Response Surface Modeling of the Steady-State Impedance Responses of Gas Sensor Arrays Comprising Functionalized Carbon Nanotubes to Detect Ozone and Nitrogen Dioxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanomaterial Synthesis and Deposition
2.1.1. Ozone Sensors
2.1.2. Nitrogen Dioxide Sensors
2.2. Sensor and Electronics Board Design
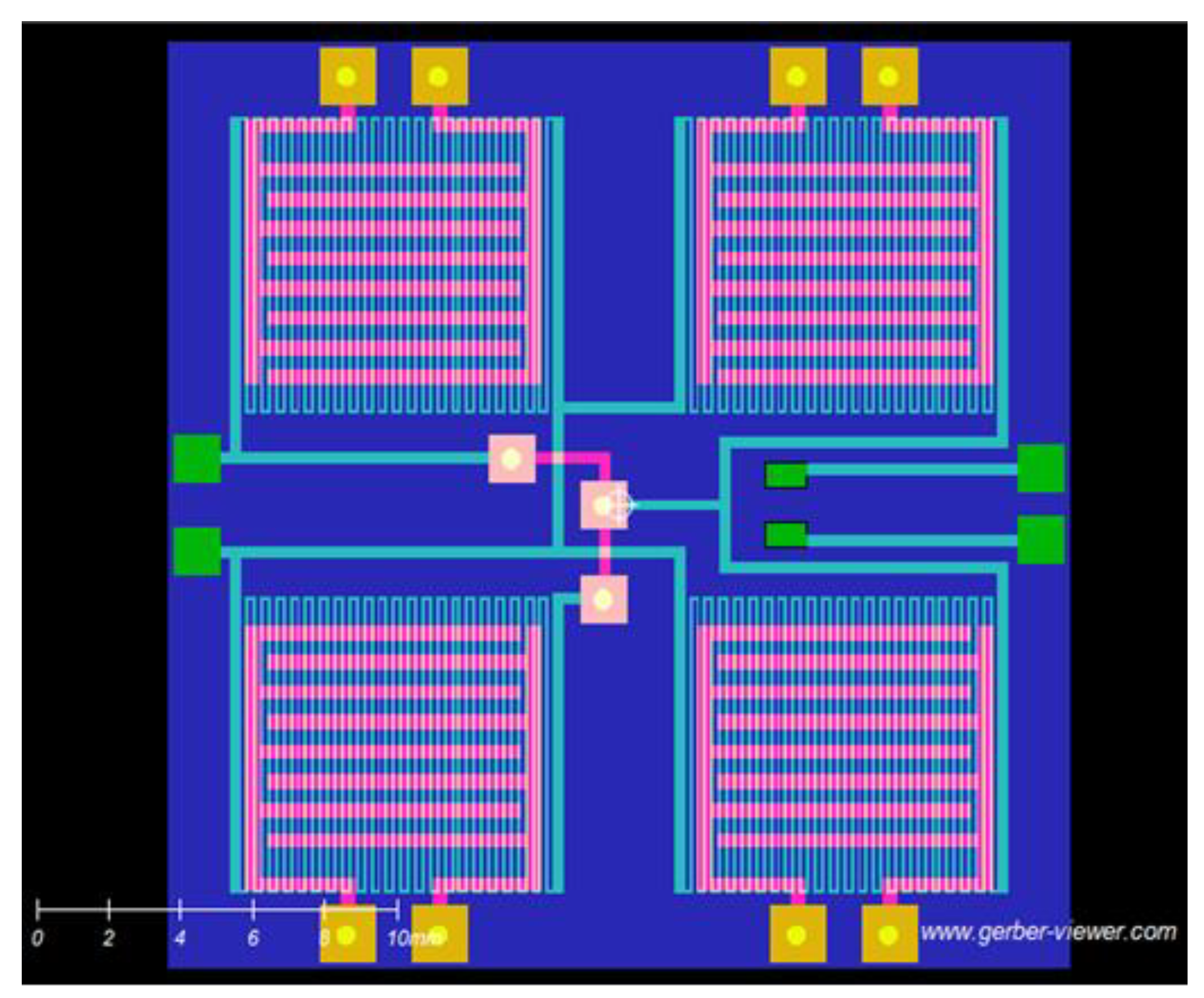
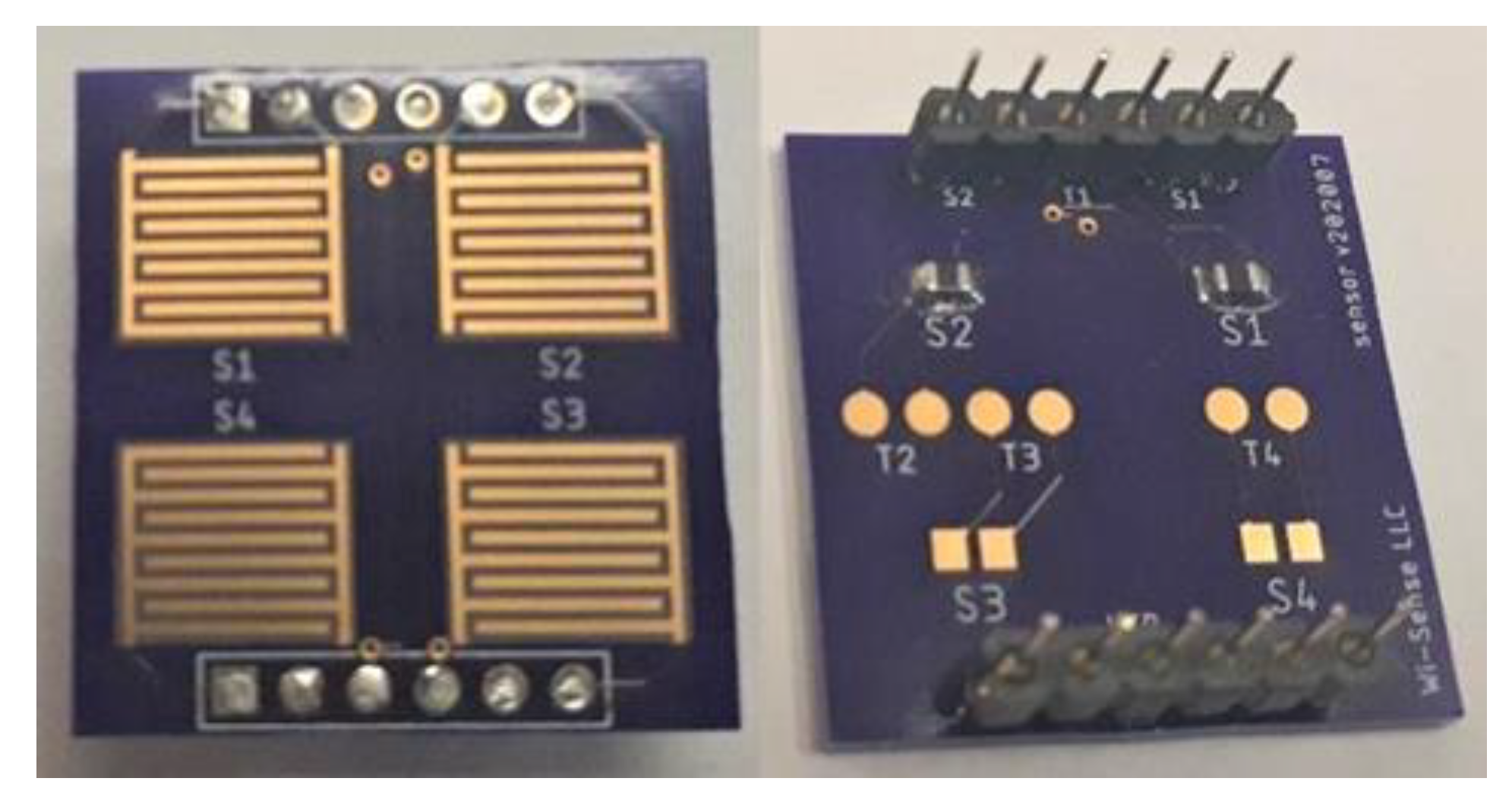
2.2.1. Sensor Array
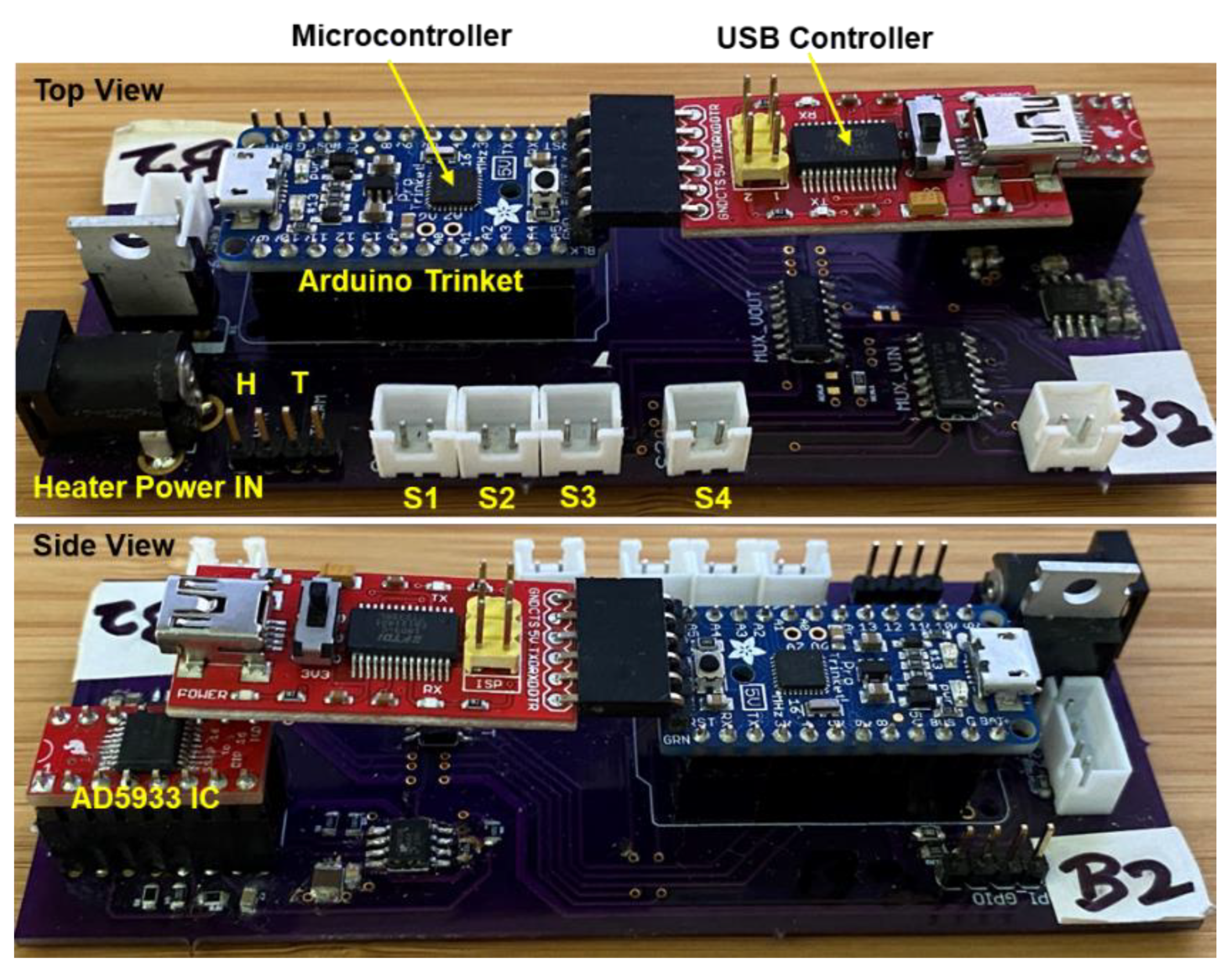
2.2.2. Impedance Measurement Circuit
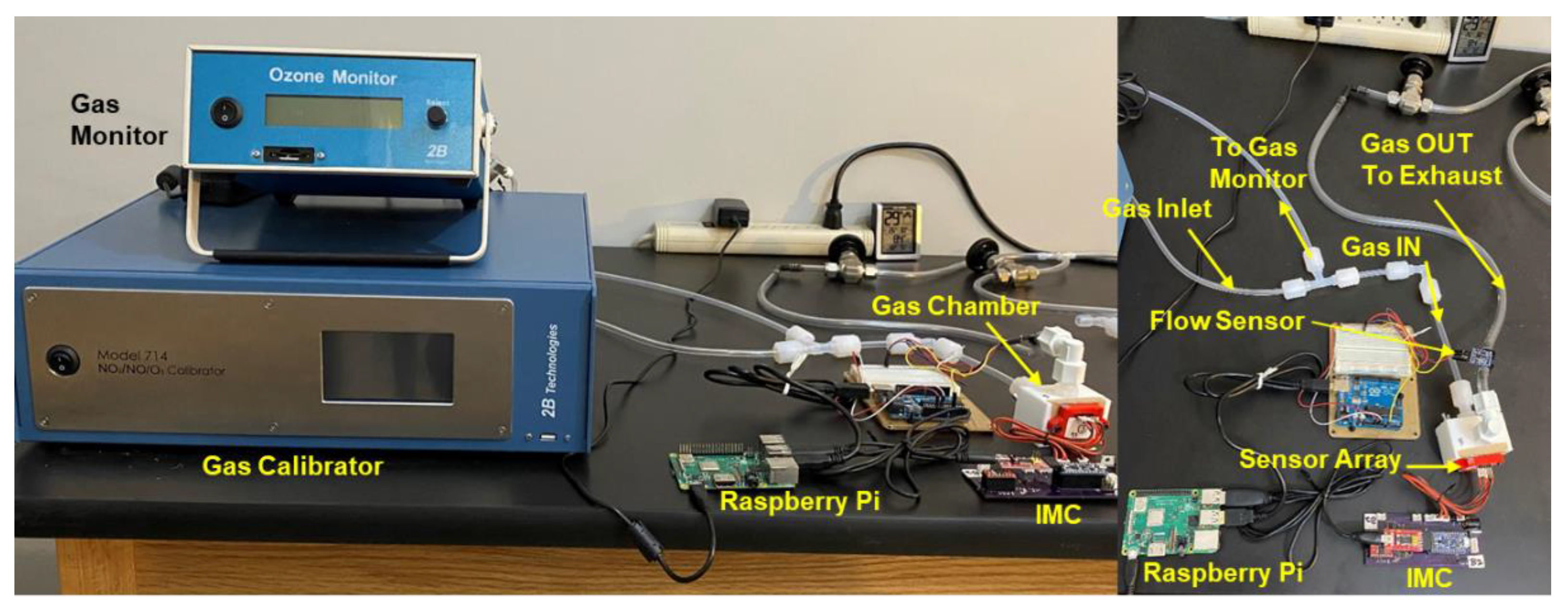
2.3. Sensor Calibration
3. Results
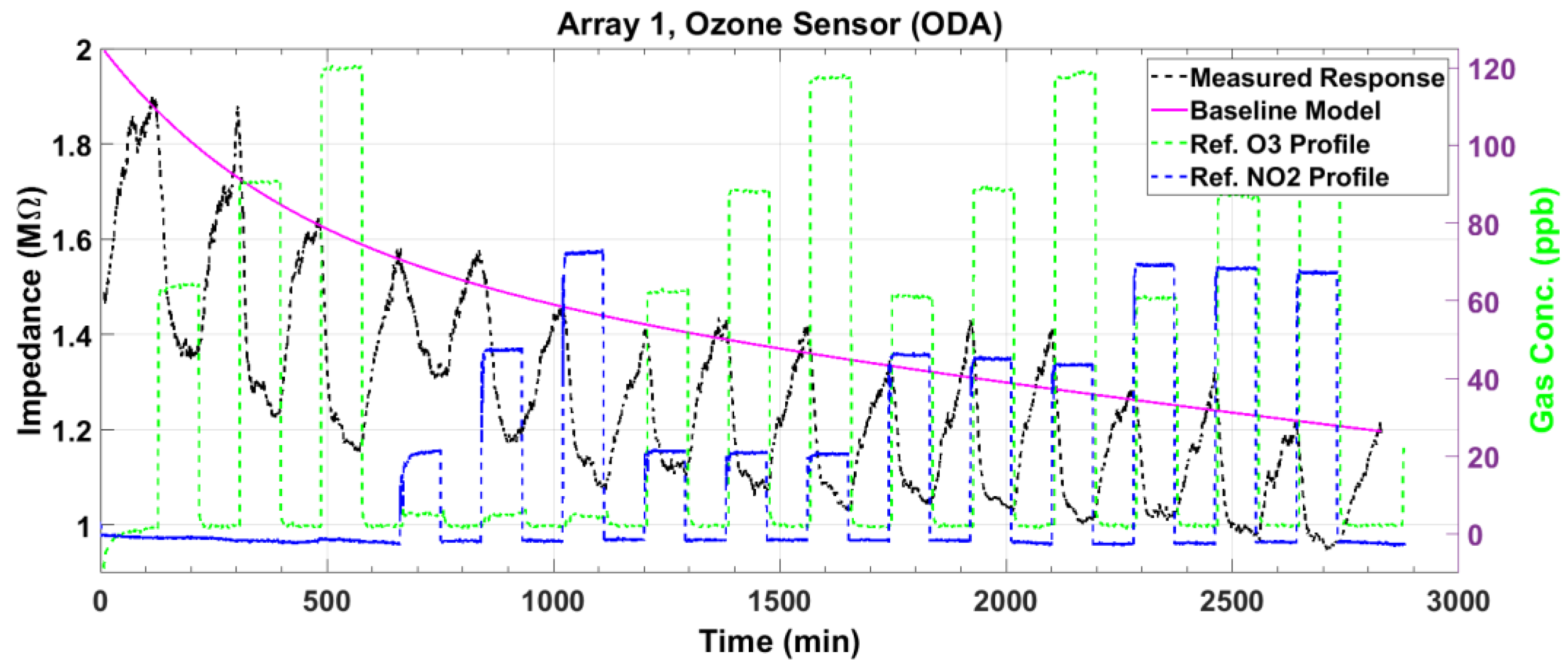
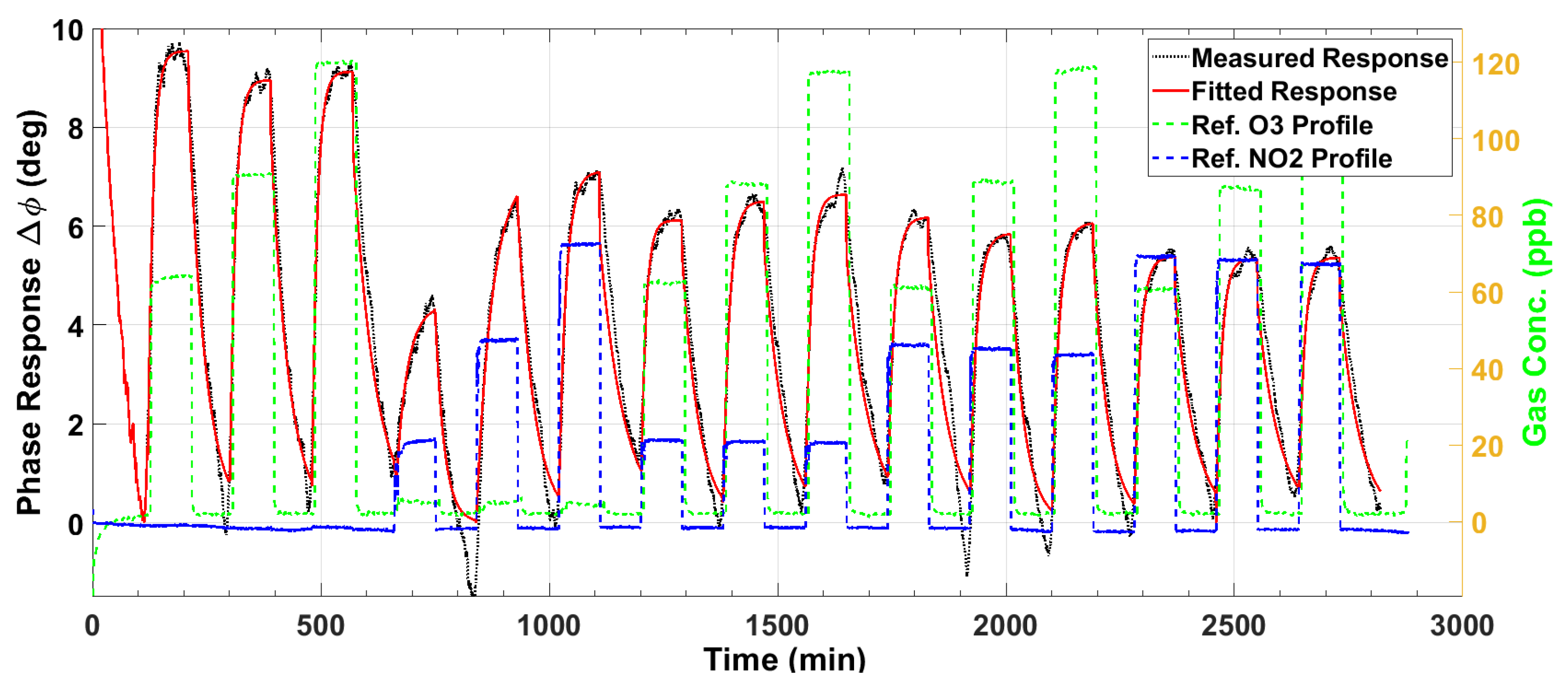
3.1. Ozone Sensor Response
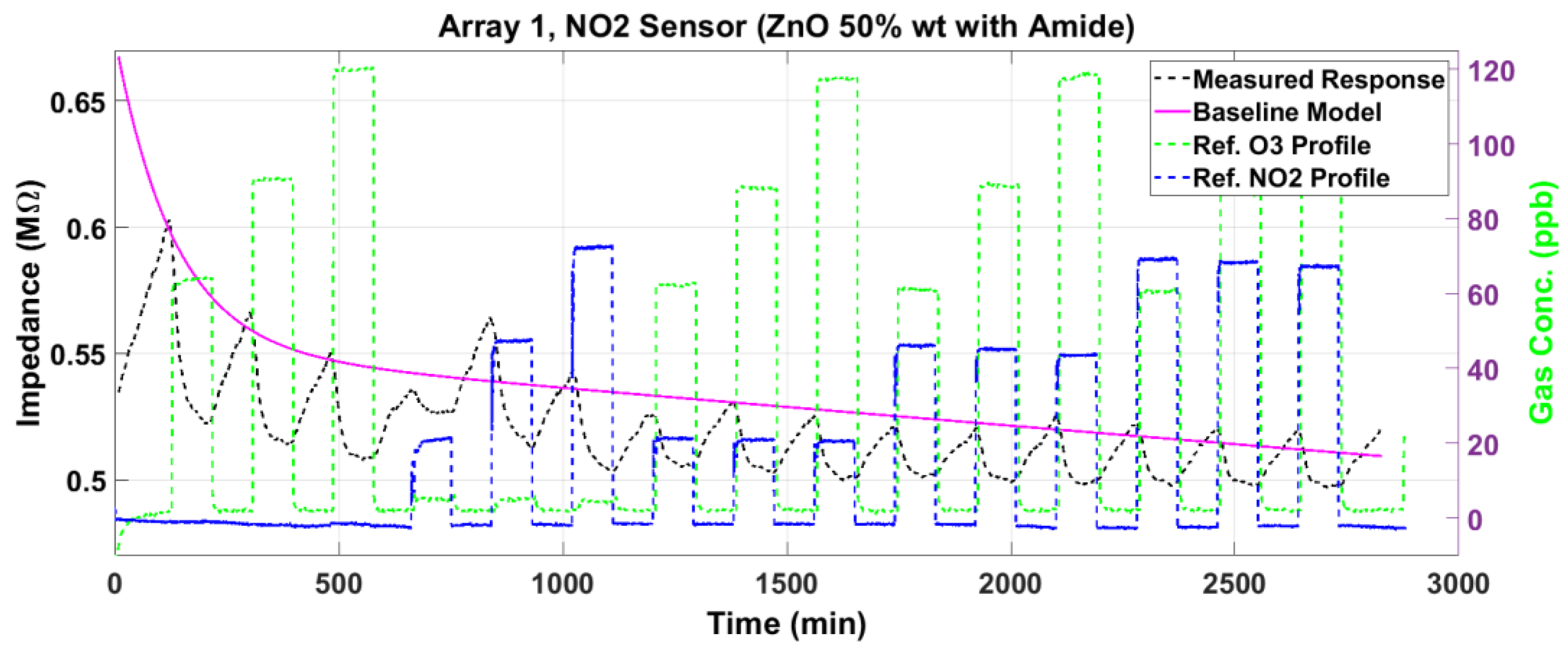
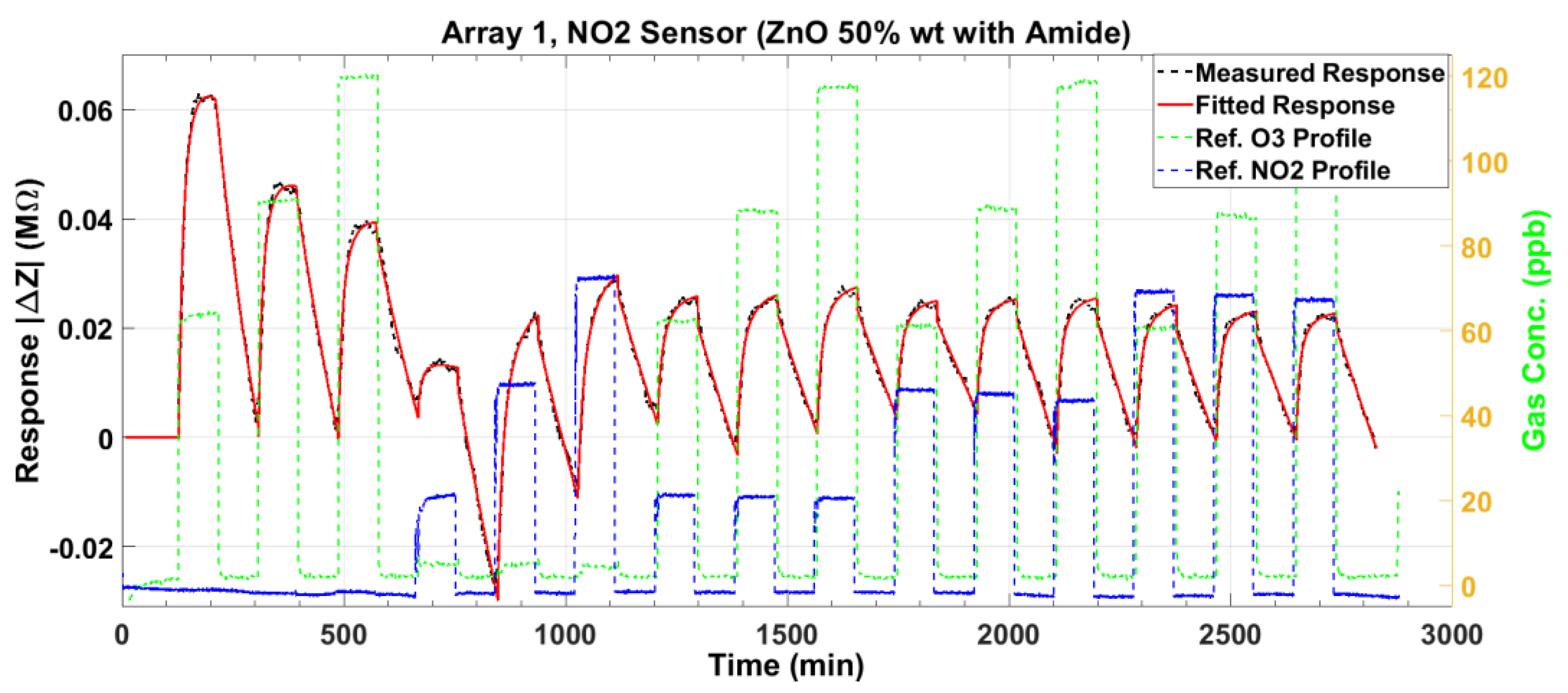
3.2. Nitrogen Dioxide Sensor Response
3.3. Maximum Response Change
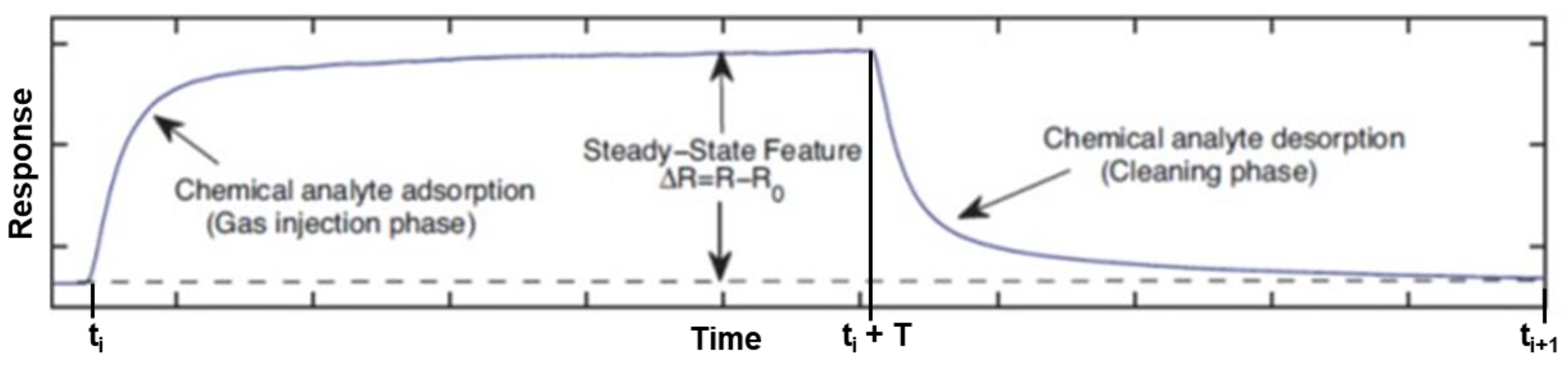
3.4. Response Time
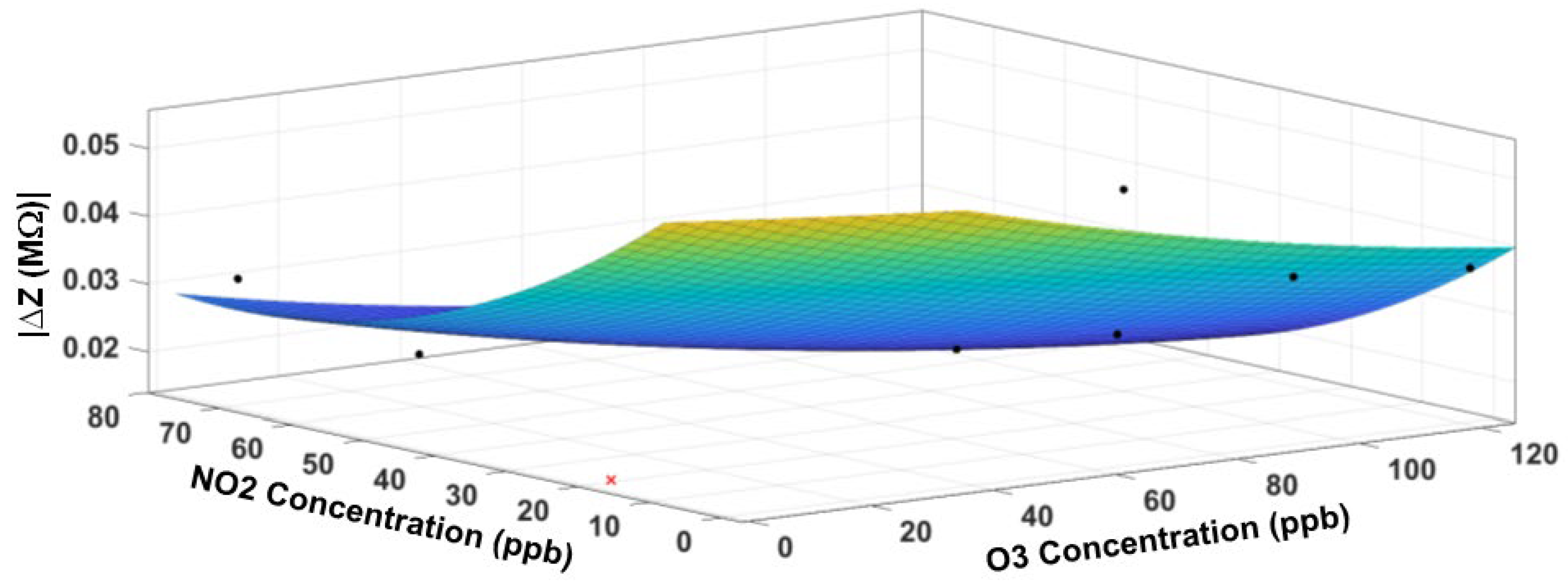
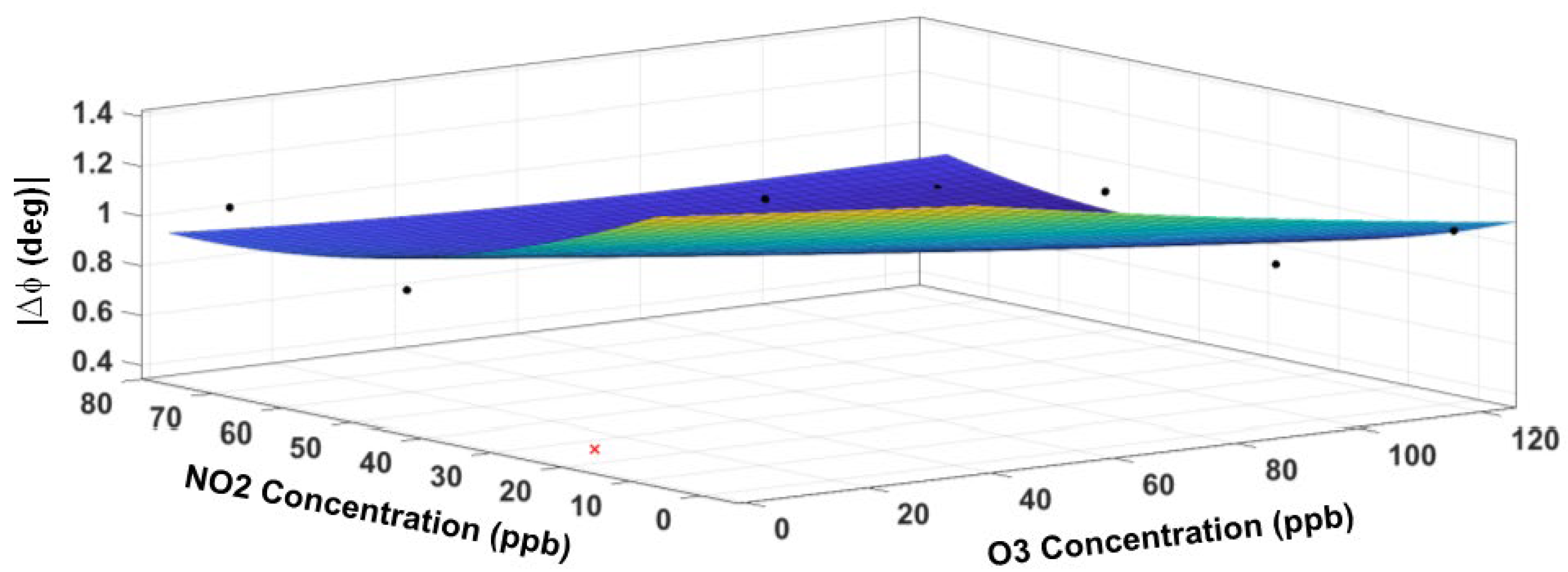
3.5. Response Surface Fitting
3.6. Sensor Performance Evaluation
3.6.1. Sensitivity
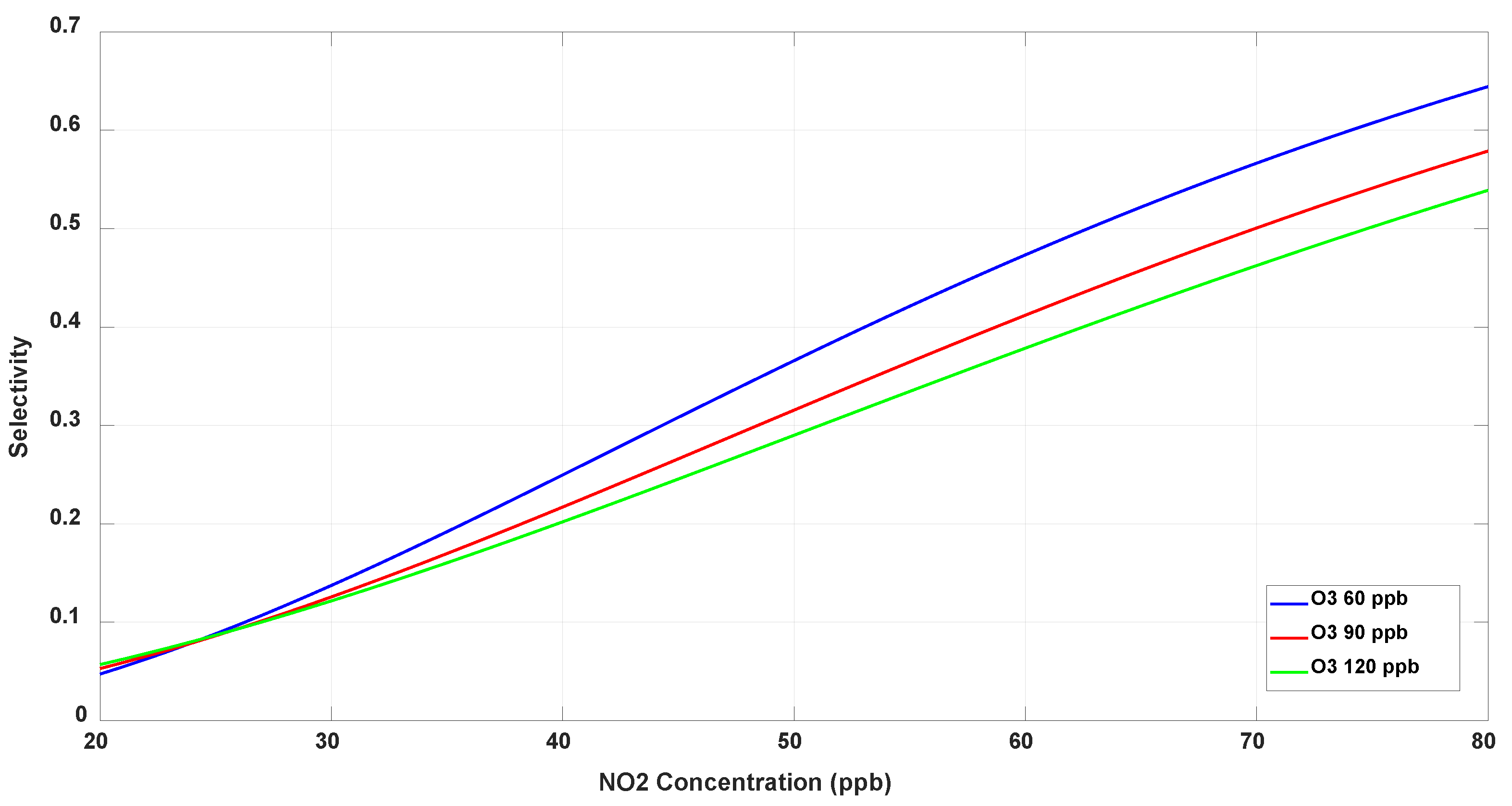
3.6.2. Selectivity
4. Discussion
Performance Comparison with Prior Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Sixth Assessment Report (AR6) of the United Nations Intergovernmental Panel on Climate Change (IPCC), April 2022. Available online: https://www.ipcc.ch/report/ar6/wg3/ (accessed on 10 January 2021).
