Assessing Spatiotemporal and Quality Alterations in Paretic Upper Limb Movements after Stroke in Routine Care: Proposal and Validation of a Protocol Using IMUs versus MoCap
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Material
2.3. Determination of Calibration Parameters
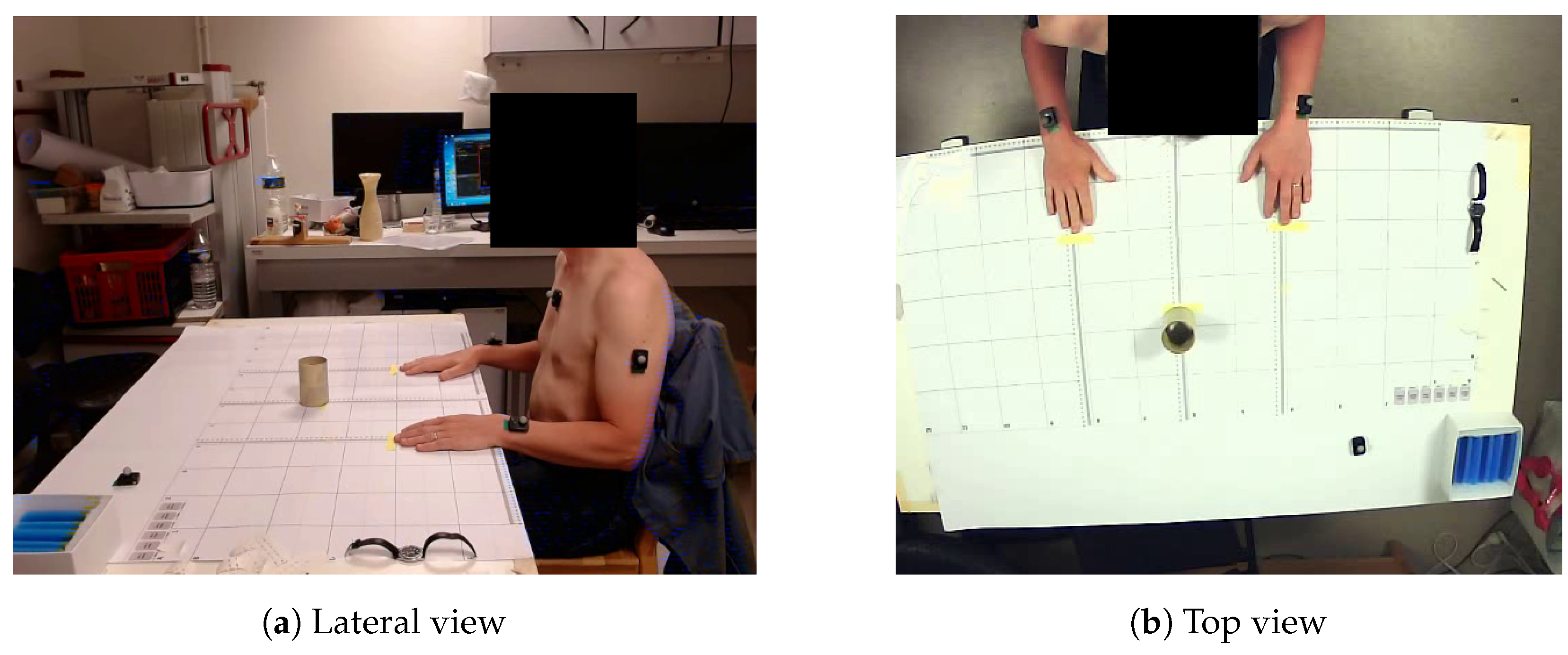
2.4. Procedure
2.4.1. Subject Setup
2.4.2. Synchronisation
2.4.3. Task Realisation
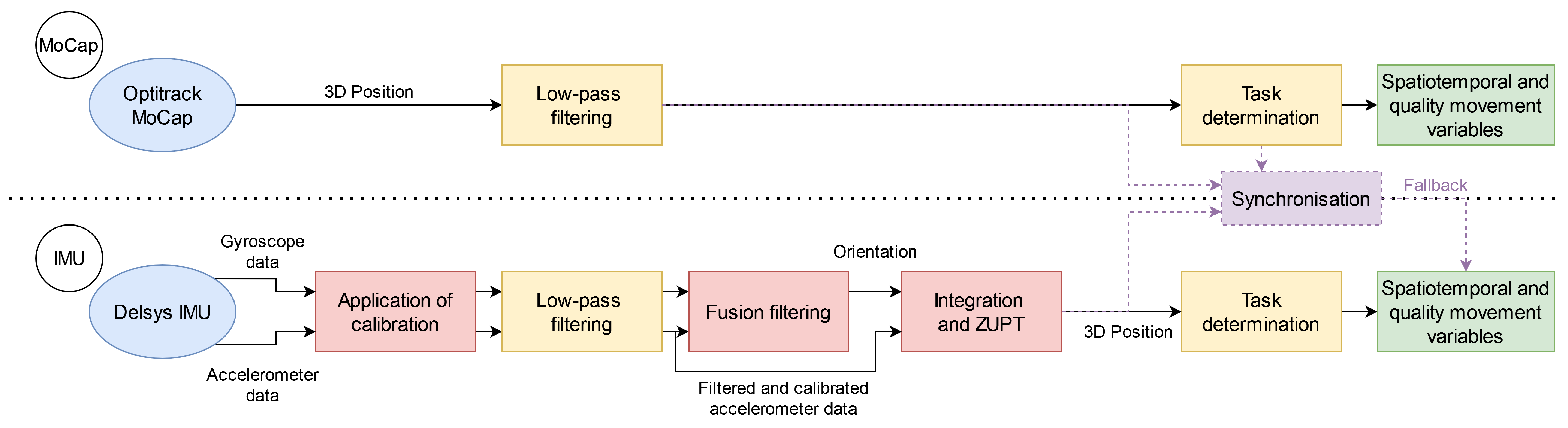
2.5. Data Processing
2.6. MoCap Data Processing
2.7. IMU Data Processing
2.7.1. Application of Calibration
2.7.2. Filtering
2.7.3. Fusion Filtering
2.7.4. Integration and ZUPT
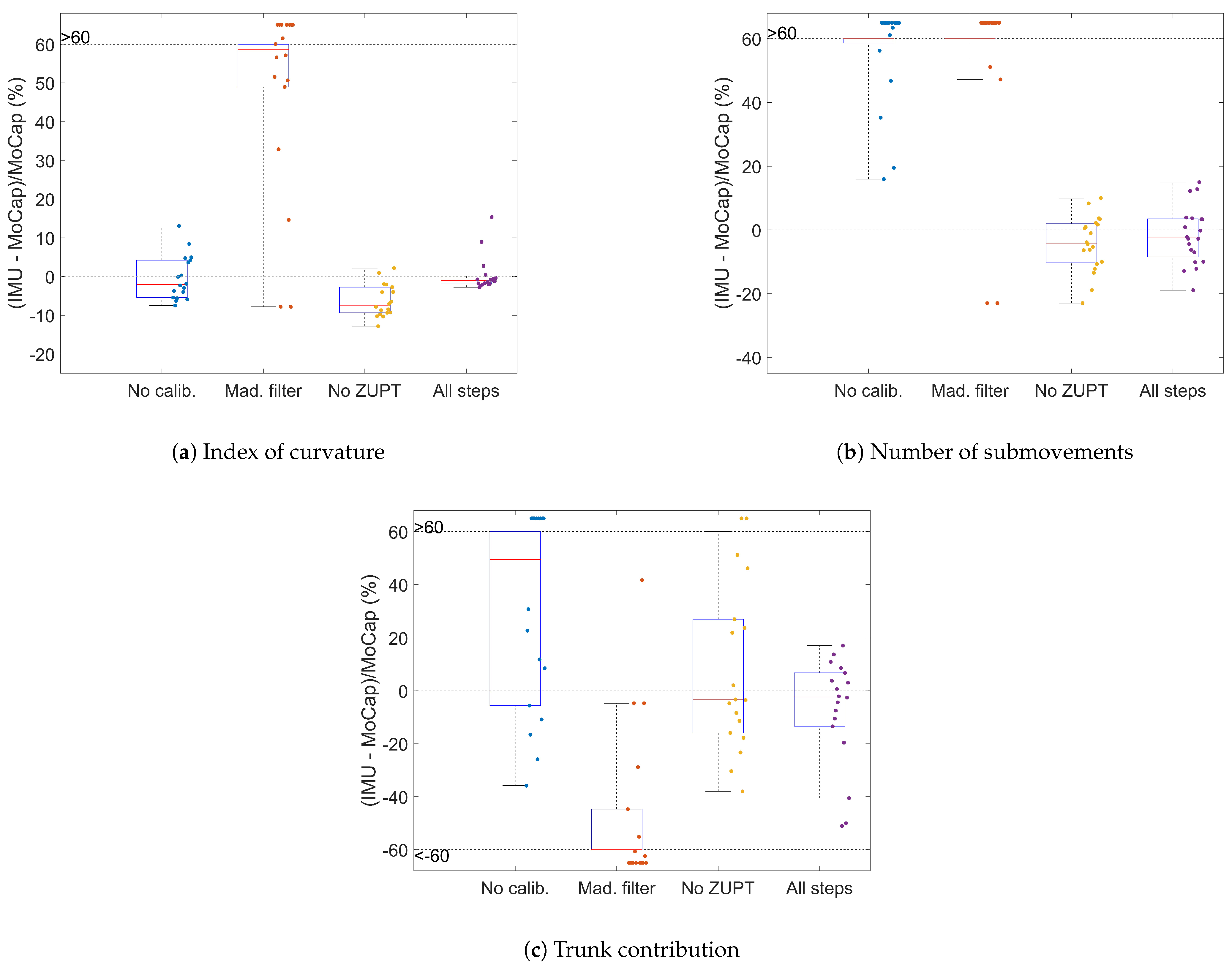
2.7.5. Influenceof the Processing Steps
- Disabling the calibration (Section 2.7.1);
- Using the Madgwick filter [28] (with parameter chosen by trial and error) as another fusion filter instead of the VQF filter (Section 2.7.3);
- Disabling ZUPT (Section 2.7.4).
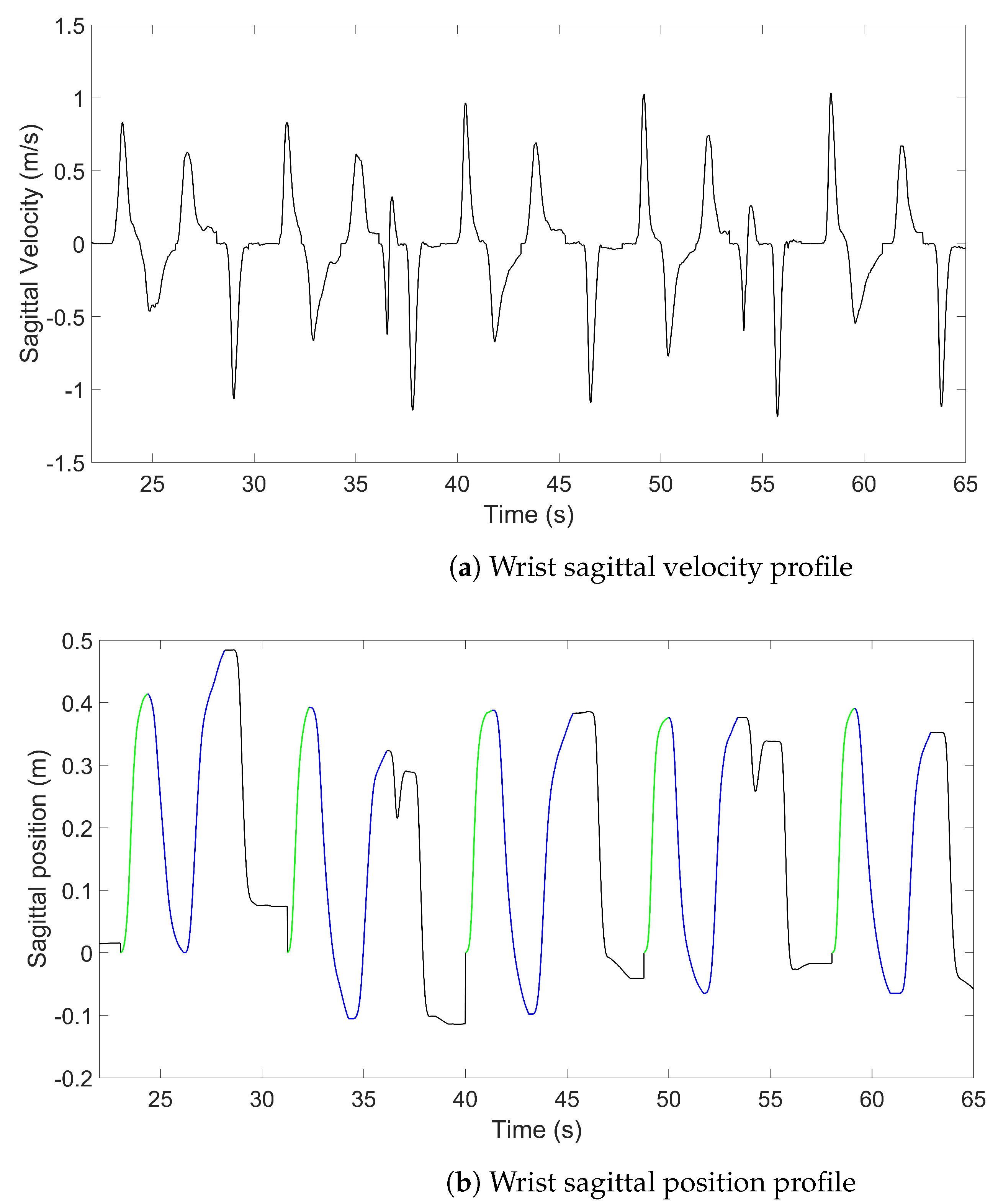
2.8. Approach Delimitation and Kinematic Variables
- The examiner defined a positive velocity threshold for the peaks, which are then automatically selected.
- Irrelevant peaks were manually removed (i.e., duplicated peaks, failed attempts, etc.).
- The approach phase was selected (the performance of the task itself, bring the glass to lips and put it down or press the switch, has not yet been analysed).
- The start and end of the approach phase were defined by a tangential velocity threshold along the antero-posterior axis > and < at 1 cm·s. The start point was determined by parsing the velocity backward until it became lower than the threshold by at least 0.2 s (this duration threshold could be manually modified). Having an adaptable threshold was required due to working with subjects with severely altered movements who might stop in the middle of the approach. The end point was determined the same way by parsing the velocity forward.
