Validating a Consumer Smartwatch for Nocturnal Respiratory Rate Measurements in Sleep Monitoring
Abstract
:1. Introduction
2. Methods
2.1. Device under Test
2.2. Participants and Study Procedure
2.3. Signal Processing Method
2.4. Validation Analysis
3. Results
3.1. Participants
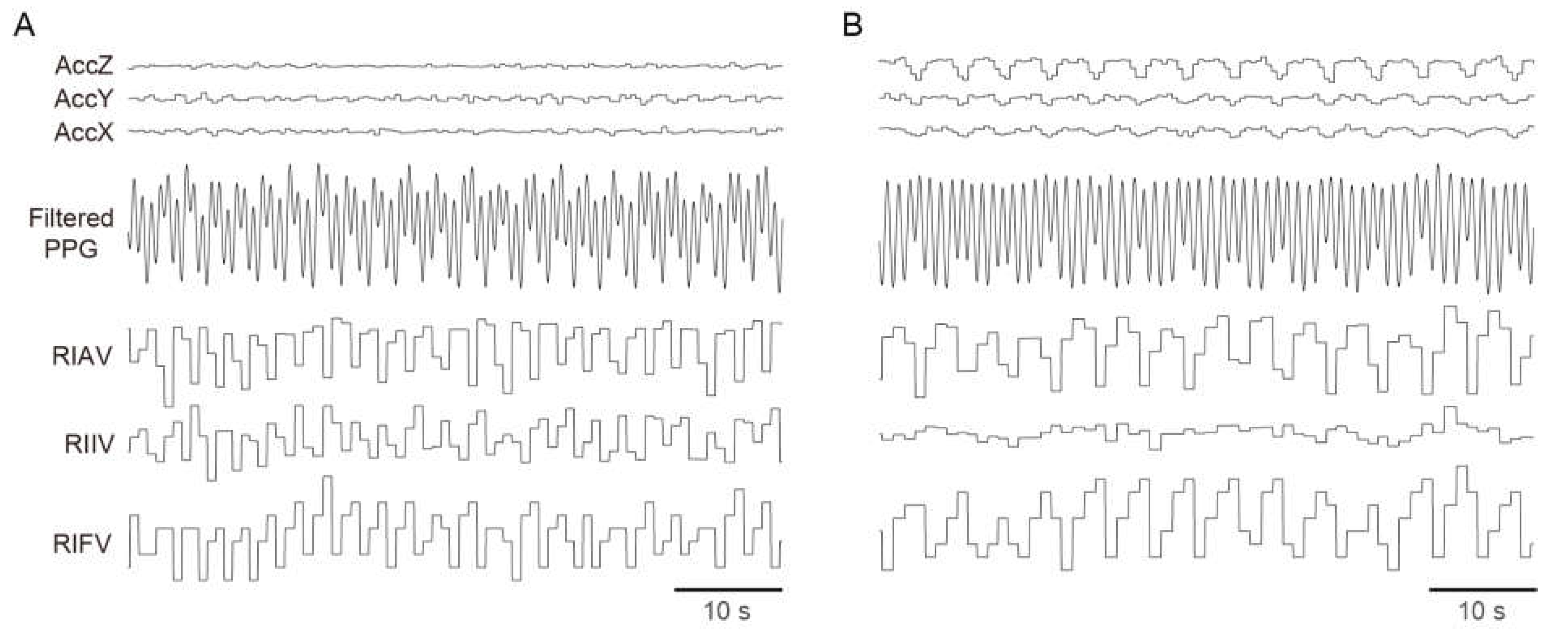
3.2. Breathing Signals Captured by a Wrist-Worn Device
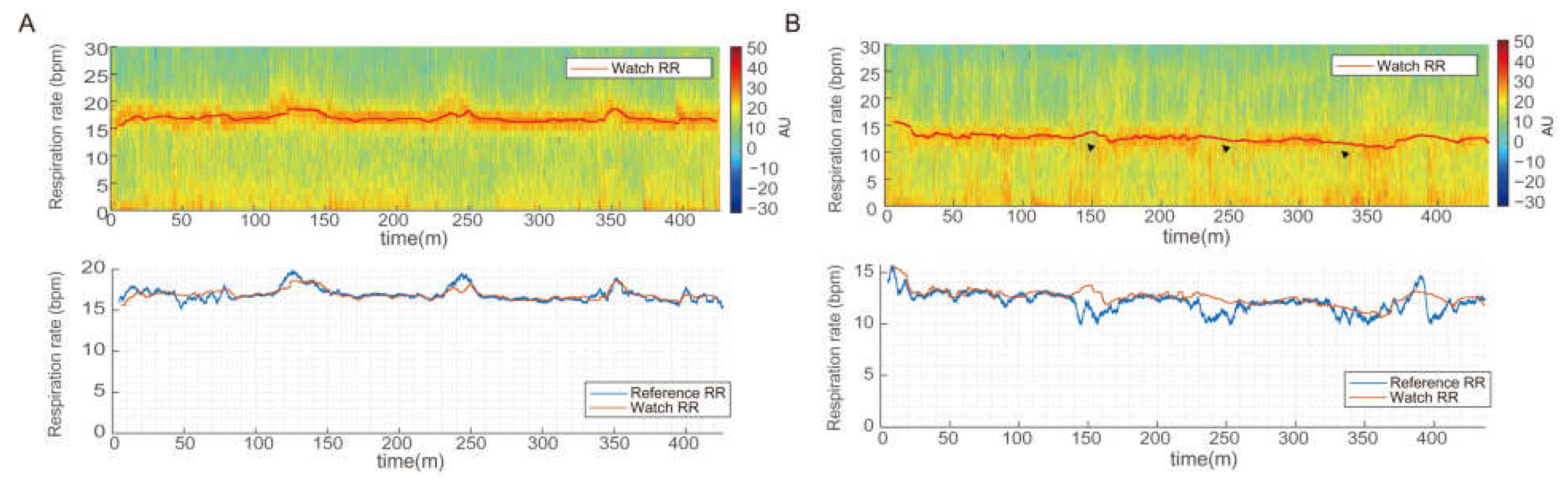
3.3. Respiratory Rate Measurements during Sleep
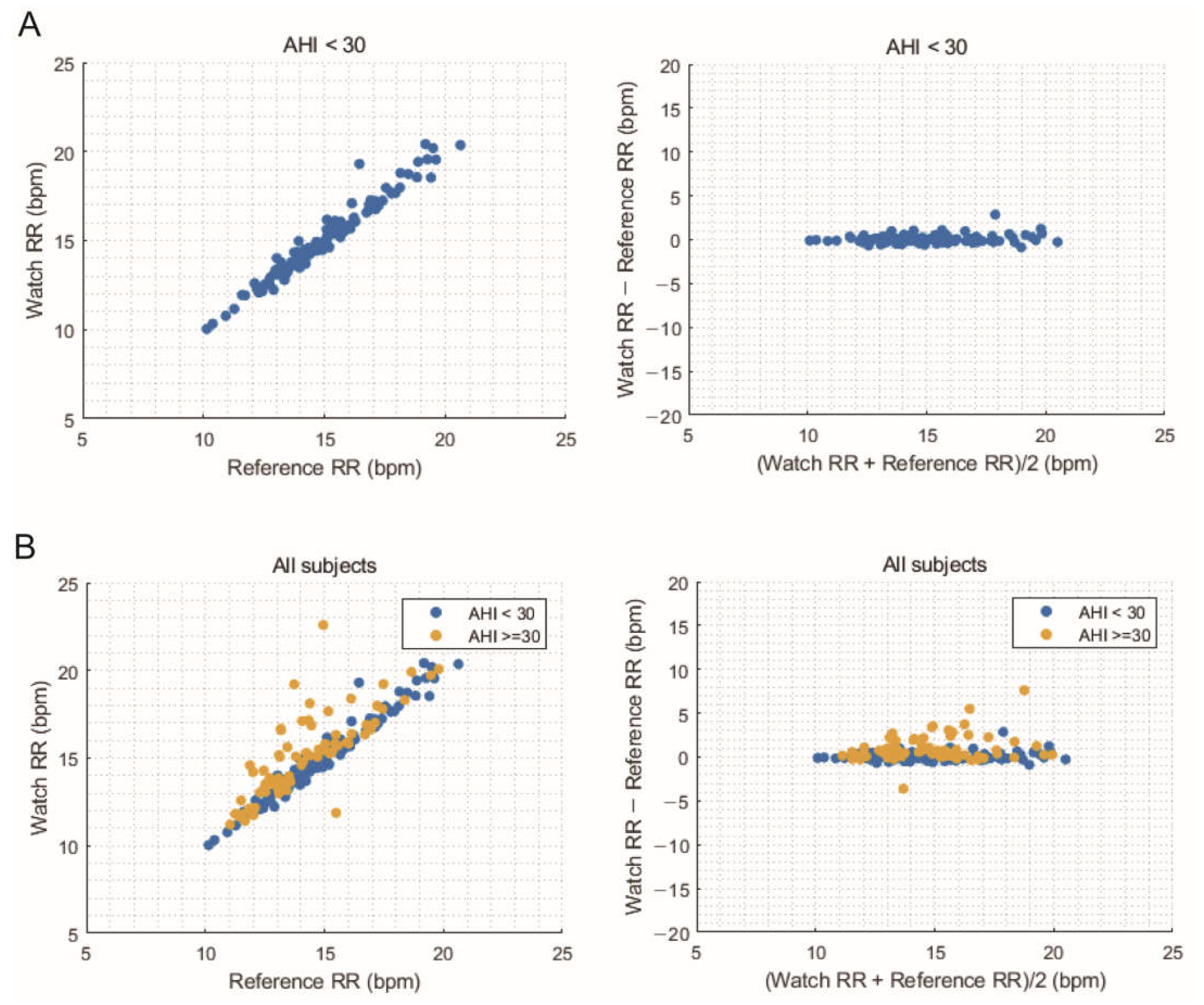
3.4. Comparison of Average Overnight Respiratory Rate
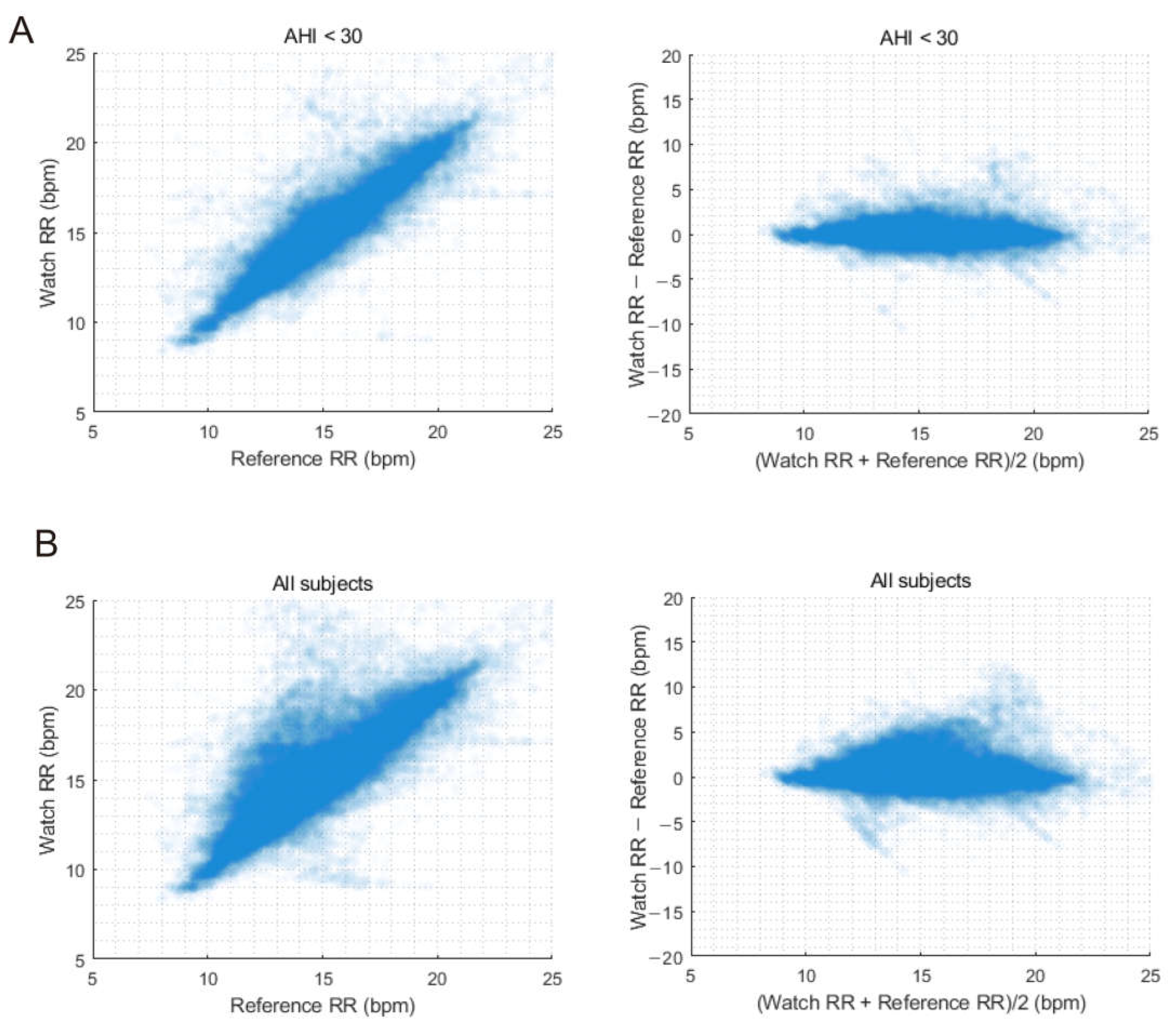
3.5. Comparison of Continuous Respiratory Rates
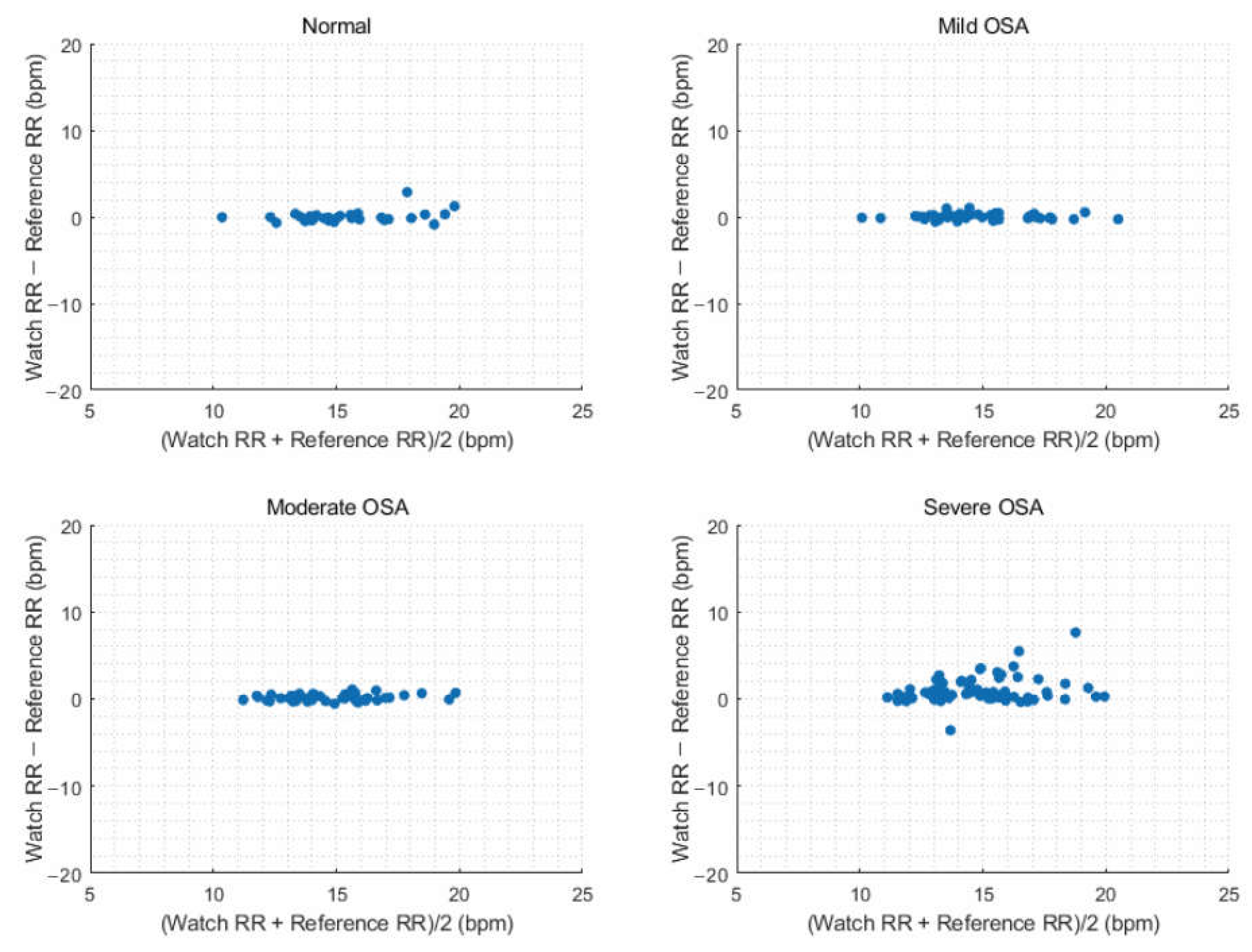
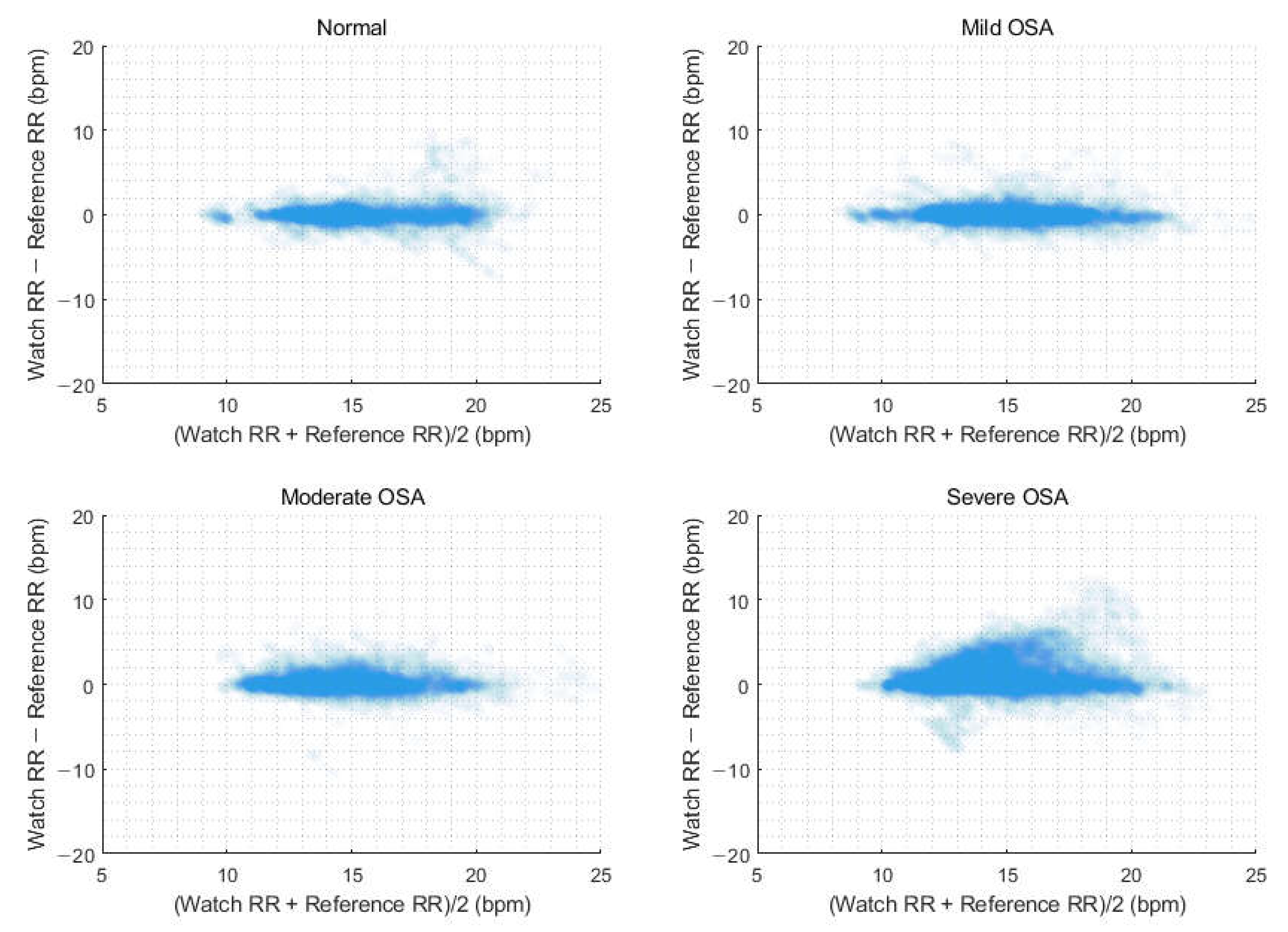
3.6. Estimation of Respiratory Rate According to Obstructive Sleep Apnea Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chung, G.S.; Choi, B.H.; Lee, J.-S.; Lee, J.S.; Jeong, D.-U.; Park, K.W.S. REM sleep estimation only using respiratory dynamics. Physiol. Meas. 2009, 30, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, G.; Williams, J.; Alrehaili, G.A.; McLean, A.; Pirouz, R.; Amdur, R.; Jain, V.; Ahari, J.; Bawa, A.; Kimbro, S. Respiratory rate variability in sleeping adults without obstructive sleep apnea. Physiol. Rep. 2016, 4, e12949. