Comparison of Slate Safety Wearable Device to Ingestible Pill and Wearable Heart Rate Monitor
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Participants
3.2. Qualitative Data
4. Discussion
4.1. Comfort
4.2. Continuous Monitoring
4.3. Limitations
5. Conclusions
Generalizability
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Heart, Lung, and Blood Institute. Extreme Heat in the U.S. Linked to an Increased Number of Deaths. 2022. Available online: https://www.nhlbi.nih.gov/news/2022/extreme-heat-us-linked-increased-number-deaths (accessed on 25 October 2022).
- US Department of Labor. US Department of Labor Reminds Southwest Employers That Workers Need Protection from the Dangers of Heat Illness. 2022. Available online: https://www.osha.gov/news/newsreleases/region6/05112022web (accessed on 25 October 2022).
- NIOSH. Heat Stress. Available online: https://www.cdc.gov/niosh/topics/heatstress/default.html#:~:text=Heat%20stress%20can%20result%20in,with%20hot%20surfaces%20or%20steam (accessed on 5 April 2022).
- Morris, N.B.; Jay, O.; Flouris, A.D.; Casanueva, A.; Gao, C.; Foster, J.; Havenith, G.; Nybo, L. Sustainable solutions to mitigate occupational heat strain—An umbrella review of physiological effects and global health perspectives. Environ. Health A Glob. Access Sci. Source 2020, 19, 95. [Google Scholar] [CrossRef]
- Most, M.S.; Yates, D.T. Inflammatory Mediation of Heat Stress-Induced Growth Deficits in Livestock and Its Potential Role as a Target for Nutritional Interventions: A Review. Animals 2021, 11, 3539. [Google Scholar] [CrossRef]
- Castillo, E.C.; Vazquez-Garza, E.; Yee-Trejo, D.; Garcia-Rivas, G.; Torre-Amione, G. What Is the Role of the Inflammation in the Pathogenesis of Heart Failure? Curr. Cardiol. Rep. 2020, 22, 139. [Google Scholar] [CrossRef]
- Ioannou, L.G.; Mantzios, K.; Tsoutsoubi, L.; Nintou, E.; Vliora, M.; Gkiata, P.; Dallas, C.N.; Gkikas, G.; Agaliotis, G.; Sfakianakis, K.; et al. Occupational Heat Stress: Multi-Country Observations and Interventions. Int. J. Environ. Res. Public Health 2021, 18, 6303. [Google Scholar] [CrossRef]
- National Safety Council, Heat Stress Wearables. Available online: https://www.nsc.org/workplace/safety-topics/work-to-zero/safety-technologies/heat-stress-wearables (accessed on 25 October 2022).
- Charkoudian, N. Skin blood flow in adult human thermoregulation: How it works, when it does not, and why. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2003; Volume 78, pp. 603–612. [Google Scholar] [CrossRef]
- Hales, J.R.S.; Hubbard, R.W.; Gaffin, S.L. Limitation of Heat Tolerance. In Comprehensive Physiology; Wiley, American Physiological Society: Hoboken, NJ, USA, 2011; pp. 285–355. [Google Scholar]
- ACGIH. Heat Stress and Strain. Available online: https://www.acgih.org/heat-stress-and-strain-2/ (accessed on 2 January 2023).