- Movement time (MT) (in seconds): duration between the starting point and the end point of the approach.
- Index of curvature (IoC): ratio of the movement arc length to the shortest distance between the start and end points. Its value theoretically goes from 1 (perfectly straight trajectory) to .
- Number of submovements (nSUB): number of sagittal velocity peaks; it is an integer from 1 to , and higher values mean lower smoothness.
- Trunk contribution (TC) (in %): ratio of the sagittal length covered by the trunk to the sagittal length convered by the wrist.
2.9. Statistics
3. Results
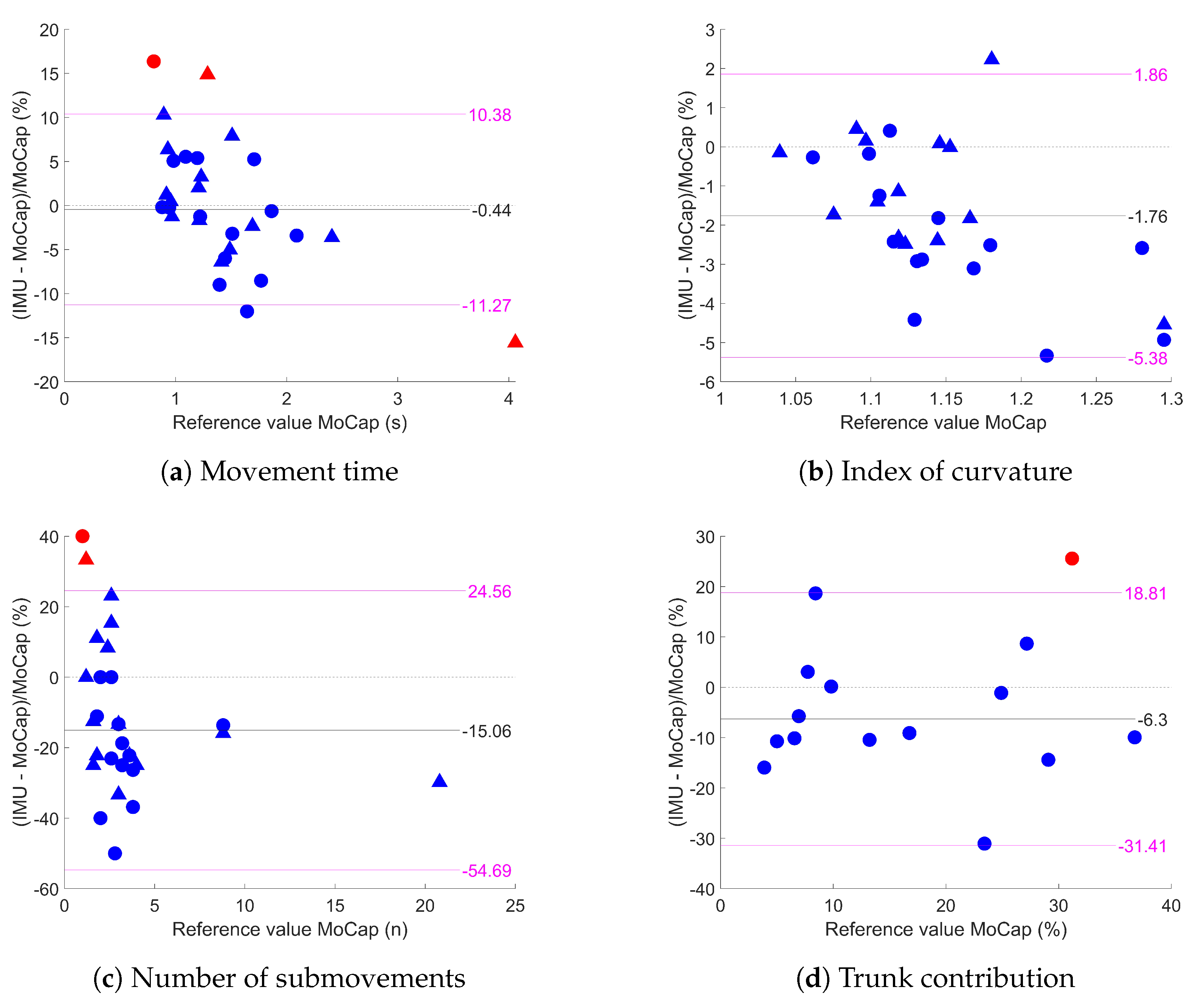
3.1. Agreement between IMUs and MoCap
3.2. Influence of the Processing Steps
4. Discussion
4.1. Agreement between IMUs and MoCap
4.2. Influence of the Processing Steps
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Kordelaar, J.; van Wegen, E.E.; Nijland, R.H.; de Groot, J.H.; Meskers, C.G.; Harlaar, J.; Kwakkel, G. Assessing Longitudinal Change in Coordination of the Paretic Upper Limb Using On-Site 3-Dimensional Kinematic Measurements. Phys. Ther. 2012, 92, 142–151. [Google Scholar] [CrossRef] [PubMed]
- van Dokkum, L.; Hauret, I.; Mottet, D.; Froger, J.; Métrot, J.; Laffont, I. The Contribution of Kinematics in the Assessment of Upper Limb Motor Recovery Early After Stroke. Neurorehabilit. Neural Repair 2013, 28, 4–12. [Google Scholar] [CrossRef]
- Kwakkel, G.; Lannin, N.A.; Borschmann, K.; English, C.; Ali, M.; Churilov, L.; Saposnik, G.; Winstein, C.; van Wegen, E.E.H.; Wolf, S.L.; et al. Standardized Measurement of Sensorimotor Recovery in Stroke Trials: Consensus-Based Core Recommendations from the Stroke Recovery and