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Su, H.-W.; Heneghan, C.; Blunt, L.; O’connor, C.; Niehaus, L. Measurement of respiratory rate using wearable devices and applications to COVID-19 detection. NPJ Digit. Med. 2021, 4, 136. [Google Scholar] [CrossRef] [PubMed]
- Ballal, T.; Heneghan, C.; Zaffaroni, A.; Boyle, P.; De Chazal, P.; Shouldice, R.; McNicholas, W.T.; Donnelly, S.C. A pilot study of the nocturnal respiration rates in COPD patients in the home environment using a non-contact biomotion sensor. Physiol. Meas. 2014, 35, 2513–2527. [Google Scholar] [CrossRef] [PubMed]
- Eick, C.; Groga-Bada, P.; Reinhardt, K.; Duckheim, M.; Mizera, L.; Böhm, K.; Götz, N.; Gawaz, M.; Zürn, C. Nocturnal respiratory rate as a predictor of mortality in patients with acute coronary syndrome. Open Heart 2018, 5, e000887. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Allen, J.; Zheng, D.; Chen, F. Recent development of respiratory rate measurement technologies. Physiol. Meas. 2019, 40, 07TR01. [Google Scholar] [CrossRef]
- Fang, Y.; Jiang, Z.; Wang, H. A Novel Sleep Respiratory Rate Detection Method for Obstructive Sleep Apnea Based on Characteristic Moment Waveform. J. Healthc. Eng. 2018, 2018, 1902176. [Google Scholar] [CrossRef]