- Notley, S.R.; Flouris, A.D.; Kenny, G.P. On the use of wearable physiological monitors to assess heat strain during occupational heat stress. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2018, 43, 869–881. [Google Scholar] [CrossRef]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 21. [Google Scholar] [CrossRef]
- Relf, R.; Eichhorn, G.; Waldock, K.; Flint, M.S.; Beale, L.; Maxwell, N. Validity of a wearable sweat rate monitor and routine sweat analysis techniques using heat acclimation. J. Therm. Biol. 2020, 90, 102577. [Google Scholar] [CrossRef]
- Relf, R.; Willmott, A.; Flint, M.S.; Beale, L.; Maxwell, N. Reliability of a wearable sweat rate monitor and routine sweat analysis techniques under heat stress in females. J. Therm. Biol. 2019, 79, 209–217. [Google Scholar] [CrossRef]
- Kalasin, S.; Sangnuang, P.; Surareungchai, W. Satellite-Based Sensor for Environmental Heat-Stress Sweat Creatinine Monitoring: The Remote Artificial Intelligence-Assisted Epidermal Wearable Sensing for Health Evaluation. ACS Biomater. Sci. Eng. 2021, 7, 322–334. [Google Scholar] [CrossRef]
- Moyen, N.E.; Bapat, R.C.; Tan, B.; Hunt, L.A.; Jay, O.; Mundel, T. Accuracy of Algorithm to Non-Invasively Predict Core Body Temperature Using the Kenzen Wearable Device. Int. J. Environ. Res. Public Health 2021, 18, 13126. [Google Scholar] [CrossRef]
- Mundt, C.W.; Montgomery, K.; Udoh, U.E.; Barker, V.N.; Thonier, G.C.; Tellier, A.M.; Ricks, R.D.; Darling, R.B.; Cagle, Y.D.; Cabrol, N.A.; et al. A multiparameter wearable physiologic monitoring system for space and terrestrial applications. IEEE Trans. Inf. Technol. Biomed. 2005, 9, 382–391. [Google Scholar] [CrossRef]
- Pandian, P.S.; Mohanavelu, K.; Safeer, K.P.; Kotresh, T.M.; Shakunthala, D.T.; Gopal, P.; Padaki, V.C. Smart Vest: Wearable multi-parameter remote physiological monitoring system. Med. Eng. Phys. 2008, 30, 466–477. [Google Scholar] [CrossRef]
- Appelboom, G.; Camacho, E.; Abraham, M.E.; Bruce, S.S.; Dumont, E.L.P.; Zacharia, B.E.; D’Amico, R.; Slomian, J.; Reginster, J.Y.; Bruyère, O.; et al. Smart wearable body sensors for patient self-assessment and monitoring. Arch. Public Health 2014, 72, 28. [Google Scholar] [CrossRef]
- Srinivasa, M.G.; Pandian, P.S. Wireless wearable remote physiological signals monitoring system. In Proceedings of the 2016 International Conference on Circuits, Controls, Communications and Computing (I4C), Bangalore, India, 4–6 October 2016; pp. 1–5. [Google Scholar]
- Majumder, S.; Mondal, T.; Deen, M.J. Wearable Sensors for Remote Health Monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef]
- Soon, S.; Svavarsdottir, H.; Downey, C.; Jayne, D.G. Wearable devices for remote vital signs monitoring in the outpatient setting: An overview of the field. BMJ Innov. 2020, 6, 55–71. [Google Scholar] [CrossRef]
- Kamisalic, A.; Fister, I., Jr.; Turkanovic, M.; Karakatic, S. Sensors and Functionalities of Non-Invasive Wrist-Wearable Devices: A Review. Sensors 2018, 18, 1714. [Google Scholar] [CrossRef]
- Matsunaga, D.; Tanaka, Y.; Seyama, M.; Nagashima, K. Non-invasive and wearable thermometer for continuous monitoring of core body temperature under various convective conditions. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 4377–4380. [Google Scholar]
- Shan, C.; Hu, J.; Zou, J.; Zhang, A. Wearable Personal Core Body Temperature Measurement Considering Individual Differences and Dynamic Tissue Blood Perfusion. IEEE J. Biomed. Health Inform. 2022, 26, 2158–2168. [Google Scholar] [CrossRef]
- Haines, W.; Momenroodaki, P.; Berry, E.; Fromandi, M.; Popovic, Z. Wireless system for continuous monitoring of core body temperature. In Proceedings of the 2017 IEEE MTT-S International Microwave Symposium (IMS), Honolulu, HI, USA, 4–9 June 2017; pp. 541–543. [Google Scholar]
- Gilgen-Ammann, R.; Schweizer, T.; Wyss, T. RR interval signal quality of a heart rate monitor and an ECG Holter at rest and during exercise. Eur. J. Appl. Physiol. 2019, 119, 1525–1532. [Google Scholar] [CrossRef]
- Etiwy, M.; Akhrass, Z.; Gillinov, L.; Alashi, A.; Wang, R.; Blackburn, G.; Gillinov, S.M.; Phelan, D.; Gillinov, A.M.; Houghtaling, P.L.; et al. Accuracy of wearable heart rate monitors in cardiac rehabilitation. Cardiovasc. Diagn. Ther. 2019, 9, 262–271. [Google Scholar] [CrossRef]
- Schaffarczyk, M.; Rogers, B.; Reer, R.; Gronwald, T. Validity of the Polar H10 Sensor for Heart Rate Variability Analysis during Resting State and Incremental Exercise in. Recreational Men and Women. Sensors 2022, 22, 6536. [Google Scholar] [CrossRef]
- SlateSafety. Real-Time Connected Worker Safety. Available online: https://slatesafety.com/industrial/ (accessed on 3 January 2023).