- Pyrgou, A.; Hadjinicolaou, P.; Santamouris, M. Enhanced near-surface ozone under heatwave conditions in a Mediterranean island. Sci. Rep. 2018, 8, 9191. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.; Mickley, L.J.; Sulprizio, M.P.; Dominici, F.; Yue, X.; Ebisu, K.; Anderson, G.B.; Khan, R.F.A.; Bravo, M.A.; Bell, M.L. Particulate air pollution from wildfires in the Western US under climate change. Clim. Chang. 2016, 138, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Rev. Artic. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- The National Academies Press. Exposure Science in the 21st Century: A Vision and a Strategy. In National Research Council Report; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- World Health Organization. Air Quality Guidelines for Europe, 2nd ed.; WHO Regional Publications European Series; World Health Organization: Geneva, Switzerland, 2000; Volume 91, pp. 1–287. [Google Scholar]
- EPA/600/R-10/076F; U.S. Environmental Protection Agency Final Report, Integrated Science Assessment (ISA) for Ozone and Related Photochemical Oxidants. U.S. Environmental Protection Agency: Washington, DC, USA, 2013.
- EPA/600/R-15/068; U.S. Environmental Protection Agency Final Report, Integrated Science Assessment (ISA) for Oxides of Nitrogen—Health Criteria. U.S. Environmental Protection Agency: Washington, DC, USA, 2016.
- U.S. Environmental Protection Agency. Indoor Air Quality (IAQ). Available online: https://www.epa.gov/indoor-air-quality-iaq (accessed on 10 January 2021).