- Do, W.; Russell, R.; Wheeler, C.; Lockwood, M.; De Vos, M.; Pavord, I.; Bafadhel, M. Performance of Contactless Respiratory Rate Monitoring by Albus HomeTM, an Automated System for Nocturnal Monitoring at Home: A Validation Study. Sensors 2022, 22, 7142. [Google Scholar] [CrossRef]
- Sun, X.; Qiu, L.I.; Wu, Y.; Tang, Y.; Cao, G. SleepMonitor: Monitoring Respiratory Rate and Body Position During Sleep Using Smartwatch. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2017, 1, 104. [Google Scholar] [CrossRef]
- Hernandez, J.; McDuff, D.; Picard, R.W. Biowatch: Estimation of heart and breathing rates from wrist motions. In Proceedings of the 2015 9th International Conference on Pervasive Computing Technologies for Healthcare, Pervasive Health, Istanbul, Turkey, 20–23 May 2015; pp. 169–176. [Google Scholar] [CrossRef]
- Singh, G.; Tee, A.; Trakoolwilaiwan, T.; Taha, A.; Olivo, M. Method of respiratory rate measurement using a unique wearable platform and an adaptive optical-based approach. Intensive Care Med. Exp. 2020, 8, 15. [Google Scholar] [CrossRef]
- Fuller, D.; Colwell, E.; Low, J.; Orychock, K.; Tobin, M.A.; Simango, B.; Buote, R.; Van Heerden, D.; Luan, H.; Cullen, K.; et al. Reliability and Validity of Commercially Available Wearable Devices for Measuring Steps, Energy Expenditure, and Heart Rate: Systematic Review. JMIR mHealth uHealth 2020, 8, e18694. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kim, D.; Lee, W.; Seo, H.; Seo, J.; Choi, J.; Joo, E.Y. Performance evaluation of a wrist-worn reflectance pulse oximeter during sleep. Sleep Health 2022, 8, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Walch, O.; Huang, Y.; Forger, D.; Goldstein, C. Sleep stage prediction with raw acceleration and photoplethysmography heart rate data derived from a consumer wearable device. Sleep 2019, 42, zsz180. [Google Scholar] [CrossRef] [PubMed]
- Doheny, E.P.; Lowery, M.M.; Russell, A.; Ryan, S. Estimation of respiration rate and sleeping position using a wearable accelerometer. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Montreal, QC, Canada, 20–24 July 2020; pp. 4668–4671. [Google Scholar] [CrossRef]
- Dehkordi, P.; Garde, A.; Molavi, B.; Ansermino, J.M.; Dumont, G.A. Extracting Instantaneous Respiratory Rate from Multiple Photoplethysmogram Respiratory-Induced Variations. Front. Physiol. 2018, 9, 948. [Google Scholar] [CrossRef] [PubMed]
- Karlen, W.; Ansermino, J.M.; Dumont, G. Adaptive pulse segmentation and artifact detection in photoplethysmography for mobile applications. Ann. Int. Conf. IEEE Eng. Med. Biol. Soc. 2012, 2012, 3131–3134. [Google Scholar] [CrossRef]