Rehabilitation Roundtable. Neurorehabilit. Neural Repair 2017, 31, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.A.; Willén, C.; Sunnerhagen, K.S. Kinematic variables quantifying upper-extremity performance after stroke during reaching and drinking from a glass. Neurorehabilit. Neural Repair 2011, 25, 71–80. [Google Scholar] [CrossRef] [PubMed]
- de los Reyes-Guzmán, A.; Dimbwadyo-Terrer, I.; Trincado-Alonso, F.; Monasterio-Huelin, F.; Torricelli, D.; Gil-Agudo, A. Quantitative assessment based on kinematic measures of functional impairments during upper extremity movements: A review. Clin. Biomech. (Bristol, Avon) 2014, 29, 719–727. [Google Scholar] [CrossRef]
- Eichelberger, P.; Ferraro, M.; Minder, U.; Denton, T.; Blasimann, A.; Krause, F.; Baur, H. Analysis of accuracy in optical motion capture—A protocol for laboratory setup evaluation. J. Biomech. 2016, 49, 2085–2088. [Google Scholar] [CrossRef]
- Geelen, J.E.; Branco, M.P.; Ramsey, N.F.; van der Helm, F.C.T.; Mugge, W.; Schouten, A.C. MarkerLess Motion Capture: ML-MoCap, a low-cost modular multi-camera setup. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021. [Google Scholar] [CrossRef]
- Longo, U.G.; Salvatore, S.D.; Carnevale, A.; Tecce, S.M.; Bandini, B.; Lalli, A.; Schena, E.; Denaro, V. Optical Motion Capture Systems for 3D Kinematic Analysis in Patients with Shoulder Disorders. Int. J. Environ. Res. Public Health 2022, 19, 12033. [Google Scholar] [CrossRef]
- Ghorbani, S.; Etemad, A.; Troje, N.F. Auto-labelling of Markers in Optical Motion Capture by Permutation Learning. In Advances in Computer Graphics; Gavrilova, M., Chang, J., Thalmann, N., Hitzer, E., Ishikawa, H., Eds.; Springer: Cham, Switzerland, 2019; pp. 167–178. [Google Scholar] [CrossRef]