- U.S. Environmental Protection Agency. “Volatile Organic Compounds’ Impact on Indoor Air Quality”. Available online: https://www.epa.gov/indoor-air-quality-iaq/volatile-organic-compounds-impact-indoor-air-quality (accessed on 10 January 2021).
- Pazo, D.Y.; Moliere, F.; Sampson, M.M.; Reese, C.M.; Agnew-Heard, K.A.; Walters, M.J.; Holman, M.R.; Blount, B.C.; Watson, C.H.; Chambers, D.M. Mainstream Smoke Levels of Volatile Organic Compounds in 50 U.S. Domestic Cigarette Brands Smoked With the ISO and Canadian Intense Protocols. Nicotine Tob. Res. 2016, 18, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Missia, D.A.; Demetriou, E.; Michael, N.; Tolis, E.I.; Bartzis, J.G. Indoor exposure from building materials: A field study. Atmos. Environ. 2010, 44, 4388–4395. [Google Scholar] [CrossRef]
- Yeatts, K.B.; El-Sadig, M.; Leith, D.; Kalsbeek, W.; Al-Maskari, F.; Couper, D.; Funk, W.E.; Zoubeidi, T.; Chan, R.L.; Trent, C.B.; et al. Indoor Air Pollutants and Health in the United Arab Emirates. Environ. Health Perspect. 2012, 120, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hinds, W.C.; Kim, S.; Shen, S.; Sioutas, C. Study of ultrafine particles near a major highway with heavy-duty diesel traffic. Atmos. Environ. 2002, 36, 4323–4335. [Google Scholar] [CrossRef]