- Karlen, W.; Raman, S.; Ansermino, J.M.; Dumont, G.A. Multiparameter respiratory rate estimation from the photoplethysmogram. IEEE Trans. Biomed. Eng. 2013, 60, 1946–1953. [Google Scholar] [CrossRef]
- Hernando, A.; Peláez-Coca, M.D.; Lozano, M.T.; Lazaro, J.; Gil, E. Finger and forehead PPG signal comparison for respiratory rate estimation. Physiol. Meas. 2019, 40, 095007. [Google Scholar] [CrossRef]
- Knorr-Chung, B.R.; McGrath, S.P.; Blike, G.T. Identifying airway obstructions using photoplethysmography (PPG). J. Clin. Monit. Comput. 2008, 22, 95–101. [Google Scholar] [CrossRef]
- Nilsson, L.M. Respiration signals from photoplethysmography. Anesth. Analg. 2013, 117, 859–865. [Google Scholar] [CrossRef]
- Staats, B.A.; Bonekat, H.W.; Harris, C.D.; Offord, K.P. Chest wall motion in sleep apnea. Am. Rev. Respir. Dis. 1984, 130, 59–63. [Google Scholar]
- Cinel, G.; Tarim, E.A.; Tekin, H.C. Wearable respiratory rate sensor technology for diagnosis of sleep apnea. In Proceedings of the TIPTEKNO 2020—Tip Teknolojileri Kongresi—2020 Medical Technologies Congress, TIPTEKNO, Antalya, Turkey, 19–20 November 2020. [Google Scholar] [CrossRef]
