Wearable System Based on Ultra-Thin Parylene C Tattoo Electrodes for EEG Recording
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aroganam, G.; Manivannan, N.; Harrison, D. Review on Wearable Technology Sensors Used in Consumer Sport Applications. Sensors 2019, 19, 1983. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, X.; Cao, T.; Wan, F.; Mak, P.U.; Mak, P.I.; Vai, M.I. Implementation of SSVEP Based BCI with Emotiv EPOC. In Proceedings of the IEEE International Conference on Virtual Environments, Human-Computer Interfaces, and Measurement Systems, VECIMS, Tianjin, China, 2–4 July 2012; IEEE Computer Society: Washington, DC, USA, 2012; pp. 34–37. [Google Scholar] [CrossRef]
- Williams, N.S.; Mcarthur, G.M.; Badcock, N.A. 10 Years of EPOC: A Scoping Review of Emotiv’s Portable EEG Device. BioRxiv 2020. [Google Scholar] [CrossRef]
- Krigolson, O.E.; Williams, C.C.; Norton, A.; Hassall, C.D.; Colino, F.L. Choosing MUSE: Validation of a Low-Cost, Portable EEG System for ERP Research. Front. Neurosci. 2017, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Baltatzis, V.; Bintsi, K.M.; Apostolidis, G.K.; Hadjileontiadis, L.J. Bullying Incidences Identification within an Immersive Environment Using HD EEG-Based Analysis: A Swarm Decomposition and Deep Learning Approach. Sci. Rep. 2017, 7, 17292. [Google Scholar] [CrossRef]
- Hehenberger, L.; Kobler, R.J.; Lopes-Dias, C.; Srisrisawang, N.; Tumfart, P.; Uroko, J.B.; Torke, P.R.; Müller-Putz, G.R. Long-Term Mutual Training for the CYBATHLON BCI Race with a Tetraplegic Pilot: A Case Study on Inter-Session Transfer and Intra-Session Adaptation. Front. Hum. Neurosci. 2021, 15, 635777. [Google Scholar] [CrossRef]
- Qing, C.; Qiao, R.; Xu, X.; Cheng, Y. Interpretable Emotion Recognition Using EEG Signals. IEEE Access 2019, 7, 94160–94170. [Google Scholar] [CrossRef]
- Katsigiannis, S.; Ramzan, N. DREAMER: A Database for Emotion Recognition Through EEG and ECG Signals from Wireless Low-Cost Off-the-Shelf Devices. IEEE J. Biomed. Health Inform. 2018, 22, 98–107. [Google Scholar] [CrossRef]