- Greenwald, R.; Bergin, M.H.; Yip, F.; Boehmer, T.; Kewada, P.; Shafer, M.M.; Schauer, J.J.; Sarnat, J.A. On-roadway in-cabin exposure to particulate matter: Measurement results using both continuous and time-integrated sampling approaches. Aerosol Sci. Technol. 2014, 48, 664–675. [Google Scholar] [CrossRef]
- Knibbs, L.D.; Cole-Hunter, T.; Morawska, L. A review of commuter exposure to ultrafine particles and its health effects. Atmos. Environ. 2011, 45, 2611–2622. [Google Scholar] [CrossRef]
- Brook, R.D. Cardiovascular effects of air pollution. Clin. Sci. 2008, 115, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Prunicki, M.; Haddad, F.; Dant, C.; Sampath, V.; Patel, R.; Smith, E.; Akdis, C.; Balmes, J.; Snyder, M.P.; et al. Cumulative Lifetime Burden of Cardiovascular Disease From Early Exposure to Air Pollution. J. Am. Hear. Assoc. 2020, 9, e014944. [Google Scholar] [CrossRef] [PubMed]
- Gasana, J.; Dillikar, D.; Mendy, A.; Forno, E.; Vieira, E.R. Motor vehicle air pollution and asthma in children: A meta-analysis. Environ. Res. 2012, 117, 36–45. [Google Scholar] [CrossRef]
- Gautier, C.; Charpin, D. Environmental triggers and avoidance in the management of asthma. J. Asthma Allergy 2017, 10, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Schultz, E.S.; Litonjua, A.A.; Melén, E. Effects of Long-Term Exposure to Traffic-Related Air Pollution on Lung Function in Children. Curr. Allergy Asthma Rep. 2017, 17, 41. [Google Scholar] [CrossRef]
- The Global Asthma Report 2018, Global Asthma Network. Available online: http://globalasthmareport.org/resources/Global_Asthma_Report_2018.pdf (accessed on 10 January 2021).