- Oliva-Lozano, J.M.; Martín-Fuentes, I.; Muyor, J.M. Validity and Reliability of a New Inertial Device for Monitoring Range of Motion at the Pelvis during Sexual Intercourse. Int. J. Environ. Res. Public Health 2020, 17, 2884. [Google Scholar] [CrossRef]
- Amarantini, D.; Rieu, I.; Castelnovo, G.; Fluchère, F.; Laurencin, C.; Degos, B.; Poujois, A.; Kreisler, A.; Sangla, S.; Tir, M.; et al. Quantification of Head Tremors in Medical Conditions: A Comparison of Analyses Using a 2D Video Camera and a 3D Wireless Inertial Motion Unit. Sensors 2022, 22, 2385. [Google Scholar] [CrossRef]
- Patel, M.; Pavic, A.; Goodwin, V.A. Wearable inertial sensors to measure gait and posture characteristic differences in older adult fallers and non-fallers: A scoping review. Gait Posture 2020, 76, 110–121. [Google Scholar] [CrossRef]
- Walmsley, C.P.; Williams, S.A.; Grisbrook, T.; Elliott, C.; Imms, C.; Campbell, A. Measurement of Upper Limb Range of Motion Using Wearable Sensors: A Systematic Review. Sport. Med.-Open 2018, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Bonato, P. Advances in wearable technology and its medical applications. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010. [Google Scholar] [CrossRef]
- Schepers, H.; van Asseldonk, E.; Buurke, J.; Veltink, P. Ambulatory Estimation of Center of Mass Displacement During Walking. IEEE Trans. Biomed. Eng. 2009, 56, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Pepper, M.G.; Wang, Z.; Mulvenna, M.D.; Bond, R.R.; Finlay, D.; Zheng, H. Upper Limb Position Tracking with a Single Inertial Sensor Using Dead Reckoning Method with Drift Correction Techniques. Sensors 2022, 23, 360. [Google Scholar] [CrossRef] [PubMed]
- Ru, X.; Gu, N.; Shang, H.; Zhang, H. MEMS Inertial Sensor Calibration Technology: Current Status and Future Trends. Micromachines 2022, 13, 879. [Google Scholar] [CrossRef]