- Song, T.; Zheng, W.; Song, P.; Cui, Z. EEG Emotion Recognition Using Dynamical Graph Convolutional Neural Networks. IEEE Trans. Affect. Comput. 2020, 11, 532–541. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, Z.; Chen, P.; Nichele, S. Emotion Recognition Using Multi-Modal Data and Machine Learning Techniques: A Tutorial and Review. Inf. Fusion 2020, 59, 103–126. [Google Scholar] [CrossRef]
- Seeck, M.; Koessler, L.; Bast, T.; Leijten, F.; Michel, C.; Baumgartner, C.; He, B.; Beniczky, S. The Standardized EEG Electrode Array of the IFCN. Clin. Neurophysiol. 2017, 128, 2070–2077. [Google Scholar] [CrossRef]
- Goverdovsky, V.; Looney, D.; Kidmose, P.; Mandic, D.P. In-Ear EEG from Viscoelastic Generic Earpieces: Robust and Unobtrusive 24/7 Monitoring. IEEE Sens. J. 2016, 16, 271–277. [Google Scholar] [CrossRef]
- Debener, S.; Emkes, R.; de Vos, M.; Bleichner, M. Unobtrusive Ambulatory EEG Using a Smartphone and Flexible Printed Electrodes around the Ear. Sci. Rep. 2015, 5, 16743. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.B.; Kappel, S.L.; Mandic, D.P.; Kidmose, P. EEG Recorded from the Ear: Characterizing the Ear-EEG Method. Front. Neurosci. 2015, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- Bleichner, M.G.; Debener, S. Concealed, Unobtrusive Ear-Centered EEG Acquisition: Ceegrids for Transparent EEG. Front. Hum. Neurosci. 2017, 11, 438. [Google Scholar] [CrossRef]
- Acar, G.; Ozturk, O.; Golparvar, A.J.; Elboshra, T.A.; Böhringer, K.; Kaya Yapici, M. Wearable and Flexible Textile Electrodes for Biopotential Signal Monitoring: A Review. Electronics 2019, 8, 479. [Google Scholar] [CrossRef]
- Casson, A.J. Wearable EEG and Beyond. Biomed. Eng. Lett. 2019, 9, 53–71. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal Electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef]
- Stauffer, F.; Thielen, M.; Sauter, C.; Chardonnens, S.; Bachmann, S.; Tybrandt, K.; Peters, C.; Hierold, C.; Vörös, J. Skin Conformal Polymer Electrodes for Clinical ECG and EEG Recordings. Adv. Healthc. Mater. 2018, 7, e1700994. [Google Scholar] [CrossRef]