- Belval, L.N.; Giersch, G.E.W.; Adams, W.M.; Hosokawa, Y.; Jardine, J.F.; Katch, R.K.; Stearns, R.L.; Casa, D.J. Age- and Sex-Based Differences in Exertional Heat Stroke Incidence in a 7-Mile Road Race. J. Athl. Train. 2020, 55, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Iyoho, A.E.; Ng, L.J.; MacFadden, L. Modeling of Gender Differences in Thermoregulation. Mil. Med. 2017, 182, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kronsberg, S.; Bouret, J.R.; Brett, A.L. Lived experiences of male nurses: Dire consequences for the nursing profession. J. Nurs. Educ. Pract. 2018, 8, 46–53. [Google Scholar] [CrossRef]
- Health, National Institute of Occupational Health and Safety Acclimatization. Available online: https://www.cdc.gov/niosh/topics/heatstress/acclima.html#:~:text=These%20physiological%20adaptations%20include%3A,core%20temperature%20and%20heart%20rate (accessed on 21 November 2022).
- Periard, J.D.; Travers, G.J.S.; Racinais, S.; Sawka, M.N. Cardiovascular adaptations supporting human exercise-heat acclimation. Auton. Neurosci. 2016, 196, 52–62. [Google Scholar] [CrossRef]
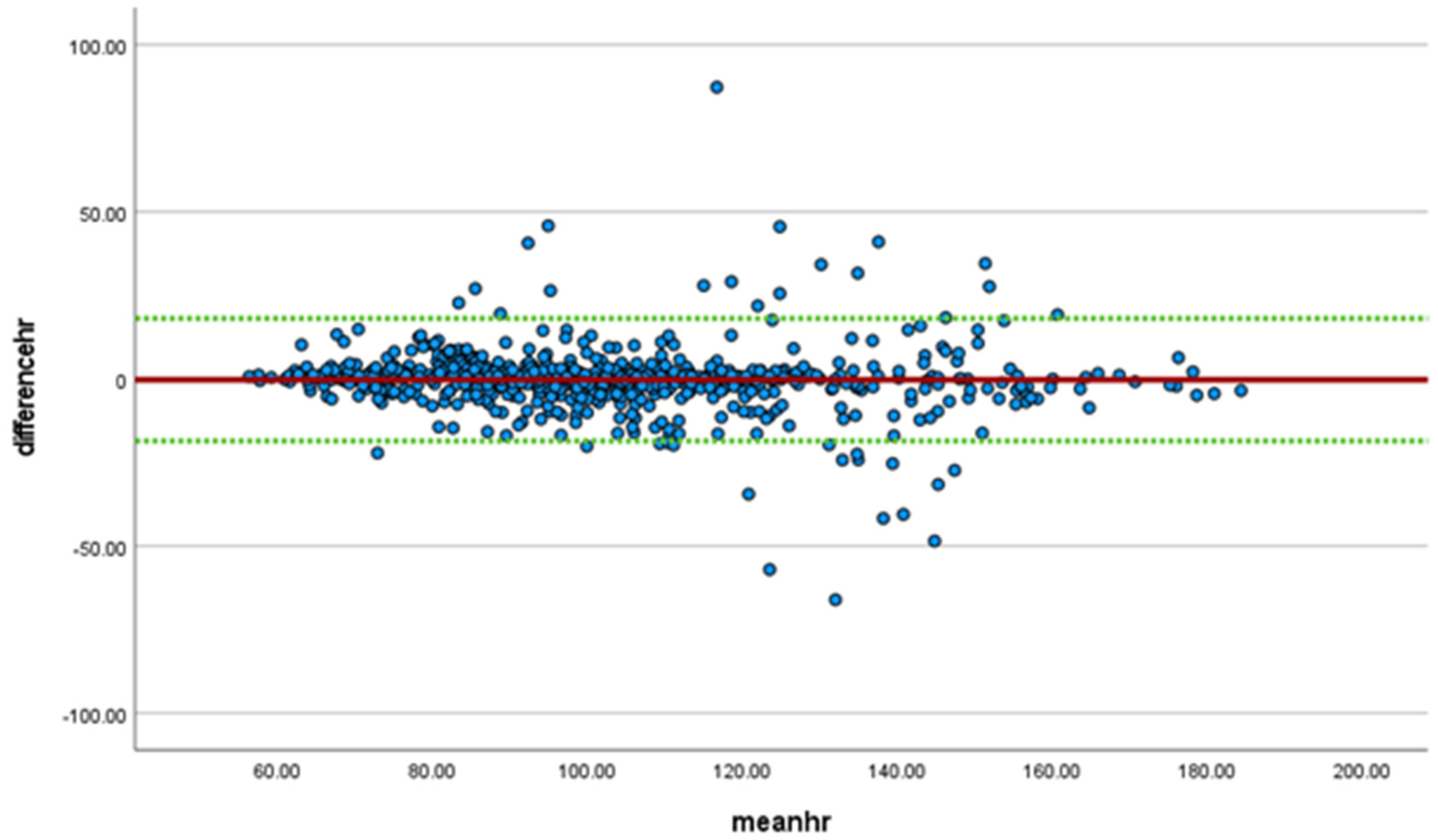
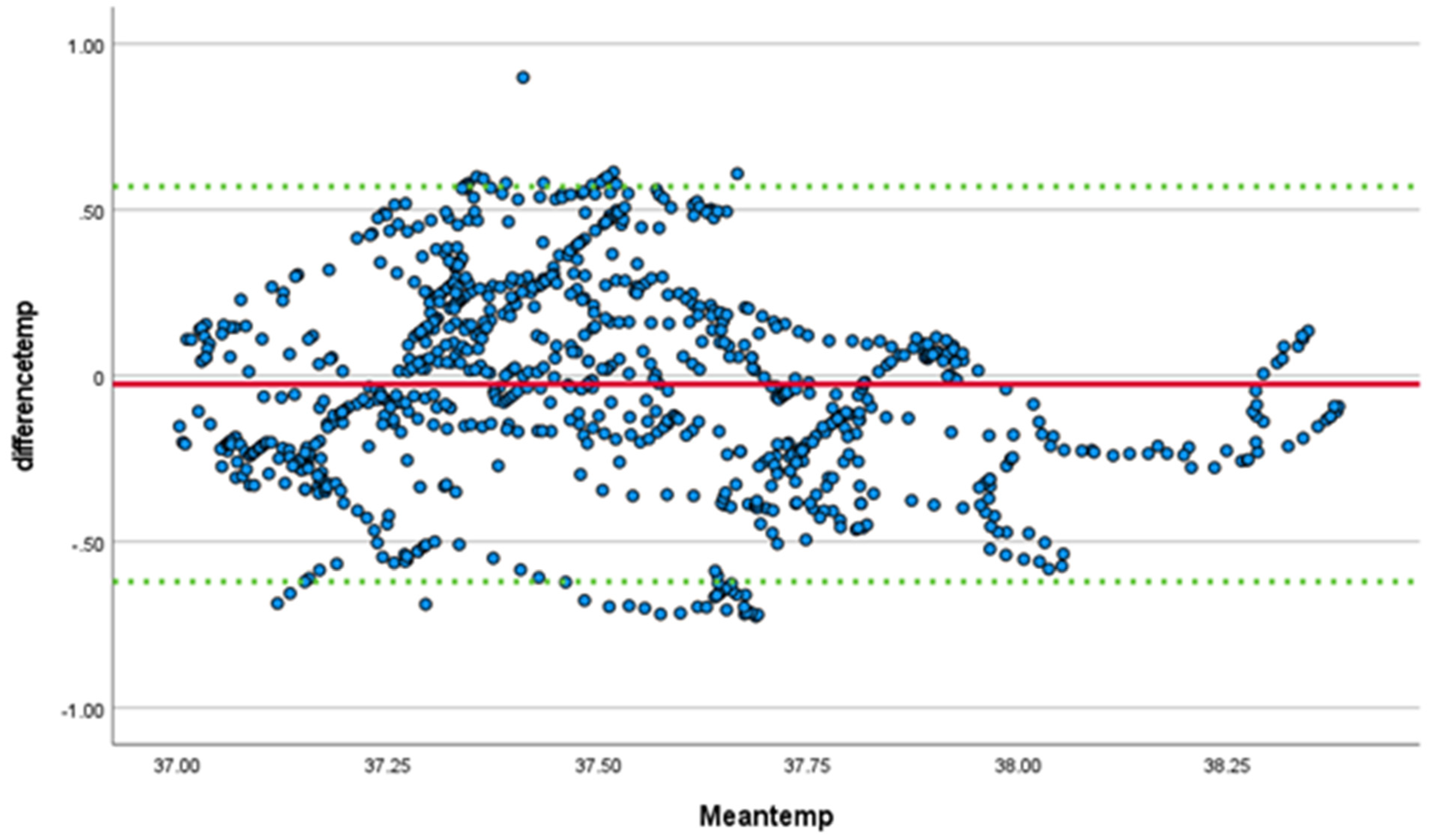
| Part ID | Temp | Sex | Age | Weight in | Weight out |
|---|---|---|---|---|---|
| 40 | mod | F | 31 | 378.4 | 379.2 |
| 29 | mod | F | 21 | 159 | 160.2 |
| 52 | mod | F | 22 | 173 | 173 |
| 58 | mod | F | 23 | 146.2 | 146.6 |