- Kok, M.; Hol, J.D.; Schön, T.B. Using Inertial Sensors for Position and Orientation Estimation. Found. Trends Signal Process. 2017, 11, 1–153. [Google Scholar] [CrossRef]
- Bhagubai, M.M.C.; Wolterink, G.; Schwarz, A.; Held, J.P.O.; Beijnum, B.J.F.V.; Veltink, P.H. Quantifying Pathological Synergies in the Upper Extremity of Stroke Subjects With the Use of Inertial Measurement Units: A Pilot Study. IEEE J. Transl. Eng. Health Med. 2021, 9, 1–11. [Google Scholar] [CrossRef]
- Callejas-Cuervo, M.; Gutierrez, R.M.; Hernandez, A.I. Joint amplitude MEMS based measurement platform for low cost and high accessibility telerehabilitation: Elbow case study. J. Bodyw. Mov. Ther. 2017, 21, 574–581. [Google Scholar] [CrossRef]
- Schwarz, A.; Bhagubai, M.M.C.; Wolterink, G.; Held, J.P.O.; Luft, A.R.; Veltink, P.H. Assessment of Upper Limb Movement Impairments after Stroke Using Wearable Inertial Sensing. Sensors 2020, 20, 4770. [Google Scholar] [CrossRef]
- Cutti, A.G.; Giovanardi, A.; Rocchi, L.; Davalli, A.; Sacchetti, R. Ambulatory measurement of shoulder and elbow kinematics through inertial and magnetic sensors. Med. Biol. Eng. Comput. 2007, 46, 169–178. [Google Scholar] [CrossRef]
- Bai, L.; Pepper, M.G.; Yan, Y.; Phillips, M.; Sakel, M. Low Cost Inertial Sensors for the Motion Tracking and Orientation Estimation of Human Upper Limbs in Neurological Rehabilitation. IEEE Access 2020, 8, 54254–54268. [Google Scholar] [CrossRef]
- Bouvier, B.; Duprey, S.; Claudon, L.; Dumas, R.; Savescu, A. Upper Limb Kinematics Using Inertial and Magnetic Sensors: Comparison of Sensor-to-Segment Calibrations. Sensors 2015, 15, 18813–18833. [Google Scholar] [CrossRef]