- Sadek, I.; Biswas, J.; Abdulrazak, B. Ballistocardiogram signal processing: A review. Health Inf. Sci. Syst. 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Li, M.; Akbarian, S.; Hafezi, M.; Yadollahi, A.; Taati, B. Vision-Based Heart and Respiratory Rate Monitoring during Sleep—A Validation Study for the Population at Risk of Sleep Apnea. IEEE J. Transl. Eng. Health Med. 2019, 7, 1900708. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.Y.; Ryan, N.P.; Chen, D.; McNeil, J.; Hopper, I. Novel wearable and contactless heart rate, respiratory rate, and oxygen saturation monitoring devices: A systematic review and meta-analysis. Anaesthesia 2022, 77, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
| Variable | All (n = 195) | AHI < 30 (n = 122) | AHI ≥ 30 (n = 73) | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 48.9 ± 13.3 | 47.6 ± 13.9 | 51.1 ± 12.1 | 0.067 |
| Male, n (%) | 143 (73.3) | 78 (63.9) | 65 (89.0) | <0.001 |
| BMI, kg/m2 | 25.9 ± 4.0 | 24.7 ± 3.6 | 28.1 ± 3.8 | <0.001 |
| Polysomnographic parameters | ||||
| TST, min | 339.7 ± 61.9 | 350.2 ± 59.0 | 322.1 ± 63.0 | 0.002 |
| Sleep latency, min | 11.5 ± 13.0 | 11.5 ± 12.5 | 11.4 ± 13.8 | 0.966 |
| WASO, % | 16.5 ± 11.8 | 15.8 ± 11.9 | 17.8 ± 11.6 | 0.265 |
| Sleep efficiency, % | 81.3 ± 12.2 | 82.0 ± 12.1 | 80.0 ± 12.4 | 0.283 |
| N1/TST, % | 20.5 ± 12.1 | 15.4 ± 6.9 | 28.9 ± 14.1 | <0.001 |
| N2/TST, % | 53.1 ± 11.2 | 56.1 ± 9.9 | 48.2 ± 11.6 | <0.001 |