- American Lung Association. Trends in COPD (Emphysema and Chronic Bronchitis): Morbidity and Mortality 2010. Available online: http://www.lungusa.org/finding-cures/our-research/trend-reports/copd-trend-report.pdf (accessed on 10 January 2021).
- Babadjouni, R.M.; Hodis, D.M.; Radwanski, R.; Durazo, R.; Patel, A.; Liu, Q.; Mack, W.J. Clinical effects of air pollution on the central nervous system; a review. J. Clin. Neurosci. 2017, 43, 16–24. [Google Scholar] [CrossRef]
- Costa, L.G.; Cole, T.B.; Dao, K.; Chang, Y.-C.; Coburn, J.; Garrick, J.M. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol. Ther. 2020, 210, 107523. [Google Scholar] [CrossRef]
- Pru, A.; Wolf, J.; Corvala, C.; Bos, R.; Neira, M. Preventing Disease through Healthy Environments: A Global Assessment of the Burden of Disease from Environmental Risks; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Murray, C.J.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Caiazzo, F.; Ashok, A.; Waitz, I.A.; Yim, S.H.; Barrett, S.R. Air pollution and early deaths in the United States. Part I: Quantifying the impact of major sectors in 2005. Atmos. Environ. 2013, 79, 198–208. [Google Scholar] [CrossRef]
- Available online: https://www.reuters.com/article/usa-pollution-airmonitors-specialreport/special-report-u-s-air-monitors-routinely-miss-pollution-even-refinery-explosions-idUSKBN28B4RT (accessed on 29 August 2021).