| 89 | mod | M | 27 | 197.8 | 197.6 |
| 43 | Mod | M | 46 | 203.6 | 206.6 |
| 59 | mod | F | 39 | 178.4 | 179.8 |
| 61 | mod | F | 34 | 160.6 | 162.2 |
| 91 | mod | M | 28 | 206 | 206.4 |
| 62 | mod | F | 56 | 136 | 136 |
| 41 | hot | F | 22 | 136.8 | 136.6 |
| 93 | hot | F | 37 | 175.8 | 175 |
| 87 | hot | F | 38 | 198.4 | 198 |
| 64 | hot | F | 37 | 143.8 | 144.2 |
| 92 | hot | F | 23 | 168.8 | 169 |
| 84 | hot | F | 38 | 127.4 | 127.8 |
| 42 | hot | F | 42 | 139.2 | 139.4 |
| 75 | hot | F | 22 | 126 | 125.6 |
| 13 | hot | F | 22 | 188.8 | 189 |
| 28 | hot | F | 21 | 153.6 | 153.8 |
| Average | 31.45 | 174.88 | 175.3 | ||
| User | Peak Pill | Avg Pill | Peak SS | Avg SS | HR Max SS | HR Avg SS | HR Max P | HR Avg P |
|---|---|---|---|---|---|---|---|---|
| 40 | 38.03 | 37.71 | 156 | 111.6 | - | - | ||
| 29 | 37.28 | 37.265 | 37.59 | 37.41 | 165 | 93.48 | - | - |
| 52 | 37.73 | 37.49 | 37.41 | 37.05 | 133 | 77 | 135.5 | 81.1 |
| 58 | 37.97 | 37.92 | 37.62 | 37.17 | 142 | 84.25 | 138 | 85.2 |
| 89 | 37.28 | 37.15 | 37.37 | 37.15 | 116 | 82.8 | 113.8 | 81.5 |
| 43 | 37.59 | 37.45 | 37.63 | 37.11 | 138 | 102.4 | 135.6 | 101.2 |
| 59 | 37.9 | 37.75 | 37.41 | 37.22 | 111 | 86.5 | 111.8 | 87.3 |
| 61 | - | - | 37.21 | 37.01 | 102 | 75.6 | 101 | 75.1 |
| 91 | 37.05 | 36.98 | 37.32 | 37.23 | 105 | 84 | 90 | 87 |
| 62 | 37.35 | 37.13 | 38.05 | 37.67 | 158 | 113.3 | 156 | 113.3 |
| 28 | 37.87 | 37.51 | 38.34 | 37.78 | 177 | 116.4 | 174.8 | 116 |