| N3/TST, % | 6.9 ± 8.7 | 8.3 ± 9.6 | 4.4 ± 6.5 | <0.001 |
| REM/TST, % | 19.6 ± 6.7 | 20.2 ± 6.0 | 18.5 ± 7.7 | 0.106 |
| AHI, /h | 28.5 ± 24.9 | 12.3 ± 8.4 | 55.6 ± 19.2 | <0.001 |
| Total AI, /h | 29.3 ± 17.3 | 20.7 ± 7.7 | 43.5 ± 19.4 | <0.001 |
| Respiratory AI, /h | 19.2 ± 20.0 | 7.7 ± 5.9 | 38.4 ± 20.5 | <0.001 |
| Lowest saturation, % | 84.0 ± 8.4 | 87.5 ± 5.3 | 78.0 ± 9.2 | <0.001 |
| Parameter | AHI < 30 (n = 122) | All Subjects (n = 195) |
|---|---|---|
| RMSE, bpm | 0.46 | 1.13 |
| Bias, bpm | 0.08 | 0.39 |
| 95% upper limit of bias, bpm | 0.98 | 2.46 |
| 95% lower limit of bias, bpm | −0.82 | −1.68 |
| Accuracy, % | 99.18 | 91.79 |
| Parameter | AHI < 30 (n = 122) | All Subjects (n = 195) |
|---|---|---|
| Total time, mins | 51,410 | 80,567 |
| RMSE, bpm | 1.22 | 1.62 |
| Bias, bpm | 0.08 | 0.37 |
| 95% upper limit of bias, bpm | 2.46 | 3.47 |
| 95% lower limit of bias, bpm | −2.31 | −2.73 |
| Accuracy, % | 92.63 | 86.66 |
| Parameter | Normal (n = 31) | Mild OSA (n = 46) | Moderate OSA (n = 45) | Severe OSA (n = 73) |
|---|---|---|---|---|
| Average overnight RR | ||||
| RMSE, bpm | 0.64 | 0.36 | 0.39 | 1.74 |
| Bias, bpm | 0.01 | 0.06 | 0.15 | 0.93 |
| 95% upper limit of bias, bpm | 0.66 | 0.36 | 0.37 | 1.49 |
| 95% lower limit of bias, bpm | 1.29 | 0.75 | 0.87 | 3.84 |