- Chi, Y.M.; Jung, T.P.; Cauwenberghs, G. Dry-Contact and Noncontact Biopotential Electrodes: Methodological Review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119. [Google Scholar] [CrossRef]
- Yao, S.; Zhu, Y. Nanomaterial-Enabled Dry Electrodes for Electrophysiological Sensing: A Review. JOM 2016, 68, 1145–1155. [Google Scholar] [CrossRef]
- Spanu, A.; Taki, M.; Baldazzi, G.; Mascia, A.; Cosseddu, P.; Pani, D.; Bonfiglio, A. Epidermal Electrodes with Ferrimagnetic/Conductive Properties for Biopotential Recordings. Bioengineering 2022, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Pei, W.; Wang, Y.; Gong, Q.; Zhang, H.; Xing, X.; Xie, Y.; Gui, Q.; Chen, H. A Self-Wetting Paper Electrode for Ubiquitous Bio-Potential Monitoring. IEEE Sens. J. 2017, 17, 2654–2661. [Google Scholar] [CrossRef]
- Casson, A.J.; Saunders, R.; Batchelor, J.C. Five Day Attachment ECG Electrodes for Longitudinal Bio-Sensing Using Conformal Tattoo Substrates. IEEE Sens. J. 2017, 17, 2205–2214. [Google Scholar] [CrossRef]
- Bihar, E.; Roberts, T.; Zhang, Y.; Ismailova, E.; Hervé, T.; Malliaras, G.G.; de Graaf, J.B.; Inal, S.; Saadaoui, M. Fully Printed All-Polymer Tattoo/Textile Electronics for Electromyography. Flex. Print. Electron. 2018, 3, 034004. [Google Scholar] [CrossRef]
- Chandra, S.; Li, J.; Afsharipour, B.; Cardona, A.F.; Suresh, N.L.; Tian, L.; Deng, Y.; Zhong, Y.; Xie, Z.; Shen, H.; et al. Performance Evaluation of a Wearable Tattoo Electrode Suitable for High-Resolution Surface Electromyogram Recording. IEEE Trans. Biomed. Eng. 2021, 68, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Inzelberg, L.; Rand, D.; Steinberg, S.; David-Pur, M.; Hanein, Y. A Wearable High-Resolution Facial Electromyography for Long Term Recordings in Freely Behaving Humans. Sci. Rep. 2018, 8, 2058. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.J.S.; Lee, D.S.; Lee, J.W.; Lee, W.; Kwon, O.; Won, P.; Jung, S.Y.; Cheng, H.; Jeong, J.W.; Akce, A.; et al. Soft, Curved Electrode Systems Capable of Integration on the Auricle as a Persistent Brain-Computer Interface. Proc. Natl. Acad. Sci. USA 2015, 112, 3920–3925. [Google Scholar] [CrossRef]