- Kohl, C.D.; Wagner, T. Gas Sensing Fundamentals; Springer: Berlin, Germany, 2014. [Google Scholar]
- Comini, E.; Faglia, G.; Sberveglieri, G. Solid State Gas Sensing; Springer: New York, NY, USA, 2009. [Google Scholar]
- Kalantar-Zadeh, K.; Fry, B. Nanotechnology-Enabled Sensors; Springer: New York, NY, USA, 2008. [Google Scholar]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A Survey on Gas Sensing Technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef]
- Figaro Gas Sensors. Available online: https://www.figarosensor.com/technicalinfo/ (accessed on 3 March 2022).
- Sensirion Gas Sensors. Available online: https://sensirion.com/products/technology/ (accessed on 3 March 2022).
- Alphasense Gas Sensors. Available online: https://www.alphasense.com/company/technology/ (accessed on 3 March 2022).
- Spec Sensors. Available online: https://www.spec-sensors.com/ (accessed on 3 March 2022).
- Ashley, K. Developments in electrochemical sensors for occupational and environmental health applications. J. Hazard. Mater. 2003, 102, 1–12. [Google Scholar] [CrossRef]
- Chou, J. Hazardous Gas Monitors: A Practical Guide to Selection, Operation and Applications; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Park, C.O.; Fergus, J.W.; Miura, N.; Park, J.; Choi, A. Solid-state electrochemical gas sensors. Ionics 2009, 15, 261–284. [Google Scholar] [CrossRef]
- Michal Raninec, Overcoming the Technical Challenges of Electrochemical Gas Sensing, Analog Devices Application Note. 2019. Available online: https://www.analog.com/en/technical-articles/overcoming-the-technical-challenges-of-electrochemical-gas-sensing.html (accessed on 2 March 2022).
- Moretto, L.M.; Kalcher, K. Environmental Analysis by Electrochemical Sensors and Biosensors; Springer: New York, NY, USA, 2014. [Google Scholar]
- Pang, X.; Shaw, M.D.; Lewis, A.C.; Carpenter, L.J.; Batchellier, T. Electrochemical ozone sensors: A miniaturised alternative for ozone measurements in laboratory experiments and air-quality monitoring. Sens. Actuators B Chem. 2017, 240, 829–837. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Blinov, I.; Brinzari, V.; Stetter, J. Effect of air humidity on gas response of SnO2 thin film ozone sensors. Sens. Actuators B Chem. 2007, 122, 519–526. [Google Scholar] [CrossRef]
- Knake, R.; Hauser, P.C. Sensitive electrochemical detection of ozone. Anal. Chim. Acta 2002, 459, 199–207. [Google Scholar] [CrossRef]
- Electrochemical, vs. Semiconductor Gas Detection–A Critical Choice. White Paper, Emerson, 00870-0200-4928, Rev AA, January 2019. Available online: https://www.emerson.com/documents/automation/white-paper-electrochemical-vs-semiconductor-gas-detection-en-5351906.pdf (accessed on 2 March 2022).
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. In2O3- and SnO2-based Ozone Sensors: Design and Characterization. Crit. Rev. Solid State Mater. Sci. 2018, 43, 83–132. [Google Scholar] [CrossRef]
- Ziegler, D.; Marchisio, A.; Palmero, P.; Tulliani, J.-M. WO3-Doped Indium Oxide Thick Films for Ozone Detection at Low Temperature. Proceedings 2017, 1, 428. [Google Scholar] [CrossRef]
- Nguyen, V.C.; Cha, H.-Y.; Kim, H. High Selectivity Hydrogen Gas Sensor Based on WO3/Pd-AlGaN/GaN HEMTs. Sensors 2023, 23, 3465. [Google Scholar] [CrossRef]
- Vallejos, S.; Khatko, V.; Aguir, K.; Ngo, K.; Calderer, J.; Gràcia, I.; Cané, C.; Llobet, E.; Correig, X. Ozone monitoring by micro-machined sensors with WO3 sensing films. Sens. Actuators B Chem. 2007, 126, 573–578. [Google Scholar] [CrossRef]
- Wozniak, L.; Kalinowski, P.; Jasinski, G.; Jasinski, P. FFT analysis of temperature modulated semiconductor gas sensor response for the prediction of ammonia concentration under humidity interference. Microelectron. Reliab. 2018, 84, 163–169. [Google Scholar] [CrossRef]
- Zhao, W.-J.; Ding, K.-L.; Chen, Y.-S.; Xie, F.-Y.; Xu, D. Optimized Low Frequency Temperature Modulation for Improving the Selectivity and Linearity of SnO2 Gas Sensor. IEEE Sensors J. 2020, 20, 10433–10443. [Google Scholar] [CrossRef]
- Elmi, I.; Zampolli, S.; Cozzani, E.; Mancarella, F.; Cardinali, G. Development of ultra-low-power consumption MOX sensors with ppb-level VOC detection capabilities for emerging applications. Sens. Actuators B Chem. 2008, 135, 342–351. [Google Scholar] [CrossRef]
- Schütze, A.; Baur, T.; Leidinger, M.; Reimringer, W.; Jung, R.; Conrad, T.; Sauerwald, T. Highly Sensitive and Selective VOC Sensor Systems Based on Semiconductor Gas Sensors: How to? Environments 2017, 4, 20. [Google Scholar] [CrossRef]
- Liu, H.; Wu, R.; Guo, Q.; Hua, Z.; Wu, Y. Electronic Nose Based on Temperature Modulation of MOS Sensors for Recognition of Excessive Methanol in Liquors. ACS Omega 2021, 6, 30598–30606. [Google Scholar] [CrossRef] [PubMed]
- Ivanovskaya, M.; Gurlo, A.; Bogdanov, P. Mechanism of O3 and NO2 detection and selectivity of In2O3 sensors. Sens. Actuators B Chem. 2001, 77, 264–267. [Google Scholar] [CrossRef]
- Rüffer, D.; Hoehne, F.; Bühler, J. New Digital Metal-Oxide (MOx) Sensor Platform. Sensors 2018, 18, 1052. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Pauly, A.; Mazet, L.; Germain, J.; Bouvet, M.; Malezieux, B. Improvement in real time detection and selectivity of phthalocyanine gas sensors dedicated to oxidizing pollutants evaluation. Thin Solid Films 2005, 490, 28–35. [Google Scholar] [CrossRef][Green Version]
- Korotcenkov, G.; Cho, B.K. Engineering approaches for the improvement of conductometric gas sensor parameters: Part 1. Improvement of sensor sensitivity and selectivity (short survey). Sens. Actuators B Chem. 2013, 188, 709–728. [Google Scholar] [CrossRef]
- Palacio, F.; Fonollosa, J.; Burgues, J.; Gomez, J.M.; Marco, S. Pulsed-Temperature Metal Oxide Gas Sensors for Microwatt Power Consumption. IEEE Access 2020, 8, 70938–70946. [Google Scholar] [CrossRef]
- Gramm, A.; Schütze, A. High performance solvent vapor identification with a two sensor array using temperature cycling and pattern classification. Sens. Actuators B Chem. 2003, 95, 58–65. [Google Scholar] [CrossRef]
- Ngo, K.A.; Lauque, P.; Aguir, K. High performance of a gas identification system using sensor array and temperature modulation. Sens. Actuators B Chem. 2007, 124, 209–216. [Google Scholar] [CrossRef]
- Comini, E. Metal oxide nanowire chemical sensors: Innovation and quality of life. Mater. Today 2016, 19, 559–567. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Tijima, S. Helical microtubes of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme Oxygen Sensitivity of Electronic Properties of Carbon Nanotubes. Science 2000, 287, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, J.; Zhou, G.; Imakiire, H.; Ding, W.; Hara, M. Controlled fabrication of carbon nanotube NO2 gas sensor using dielectrophoretic impedance measurement. Sens. Actuators B Chem. 2005, 108, 398–403. [Google Scholar] [CrossRef]
- Zhang, T.; Mubeen, S.; Bekyarova, E.; Yoo, B.Y.; Haddon, R.C.; Myung, N.V.; Deshusses, M.A. Poly(m-aminobenzene sulfonic acid) functionalized single-walled carbon nanotubes based gas sensor. Nanotechnology 2007, 18, 165504–165509. [Google Scholar] [CrossRef]
- Brahim, S.; Colbern, S.; Gump, B.; Grigorian, L. Tailoring Gas Sensing Properties of Carbon Nanotubes. J. Appl. Phys. 2008, 104, 1–10. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Star, A. Carbon Nanotube Gas and Vapor Sensors. Angew. Chem. Int. Ed. 2008, 47, 6550–6570. [Google Scholar] [CrossRef]
- Abdellah, A.; Yaqub, A.; Ferrari, C.; Fabel, B.; Lugli, P.; Scarpa, G. Spray deposition of highly uniform CNT films and their application in gas sensing. In Proceedings of the 11th International Conference on Nanotechnology, Portland, OR, USA, 15–19 August 2011. [Google Scholar] [CrossRef]