- Cutti, A.G.; Paolini, G.; Troncossi, M.; Cappello, A.; Davalli, A. Soft tissue artefact assessment in humeral axial rotation. Gait Posture 2005, 21, 341–349. [Google Scholar] [CrossRef]
- Cahill-Rowley, K.; Rose, J. Temporal–spatial reach parameters derived from inertial sensors: Comparison to 3D marker-based motion capture. J. Biomech. 2017, 52, 11–16. [Google Scholar] [CrossRef]
- de Vries, W.; Veeger, H.; Baten, C.; van der Helm, F. Magnetic distortion in motion labs, implications for validating inertial magnetic sensors. Gait Posture 2009, 29, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Madgwick, S.O.H.; Harrison, A.J.L.; Vaidyanathan, R. Estimation of IMU and MARG orientation using a gradient descent algorithm. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011. [Google Scholar] [CrossRef]
- Laidig, D.; Seel, T. VQF: Highly accurate IMU orientation estimation with bias estimation and magnetic disturbance rejection. Inf. Fusion 2023, 91, 187–204. [Google Scholar] [CrossRef]
- Frykberg, G.E.; Grip, H.; Murphy, M.A. How many trials are needed in kinematic analysis of reach-to-grasp?—A study of the drinking task in persons with stroke and non-disabled controls. J. NeuroEng. Rehabil. 2021, 18, 101. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.M.; Kersting, U.G.; Pedersen, A.R.; Svendsen, S.W.; Nielsen, J.F. Three-dimensional kinematics of shoulder function in stroke patients: Inter- and intra-rater reliability. J. Electromyogr. Kinesiol. 2019, 47, 35–42. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Lexell, J.; Flansbjer, U.B.; Holmbäck, A.M.; Downham, D.; Patten, C. Reliability of gait performance tests in men and women with hemiparesis after stroke. J. Rehabil. Med. 2005, 37, 75–82. [Google Scholar] [CrossRef]
