
Chicago/Turabian StyleJames, Edward, and Peter R. T. Munro. 2023. "Diffuse Correlation Spectroscopy: A Review of Recent Advances in Parallelisation and Depth Discrimination Techniques" Sensors 23, no. 23: 9338. https://doi.org/10.3390/s23239338
APA StyleJames, E., & Munro, P. R. T. (2023). Diffuse Correlation Spectroscopy: A Review of Recent Advances in Parallelisation and Depth Discrimination Techniques. Sensors, 23(23), 9338. https://doi.org/10.3390/s23239338