- Ferrari, L.M.; Ismailov, U.; Badier, J.M.; Greco, F.; Ismailova, E. Conducting Polymer Tattoo Electrodes in Clinical Electro- and Magneto-Encephalography. NPJ Flex. Electron. 2020, 4, 4. [Google Scholar] [CrossRef]
- Peng, H.L.; Liu, J.Q.; Tian, H.C.; Dong, Y.Z.; Yang, B.; Chen, X.; Yang, C.S. A Novel Passive Electrode Based on Porous Ti for EEG Recording. Sens. Actuators B Chem. 2016, 226, 349–356. [Google Scholar] [CrossRef]
- Shustak, S.; Inzelberg, L.; Steinberg, S.; Rand, D.; Pur, M.D.; Hillel, I.; Katzav, S.; Fahoum, F.; de Vos, M.; Mirelman, A.; et al. Home Monitoring of Sleep with a Temporary-Tattoo EEG, EOG and EMG Electrode Array: A Feasibility Study. J. Neural Eng. 2019, 16, 026024. [Google Scholar] [CrossRef]
- Nawrocki, R.A.; Jin, H.; Lee, S.; Yokota, T.; Sekino, M.; Someya, T. Self-Adhesive and Ultra-Conformable, Sub-300 Nm Dry Thin-Film Electrodes for Surface Monitoring of Biopotentials. Adv. Funct. Mater. 2018, 28, 1803279. [Google Scholar] [CrossRef]
- Spanu, A.; Mascia, A.; Baldazzi, G.; Fenech-Salerno, B.; Torrisi, F.; Viola, G.; Bonfiglio, A.; Cosseddu, P.; Pani, D. Parylene C-Based, Breathable Tattoo Electrodes for High-Quality Bio-Potential Measurements. Front. Bioeng. Biotechnol. 2022, 10, 820217. [Google Scholar] [CrossRef]
- Ferree, T.C.; Luu, P.; Russel, G.S.; Tucker, D.M. Scalp electrode impedance, infection risk, and EEG data quality. Clin. Neurophysiol. 2001, 112, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Pani, D.; Dessi, A.; Saenz-Cogollo, J.F.; Barabino, G.; Fraboni, B.; Bonfiglio, A. Fully Textile, PEDOT:PSS Based Electrodes for Wearable ECG Monitoring Systems. IEEE Trans. Biomed. Eng. 2016, 63, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, S.; Li, M.; Duan, Y.Y. Towards Real-Life EEG Applications: Novel Superporous Hydrogel-Based Semi-Dry EEG Electrodes Enabling Automatically “charge-Discharge” Electrolyte. J. Neural Eng. 2021, 18, 046016. [Google Scholar] [CrossRef]