| 41 | 37.74 | 37.5 | 37.94 | 37.48 | 169 | 110 | 165.6 | 109 |
| 93 | 38.41 | 37.96 | 38.4 | 38.02 | 169 | 124 | 168.6 | 124.1 |
| 87 | 37.32 | 37.15 | 37.55 | 37.42 | 112 | 95.1 | - | - |
| 64 | 37.58 | 37.43 | 37.44 | 37.21 | 122 | 84.73 | - | - |
| 92 | 37.97 | 37.7 | 37.93 | 37.6 | 132 | 109.6 | 131.8 | 110 |
| 84 | 37.79 | 37.35 | 37.66 | 37.38 | 119 | 100.4 | 115.9 | 99.5 |
| 42 | 37.76 | 37.62 | 38.01 | 37.52 | 159 | 106.8 | 154.9 | 107.5 |
| 75 | 37.72 | 37.55 | 38.04 | 37.73 | 146 | 111.9 | 141.3 | 110 |
| 13 | 38.34 | 37.83 | 38.43 | 37.83 | 186 | 126.3 | 182.5 | 126.6 |
| Overall | 37.70 | 37.49 | 37.75 | 37.45 | 140.85 | 99.81 | 138.57 | 100.9 |
| HR Participant | Pearson Correlation | SIG | ci | Percent Different Peak | Percent Different Average | ||
|---|---|---|---|---|---|---|---|
| 40 | No available data | ||||||
| 29 | No available data | ||||||
| 52 | 0.945 | <0.001 | 0.907–0.967 | 1.84 | 5.06 | ||
| 58 | No available data | ||||||
| 89 | 0.953 | <0.001 | 0.915–0.973 | −1.93 | −1.60 | ||
| 43 | 0.967 | <0.001 | 0.928–0.984 | −1.77 | −1.19 | ||
| 59 | 0.788 | <0.001 | 0.636–0.877 | 0.72 | 0.91 | ||
| 61 | 0.942 | <0.001 | 0.894–0.967 | −0.99 | −0.67 | ||
| 91 | 0.332 | 0.012 | 0.073–0.545 | −16.7 | 3.44 | ||
| 62 | 0.992 | <0.001 | 0.986–0.995 | −1.28 | 0 | ||
| 28 | 0.991 | <0.001 | 0.985–0.994 | −1.26 | −0.34 | ||
| 41 | 0.698 | <0.001 | 0.534–0.807 | −2.05 | −0.92 | ||