- Bayle, N.; Lempereur, M.; Hutin, E.; Motavasseli, D.; Remy-Neris, O.; Gracies, J.M.; Cornec, G. Comparison of Various Smoothness Metrics for Upper Limb Movements in Middle-Aged Healthy Subjects. Sensors 2023, 23, 1158. [Google Scholar] [CrossRef]
- Patrizi, G.; Catelani, M.; Ciani, L.; Carratu, M.; Pietrosanto, A.; Sommella, P.; Betta, G.; Capriglione, D. Investigation of the effects of temperature variations on Inertial Measurement Units for UAV. In Proceedings of the 2022 IEEE 9th International Workshop on Metrology for AeroSpace (MetroAeroSpace), Pisa, Italy, 27–29 June 2022. [Google Scholar] [CrossRef]
- Wagner, J.M.; Rhodes, J.A.; Patten, C. Reproducibility and Minimal Detectable Change of Three-Dimensional Kinematic Analysis of Reaching Tasks in People With Hemiparesis After Stroke. Phys. Ther. 2008, 88, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.S.; Bishop, M.D.; McGuirk, T.E.; Sethi, A.; Richards, L.G. Reliability of Upper Extremity Kinematics While Performing Different Tasks in Individuals With Stroke. J. Mot. Behav. 2011, 43, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Engdahl, S.M.; Gates, D.H. Reliability of upper limb movement quality metrics during everyday tasks. Gait Posture 2019, 71, 253–260. [Google Scholar] [CrossRef] [PubMed]
| Id | Age (Years) | Sex (F/M) | Time Since Stroke (Months) | Stroke Type (I/H) | Stroke Side (R/L), Location | Pre-Stroke Dominant Limb | Paretic Side (R/L) | FMA-UE (/66) | EmNSA-SS UE (/44) | Task Analysed |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | F | 1 | I | L, brainstem | R | R | 63 | 44 | DRINK |
| 2 | 47 | F | 2 | H | L, temporo-occipital lobar | R | R | 66 | 44 | DRINK |
| 3 | 57 | M | 3 | I | L, cortical MCA | R | R | 40 | 40 | DRINK |
| 4 | 57 | M | 18 | H | L, subcortical MCA | R | R | 66 | 44 | DRINK |
| 5 | 61 | M | 36 | I | L, lenticular | R | R | 29 | 43 | DRINK |