- Novak, J.P.; Snow, E.S.; Houser, E.J.; Park, D.; Stepnowski, J.L.; McGill, R.A. Nerve agent detection using networks of single-walled carbon nanotubes. Appl. Phys. Lett. 2003, 83, 4026–4028. [Google Scholar] [CrossRef]
- Lee, C.Y.; Sharma, R.; Radadia, A.D.; Masel, R.I.; Strano, M.S. On-chip micro-gas- chromatograph enabled by a non-covalently functionalized single-walled carbon nanotube sensor array. Angew. Chem. 2008, 120, 5096–5099. [Google Scholar] [CrossRef]
- Zheng, Q.; Fu, Y.-C.; Xu, J.-Q. Advances in the chemical sensors for the detection of DMMP—A simulant for nerve agent sarin. Procedia Eng. 2010, 7, 179–184. [Google Scholar] [CrossRef]
- Varghese, O.K.; Kichambre, P.D.; Gong, D.; Ong, K.G.; Dickey, E.C.; Grimes, C.A. Gas sensing characteristics of multi-wall carbon nanotubes. Sens. Actuators B Chem. 2001, 81, 32–41. [Google Scholar] [CrossRef]
- de Araújo, E.P.; Paiva, M.P.; Moisés, L.A.; Santo, G.S.D.E.; Blanco, K.C.; Chiquito, A.J.; Amorim, C.A. Improving Hazardous Gas Detection Behavior with Palladium Decorated SnO2 Nanobelts Networks. Sensors 2023, 23, 4783. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Pham, A.; Gaillard, J.; Parker, A.; Rao, A.M. Carbon-nanotube-based resonant-circuit sensor for ammonia. Appl. Phys. Lett. 2002, 80, 4632–4634. [Google Scholar] [CrossRef]
- Someya, T.; Small, J.; Kim, P.; Nuckolls, C.; Yardley, J.T. Alcohol Vapor Sensors Based on Single-Walled Carbon Nanotube Field Effect Transistors. Nano Lett. 2003, 3, 877–881. [Google Scholar] [CrossRef]
- Snow, E.S.; Perkins, F.K.; Houser, E.J.; Badescu, S.C.; Reinecke, T.L. Chemical Detection with a Single-Walled Carbon Nanotube Capacitor. Science 2005, 307, 1942–1945. [Google Scholar] [CrossRef]
- Patel, S.; Mlsna, T.; Fruhberger, B.; Klaassen, E.; Cemalovic, S.; Baselt, D. Chemicapacitive microsensors for volatile organic compound detection. Sens. Actuators B Chem. 2003, 96, 541–553. [Google Scholar] [CrossRef]
- Delapierre, G.; Grange, H.; Chambaz, B.; Destannes, L. Polymer-based capacitive humidity sensor: Characteristics and experimental results. Sens. Actuators 1983, 4, 97–104. [Google Scholar] [CrossRef]
- Kalvoda, L.; Jakoubková, J.; Burda, M.; Kwiecien, P.; Richter, I.; Kopeček, J. Fiber Optic Sensor of Ammonia Gas Using Plasmonic Extraordinary Optical Transmission. Sensors 2023, 23, 4065. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.; Roh, S. Effects of Gas and Steam Humidity on Particulate Matter Measurements Obtained Using Light-Scattering Sensors. Sensors 2023, 23, 6199. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, R.; Staiculescu, D.; Wong, C.P.; Tentzeris, M.M. A Novel Conformal RFID-Enabled Module Utilizing Inkjet-Printed Antennas and Carbon Nanotubes for Gas-Detection Applications. IEEE Antennas Wirel. Propag. Lett. 2009, 8, 653–656. [Google Scholar] [CrossRef]
- Lee, H.; Naishadham, K.; Tentzeris, M.M.; Shaker, G. A novel highly sensitive antenna-based gas sensor utilizing carbon nanotubes and inkjet printing. In Proceedings of the IEEE Antennas and Propagation Symposium, Spokane, WA, USA, 3–8 July 2011. [Google Scholar]
- Lee, H.; Shaker, G.; Naishadham, K.; Song, X.; McKinley, M.; Wagner, B.; Tentzeris, M. Carbon-Nanotube Loaded Antenna-Based Ammonia Gas Sensor. IEEE Trans. Microw. Theory Tech. 2011, 59, 2665–2673. [Google Scholar] [CrossRef]
- Naishadham, K.; Bekyarova, E.; Savi, P. Passive nanotechnology based sensors for the remote detection of environmental pollutants impacting public health. In Proceedings of the IEEE Sensors Conference, Glasgow, Scotland, 1 November 2017. [Google Scholar] [CrossRef]
- Naishadham, K.; Song, X.; Wagner, B. Highly Sensitive Standoff Gas Sensing Using Carbon Nanotubes and Integrated Wireless Devices. U.S. Patent 20130230429 A1, September 2017. [Google Scholar]
- Gugliandolo, G.; Naishadham, K.; Neri, G.; Fernicola, V.C.; Donato, N. A Novel Sensor-Integrated Aperture Coupled Microwave Patch Resonator for Humidity Detection. IEEE Trans. Instrum. Meas. 2021, 70, 1–11. [Google Scholar] [CrossRef]
- Gugliandolo, G.; Naishadham, K.; Crupi, G.; Campobello, G.; Donato, N. Microwave Transducers for Gas Sensing: A Challenging and Promising New Frontier. IEEE Instrum. Meas. Mag. 2022, 25, 42–51. [Google Scholar] [CrossRef]
- Gugliandolo, G.; Naishadham, K.; Crupi, G.; Donato, N. Design and Characterization of a Microwave Transducer for Gas Sensing Applications. Chemosensors 2022, 10, 127. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, H.; Bekyarova, E.; Itkis, M.E.; Niyogi, S.; Haddon, R.C. Carbon nanotubes: Functionalization. In Dekker Encyclopedia of Nanoscience and Nanotechnolog; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Bekyarova, E.; Kalinina, I.; Sun, X.; Shastry, T.; Worsley, K.; Chi, X.; Itkis, M.E.; Haddon, R.C. Chemically Engineered Single-Walled Carbon Nanotube Materials for the Electronic Detection of Hydrogen Chloride. Adv. Mater. 2010, 22, 848–852. [Google Scholar] [CrossRef]
- Sensigent Intelligent Sensing Solutions. Available online: http://www.sensigent.com/products/nanosensors.html (accessed on 16 September 2021).
- Itkis, M.E.; Perea, D.E.; Niyogi, S.; Love, J.; Tang, J.; Yu, A.; Kang, C.; Jung, R.; Haddon, R.C. Optimization of the Ni−Y Catalyst Composition in Bulk Electric Arc Synthesis of Single-Walled Carbon Nanotubes by Use of Near-Infrared Spectroscopy. J. Phys. Chem. B 2004, 108, 12770–12775. [Google Scholar] [CrossRef]
- Ziegler, D.; Bekyarova, E.; Marchisio, A.; Tulliani, J.-M.; Naishadham, K. Highly Selective Ozone Sensors Based on Functionalized Carbon Nanotubes. In Proceedings of the 2018 IEEE Sensors Conference, New Delhi, India, 28–31 October 2018. [Google Scholar] [CrossRef]
- Naishadham, G.; Bekyarova, E.; Qian, Y.; Naishadham, K. Design of Low-Frequency Impedance Measurement Sensors for Respiratory Health. In Proceedings of the 2018 IEEE Sensors Conference, New Delhi, India, 28–31 October 2018. [Google Scholar] [CrossRef]
- Potyrailo, R.A. Multivariable Sensors for Ubiquitous Monitoring of Gases in the Era of Internet of Things and Industrial Internet. Chem. Rev. 2016, 116, 11877–11923. [Google Scholar] [CrossRef]
- Berger, P.D.; Maurer, R.E.; Celli, G.B. Experimental Design with Application in Management, Engineering, and the Sciences; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Vergara, A.; Fonollosa, J.; Mahiques, J.; Trincavelli, M.; Rulkov, N.; Huerta, R. On the performance of gas sensor arrays in open sampling systems using Inhibitory Support Vector Machines. Sens. Actuators B Chem. 2013, 185, 462–477. [Google Scholar] [CrossRef]
- Vergara, A.; Vembu, S.; Ayhan, T.; Ryan, M.A.; Homer, M.L.; Huerta, R. Chemical gas sensor drift compensation using classifier ensembles. Sens. Actuators B Chem. 2012, 166–167, 320–329. [Google Scholar] [CrossRef]
- Rodriguez-Lujan, I.; Fonollosa, J.; Vergara, A.; Homer, M.; Huerta, R. On the calibration of sensor arrays for pattern recognition using the minimal number of experiments. Chemom. Intell. Lab. Syst. 2014, 130, 123–134. [Google Scholar] [CrossRef]
- Fonollosa, J.; Vergara, A.; Huerta, R. Algorithmic mitigation of sensor failure: Is sensor replacement really necessary? Sens. Actuators B Chem. 2013, 183, 211–221. [Google Scholar] [CrossRef]
- Gutierrez-Osuna, R. Pattern analysis for machine olfaction: A review. IEEE Sensors J. 2002, 2, 189–202. [Google Scholar] [CrossRef]
- Araújo, T.; Silva, L.; Aguiar, A.; Moreira, A. Calibration Assessment of Low-Cost Carbon Dioxide Sensors Using the Extremely Randomized Trees Algorithm. Sensors 2023, 23, 6153. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Magna, G.; Vergara, A.; Di Natale, C. Cooperative classifiers for reconfigurable sensor arrays. Sens. Actuators B Chem. 2014, 199, 83–92. [Google Scholar] [CrossRef]
- Leveraging Artificial Intelligence in Gas Sensing. Available online: https://www.renesas.com/us/en/blogs/leveraging-artificial-intelligence-gas-sensing (accessed on 18 December 2021).