| 93 | 0.36 | 0.008 | 0.098–0.570 | −0.24 | 0.08 | ||
| 87 | No available data | ||||||
| 64 | No available data | ||||||
| 92 | 0.974 | <0.001 | 0.949–0.986 | −0.15 | 0.36 | ||
| 84 | 0.966 | <0.001 | 0.935–0.981 | −2.67 | −0.90 | ||
| 42 | 0.915 | <0.001 | 0.857–0.949 | −2.65 | 0.65 | ||
| 75 | 0.907 | <0.001 | 0.833–0.947 | −3.33 | −1.73 | ||
| 13 | 0.982 | <0.001 | 0.968–0.989 | −1.92 | 0.24 | ||
| overall | 0.926 | <0.001 | 0.915–0.935 | 0.961 (p < 0.001) | 0.955–0.966 | −1.65 | 1.08 |
| Temperature Participant | Pearson Correlation | SIG | ci | ICC | Percent Difference Peak | Percent Difference Average | |
|---|---|---|---|---|---|---|---|
| 40 | No data available | ||||||
| 29 | Two Data points only | −0.83 | −0.39 | ||||
| 52 | Limited Data | 0.85 | 1.17 | ||||
| 58 | 1.00 | <0.001 | 2 POINTS ONLY | 0.92 | 1.98 | ||
| 89 | 0.821 | <0.001 | 0.702–0.892 | −0.24 | 0 | ||
| 43 | 0.497 | <0.001 | 0.321–0.637 | −0.11 | 0.91 | ||
| 59 | 0.922 | <0.001 | 0.874–0.951 | 1.29 | 1.4 | ||
| 61 | No data available | ||||||
| 91 | 0.747 | <0.001 | 0.610–0.837 | −0.73 | −0.68 | ||
| 62 | 0.945 | <0.001 | 0.912–0.965 | −1.87 | −1.45 | ||
| 28 | 0.96 | <0.001 | 0.936–0.974 | −1.24 | −0.72 | ||
| 41 | 0.6 | <0.001 | 0.447–0.717 | −0.53 | 0.05 | ||
| 93 | 0.835 | <0.001 | 0.748–0.891 | 0.03 | −0.16 | ||
| 87 | 0.956 | <0.001 | 0.854–0.985 | −0.62 | −0.73 | ||
| 64 | 0.186 | 0.725 | −0.745–0.862 Limited data | 0.37 | 0.59 | ||