- Cannard, C.; Wahbeh, H.; Delorme, A. Validating the Wearable MUSE Headset for EEG Spectral Analysis and Frontal Alpha Asymmetry. In Proceedings of the 2021 IEEE International Conference on Bioinformatics and Biomedicine, BIBM 2021, Houston, TX, USA, 9–12 December 2021; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2021; pp. 3603–3610. [Google Scholar]
- Wilkinson, C.M.; Burrell, J.I.; Kuziek, J.W.P.; Thirunavukkarasu, S.; Buck, B.H.; Mathewson, K.E. Predicting Stroke Severity with a 3-Min Recording from the Muse Portable EEG System for Rapid Diagnosis of Stroke. Sci. Rep. 2020, 10, 18465. [Google Scholar] [CrossRef] [PubMed]
- Simar, C.; Mathieu, P.; Cebolla, A.; Leroy, A.; Bontempi, G.; Cheron, G. EEG-Based Brain-Computer Interface for Alpha Speed Control of a Small Robot Using the MUSE Headband. In Proceedings of the 2020 International Joint Conference on Neural Networks (IJCNN), Glasgow, UK, 19–24 July 2020; pp. 1–4. [Google Scholar]
- Malmivuo, J. Bioelectromagnetism—Principles and Applications of Bioelectric and Biomagnetic Fields—The Internet Version; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaraswamy, S.D. High-Frequency Brain Activity and Muscle Artifacts in MEG/EEG: A Review and Recommendations. Front. Hum. Neurosci. 2013, 7, 138. [Google Scholar] [CrossRef]
- Fraschini, M.; Demuru, M.; Crobe, A.; Marrosu, F.; Stam, C.J.; Hillebrand, A. The Effect of Epoch Length on Estimated EEG Functional Connectivity and Brain Network Organisation. J. Neural Eng. 2016, 13, 036015. [Google Scholar] [CrossRef] [PubMed]
- Pani, S.M.; Ciuffi, M.; Demuru, M.; la Cava, S.M.; Bazzano, G.; D’Aloja, E.; Fraschini, M. Subject, Session and Task Effects on Power, Connectivity and Network Centrality: A Source-Based EEG Study. Biomed. Signal Process. Control 2020, 59, 101891. [Google Scholar] [CrossRef]
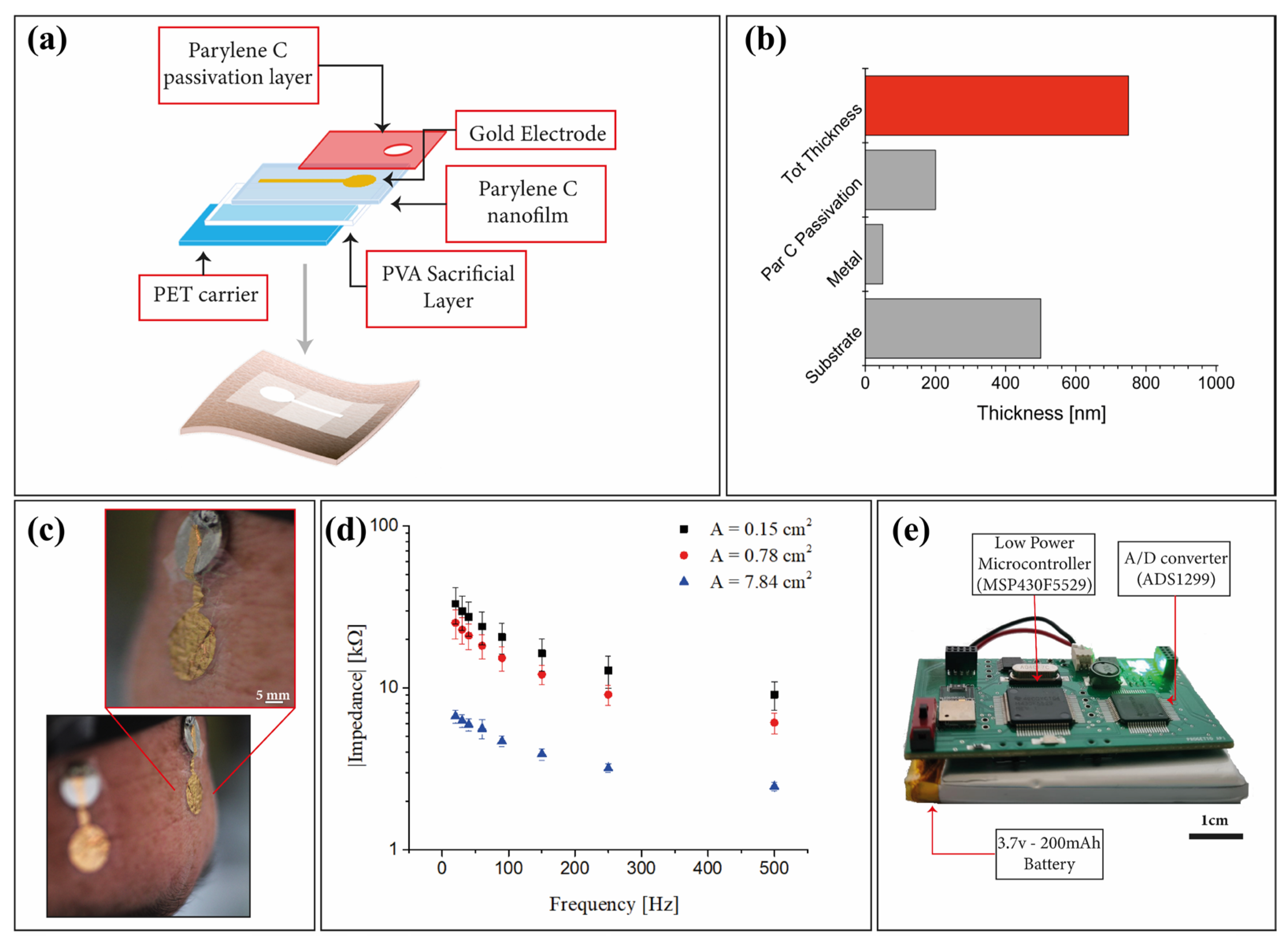
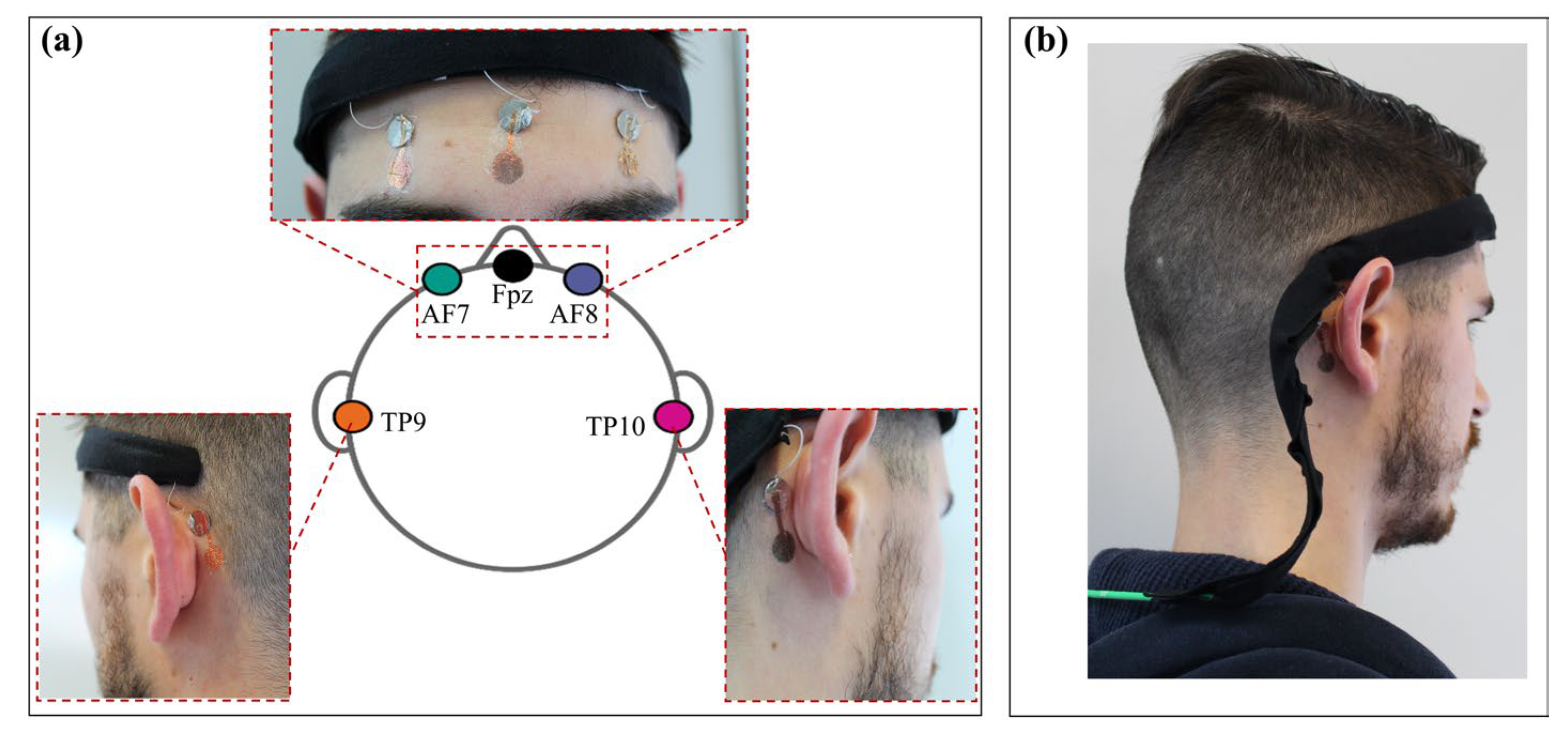


| EEG Recording Systems | N° of Recording Channels | Electrodes Placement | Brain Wave Detected/ERPs | Portability/Wearable Acquisition System | Electrodes | Electrodes Thickness [µm] |