| Accuracy, % | 96.77 | 100.00 | 100.00 | 79.45 |
| Continuous RR measurements | ||||
| Total time, mins | 13,702 | 19,241 | 18,467 | 13,702 |
| RMSE, bpm | 1.33 | 1.26 | 1.15 | 2.17 |
| Bias, bpm | 0.04 | 0.06 | 0.17 | 0.92 |
| 95% upper limit of bias, bpm | 1.33 | 1.25 | 1.14 | 1.97 |
| 95% lower limit of bias, bpm | 2.66 | 2.52 | 2.40 | 4.77 |
| Accuracy, % | 92.39 | 92.61 | 92.50 | 75.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.; Kim, D.; Choi, J.; Joo, E.Y. Validating a Consumer Smartwatch for Nocturnal Respiratory Rate Measurements in Sleep Monitoring. Sensors 2023, 23, 7976. https://doi.org/10.3390/s23187976
Jung H, Kim D, Choi J, Joo EY. Validating a Consumer Smartwatch for Nocturnal Respiratory Rate Measurements in Sleep Monitoring. Sensors. 2023; 23(18):7976. https://doi.org/10.3390/s23187976
Chicago/Turabian StyleJung, Hyunjun, Dongyeop Kim, Jongmin Choi, and Eun Yeon Joo. 2023. "Validating a Consumer Smartwatch for Nocturnal Respiratory Rate Measurements in Sleep Monitoring" Sensors 23, no. 18: 7976. https://doi.org/10.3390/s23187976
APA StyleJung, H., Kim, D., Choi, J., & Joo, E. Y. (2023). Validating a Consumer Smartwatch for Nocturnal Respiratory Rate Measurements in Sleep Monitoring. Sensors, 23(18), 7976. https://doi.org/10.3390/s23187976