- Feng, S.; Farha, F.; Li, Q.; Wan, Y.; Xu, Y.; Zhang, T.; Ning, H. Review on Smart Gas Sensing Technology. Sensors 2019, 19, 3760. [Google Scholar] [CrossRef] [PubMed]
- Bekyarova, E.; Davis, M.; Burch, T.; Itkis, M.E.; Zhao, B.; Sunshine, S.; Haddon, R.C. Chemically Functionalized Single-Walled Carbon Nanotubes for Ammonia Sensors. J. Phys. Chem. B 2004, 108, 19717–19720. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc Oxide Nanostructures for NO2 Gas–Sensor Applications: A Review. Nano-Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef]
- Park, S.; Byoun, Y.; Kang, H.; Song, Y.J.; Choi, S.W. ZnO Nanocluster-Functionalized Single-Walled Carbon Nanotubes Synthesized by Microwave Irradiation for Highly Sensitive NO2 Detection at Room Temperature. ACS Omega 2019, 4, 10677–10686. [Google Scholar] [CrossRef]
- Analog Devices AD5933 Impedance Converter, Network Analyzer. Available online: http://www.analog.com/media/en/technical-documentation/data-sheets/AD5933.pdf (accessed on 10 February 2017).
- Danzer, K. Selectivity and specificity in analytical chemistry. General considerations and attempt of a definition and quantification. Anal. Bioanal. Chem. 2001, 369, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Colindres, S.C.; Aguir, K.; Cervantes, F.; Villa, L.; Garibay, V. Carbon nanotubes functionalized by nanoparticles of platinum. Mater. Sci. Forum. 2014, 793, 45–50. [Google Scholar] [CrossRef]
- Colindres, S.C.; Aguir, K.; Sodi, F.C.; Vargas, L.V.; Salazar, J.A.M.; Febles, V.G. Ozone Sensing Based on Palladium Decorated Carbon Nanotubes. Sensors 2014, 14, 6806–6818. [Google Scholar] [CrossRef]
- Ghaddab, B.; Sanchez, J.; Mavon, C.; Paillet, M.; Parret, R.; Zahab, A.; Bantignies, J.-L.; Flaud, V.; Beche, E.; Berger, F. Detection of O3 and NH3 using hybrid tin dioxide/carbon nanotubes sensors: Influence of materials and processing on sensor’s sensitivity. Sens. Actuators B Chem. 2012, 170, 67–74. [Google Scholar] [CrossRef]
- Park, Y.; Dong, K.-Y.; Lee, J.; Choi, J.; Bae, G.-N.; Ju, B.-K. Development of an ozone gas sensor using single-walled carbon nanotubes. Sens. Actuators B Chem. 2009, 140, 407–411. [Google Scholar] [CrossRef]
- Fort, A.; Panzardi, E.; Al-Hamry, A.; Vignoli, V.; Mugnaini, M.; Addabbo, T.; Kanoun, O. Highly Sensitive Detection of NO2 by Au and TiO2 Nanoparticles Decorated SWCNTs Sensors. Sensors 2020, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Valentini, L.; Armentano, I.; Kenny, J.M.; Cantalini, C.; Lozzi, L.; Santucci, S. Sensors for sub-ppm NO2 gas detection based on carbon nanotube thin films. Appl. Phys. Lett. 2003, 82, 961–963. [Google Scholar] [CrossRef]











| Sensor | Gas/Pulse No. | Max |ΔZ|/Z0 | Max |Δφ|/φ0 | Comment |
|---|---|---|---|---|
| O3 | O3/3 | 26.8% | 32.9% | Target gas |
| O3 | NO2/6 | 26.0% | 36.4% | Interfering gas |
| NO2 | NO2/6 | 5.3% | 30.9% | Target gas |
| NO2 | O3/3 | 10.3% | 28.6% | Interfering gas |
| Feature | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | Avg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O3 Mag. | 6.38 | 13.47 | 10.33 | 10.85 | 21.24 | 13.70 | 8.40 | 9.48 | 14.83 | 11.79 | 7.99 | 9.06 | 7.44 | 7.41 | 11.15 | 10.90 |
| O3 Phase | 13.64 | 15.60 | 13.56 | 18.24 | 23.82 | 21.54 | 25.52 | 14.25 | 17.10 | 8.33 | 3.02 | 5.69 | 2.77 | 6.56 | 4.95 | 12.97 |
| NO2 Mag. | 18.42 | 13.80 | 12.68 | 15.87 | 24.55 | 25.05 | 12.06 | 14.18 | 12.98 | 14.16 | 10.08 | 7.81 | 11.40 | 8.74 | 8.40 | 14.01 |
| NO2 Phase | 14.73 | 15.64 | 18.66 | 21.84 | 32.19 | 19.47 | 16.89 | 12.79 | 17.55 | 15.43 | 14.42 | 13.8 | 15.18 | 14.4 | 19.89 | 17.52 |
| Response | R2 | RMSE | ||||||
|---|---|---|---|---|---|---|---|---|
| Magnitude | 0.581 | −2.288 × 10−3 | −7.663 × 10−3 | 1.128 × 10−5 | 6.752 × 10−6 | 6.680 × 10−5 | 0.9568 | 0.0199 |
| Phase | 10.34 | −3.379 × 10−2 | −0.1227 | 1.851 × 10−4 | 6.799 × 10−6 | 1.072 × 10−3 | 0.9376 | 0.4532 |
| Response | R2 | RMSE | ||||||
|---|---|---|---|---|---|---|---|---|
| Magnitude | 0.06259 | −3.057 × 10−4 | −9.963 × 10−4 | 8.364 × 10−7 | 1.496 × 10−6 | 7.328 × 10−6 | 0.8849 | 0.0047 |
| Phase | 1.511 | −4.888 × 10−3 | −1.565 × 10−2 | 8.616 × 10−6 | 4.364 × 10−5 | 1.093 × 10−4 | 0.7178 | 0.1050 |
| Materials | Gas | Response | Concentration (ppb) | Temperature (°C) | Response Change | Response Time (min) | Reference |
|---|---|---|---|---|---|---|---|
| ODA-SWNTs 1 | O3 | abs (impedance) | 60 | 75 | 26.8% | 6.4 | This work |
| ODA-SWNTs | O3 | impedance phase | 60 | 75 | 32.9% | 13.6 | This work |
| Pd-decorated MWNTs 2 | O3 | resistance | 100 | 120 | 1.47 | 1 | [116] |
| Hybrid SnO2/SWNTs | O3 | resistance | 174 | 300 | 10.5 | 40 | [117] |
| Thermally treated SWNTs | O3 | resistance | 1000 | 350 | 14.7 | 1.7 | [118] |
| ZnO NPs 3/amide-SWNTs | NO2 | abs (impedance) | 70 | 75 | 5.3% | 25.1 | This work |
| ZnO NPs/amide-SWNTs | NO2 | impedance phase | 70 | 75 | 30.9% | 19.5 | This work |
| Au NPs/SWNTs | NO2 | resistance | 12,000 | 240 | 10%/ppm | 0.5 | [119] |
| ZnO NPs/SWNTs | NO2 | resistance | 1000 | 25 | 70% | 3.3 | [112] |
| PECVD 4 of CNTs on Si3N4/Si substrates | NO2 | resistance | 100 | 165 | 3.3 | 65 | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naishadham, K.; Naishadham, G.; Cabrera, N.; Bekyarova, E. Response Surface Modeling of the Steady-State Impedance Responses of Gas Sensor Arrays Comprising Functionalized Carbon Nanotubes to Detect Ozone and Nitrogen Dioxide. Sensors 2023, 23, 8447. https://doi.org/10.3390/s23208447
Naishadham K, Naishadham G, Cabrera N, Bekyarova E. Response Surface Modeling of the Steady-State Impedance Responses of Gas Sensor Arrays Comprising Functionalized Carbon Nanotubes to Detect Ozone and Nitrogen Dioxide. Sensors. 2023; 23(20):8447. https://doi.org/10.3390/s23208447
Chicago/Turabian StyleNaishadham, Krishna, Gautam Naishadham, Nelson Cabrera, and Elena Bekyarova. 2023. "Response Surface Modeling of the Steady-State Impedance Responses of Gas Sensor Arrays Comprising Functionalized Carbon Nanotubes to Detect Ozone and Nitrogen Dioxide" Sensors 23, no. 20: 8447. https://doi.org/10.3390/s23208447
APA StyleNaishadham, K., Naishadham, G., Cabrera, N., & Bekyarova, E. (2023). Response Surface Modeling of the Steady-State Impedance Responses of Gas Sensor Arrays Comprising Functionalized Carbon Nanotubes to Detect Ozone and Nitrogen Dioxide. Sensors, 23(20), 8447. https://doi.org/10.3390/s23208447