|---|---|---|---|---|---|---|
| Our Work | 4 | AF7, Fpz, AF8, TP9, TP10 | Alpha and Beta | Custom portable PCB | Tattoo dry electrodes | 0.7–0.8 |
| V. Goverdovsky et al. [12] | 1 | Ear | SSVEP ASSR | Bench amplifier | Cloth electrode | // |
| S. Debener et al. [13] | 16 | Ear | Alpha, Beta and ERPSs | SMARTING (mBraintrain) mobile EEG amplifier | Flexible printed electrodes | // |
| J. J. S. Norton et al. [28] | 1 | Ear | P300 ERP | Bench amplifier | Tattoo dry electrodes | 3 |
| L. M. Ferrari et al. [29] | 1 | CZ-OZ T7-CZ | Alpha N100 | Bench amplifier | Tattoo dry electrodes | 1.5 |
| H. L. Peng et al. [30] | 1 | FP1 | N100 | Bench amplifier | Dry electrodes | 1200 |
| S.Shustak et al. [31] | 4 | F7, F8 | Alpha, k-complexes and spindles | portable PCB | Dry electrodes | // |
| Wilcoxon Signed Rank Test | |||||
|---|---|---|---|---|---|
| Measure 1 | Measure 2 | W | z | p | Rank-Biserial Correlation |
| Tattoo-EC | Tattoo-EO | 33.000 | −2.286 | 0.021 | −0.614 |
| Muse-EC | Muse-EO | 5.000 | −3.385 | <0.001 | −0.935 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mascia, A.; Collu, R.; Spanu, A.; Fraschini, M.; Barbaro, M.; Cosseddu, P. Wearable System Based on Ultra-Thin Parylene C Tattoo Electrodes for EEG Recording. Sensors 2023, 23, 766. https://doi.org/10.3390/s23020766
Mascia A, Collu R, Spanu A, Fraschini M, Barbaro M, Cosseddu P. Wearable System Based on Ultra-Thin Parylene C Tattoo Electrodes for EEG Recording. Sensors. 2023; 23(2):766. https://doi.org/10.3390/s23020766
Chicago/Turabian StyleMascia, Antonello, Riccardo Collu, Andrea Spanu, Matteo Fraschini, Massimo Barbaro, and Piero Cosseddu. 2023. "Wearable System Based on Ultra-Thin Parylene C Tattoo Electrodes for EEG Recording" Sensors 23, no. 2: 766. https://doi.org/10.3390/s23020766
APA StyleMascia, A., Collu, R., Spanu, A., Fraschini, M., Barbaro, M., & Cosseddu, P. (2023). Wearable System Based on Ultra-Thin Parylene C Tattoo Electrodes for EEG Recording. Sensors, 23(2), 766. https://doi.org/10.3390/s23020766