| 92 | 0.961 | <0.001 | 0.932–0.977 | 0.11 | 0.27 | ||
| 84 | 0.126 | 0.295 | −0.111–0.348 | 0.34 | −0.08 | ||
| 42 | 0.115 | 0.351 | −0.128–0.343 | −0.66 | 0.27 | ||
| 75 | 0.486 | <0.001 | 0.239–0.669 | −0.85 | −0.48 | ||
| 13 | 0.903 | <0.001 | 0.846–0.938 | −0.23 | 0 | ||
| overall | 0.594 | <0.001 | 0.550–0.635 | 0.742 (p < 0.001) | 0.705–0.774 | −0.13 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callihan, M.; Cole, H.; Stokley, H.; Gunter, J.; Clamp, K.; Martin, A.; Doherty, H. Comparison of Slate Safety Wearable Device to Ingestible Pill and Wearable Heart Rate Monitor. Sensors 2023, 23, 877. https://doi.org/10.3390/s23020877
Callihan M, Cole H, Stokley H, Gunter J, Clamp K, Martin A, Doherty H. Comparison of Slate Safety Wearable Device to Ingestible Pill and Wearable Heart Rate Monitor. Sensors. 2023; 23(2):877. https://doi.org/10.3390/s23020877
Chicago/Turabian StyleCallihan, Michael, Heather Cole, Holly Stokley, Joshua Gunter, Kaitlyn Clamp, Alexis Martin, and Hannah Doherty. 2023. "Comparison of Slate Safety Wearable Device to Ingestible Pill and Wearable Heart Rate Monitor" Sensors 23, no. 2: 877. https://doi.org/10.3390/s23020877
APA StyleCallihan, M., Cole, H., Stokley, H., Gunter, J., Clamp, K., Martin, A., & Doherty, H. (2023). Comparison of Slate Safety Wearable Device to Ingestible Pill and Wearable Heart Rate Monitor. Sensors, 23(2), 877. https://doi.org/10.3390/s23020877