| 6 | 47 | F | 2 | I | L, capsulo-lenticular | R | R | 16 | 43 | LIGHT |
| 7 | 65 | M | 1 | I | L, cortical and subcortical MCA | R | R | 66 | 44 | DRINK |
| 8 | 55 | M | 13 | I | R, subcortical MCA | R | L | 66 | 44 | DRINK |
| 9 | 53 | M | 8 | I | R, pons and lower cerebellum | R | R | 65 | 40 | DRINK |
| 10 | 58 | M | 8 | I | L, cortical MCA | L | R | MD | MD | DRINK |
| 11 | 42 | F | 1 | I | L, cortical and subcortical MCA | R | R | MD | 44 | DRINK |
| 12 | 48 | F | 3 | H | Median, pons | R | R | 46 | 8 | LIGHT |
| 13 | 56 | M | 18 | I | L, posterior cortical MCA | R | R | 55 | 38 | DRINK |
| 14 | 52 | F | 1 | I | L, subcortical MCA | R | R | 32 | 44 | DRINK |
| 15 | 53 | F | 29 | I | L, subcortical MCA | R | R | 62 | 43 | DRINK |
| Variable (Unit) | r (p-Value) | Number of Outliers | MoCap Mean Value (SD) | IMU Mean Value (SD) | ICC [95%CI] | Bias [95%CI] | MDC |
|---|---|---|---|---|---|---|---|
| MT (s) | 0.77 (<0.01 ) | 3 | 1.35 (0.40) | 1.34 (0.36) | 0.989 [0.977;0.995] | −0.44% [−2.61;1.72] | 10.83% |
| IoC | 0.92 (<0.01 ) | 2 | 1.14 (0.06) | 1.12 (0.05) | 0.935 [0.602;0.979] | −1.76% [−2.46;−1.06] | 3.62% |
| nSUB (n) | 0.91 (<0.01 ) | 2 | 3.71 (3.79) | 3.01 (2.73) | 0.955 [0.870;0.982] | −15.06% [−21.77;−8.36] | 39.62% |
| TC (%) | 0.69 (<0.01 ) | 1 | 15.69 (10.63) | 14.52 (9.77) | 0.983 [0.944;0.995] | −6.30% [−13.18;0.58] | 25.11% |
| Variable (Unit) | r (p-Value) | Number Outliers | MoCap Mean Value (SD) a | IMU Mean Value (SD) | ICC [95% CI] | Bias [95% CI] | MDC b |
|---|---|---|---|---|---|---|---|
| No calibration | |||||||
| IoC | 0.97 (<0.01 ) | 2 | 1.14 (0.07) | 1.11 (0.06) | 0.537 [0.050;0.780] | −2.85% [−5.10;−0.61] | 11.69% |
| nSUB (n) | 0.88 (<0.01 ) | 1 | 4.10 (5.72) | 5.16 (3.53) | 0.906 [0.791;0.957] | 62.53% [41.76;83.31] | 71.49% |
| TC (%) | 0.92 (<0.01 ) | 0 | 16.74 (10.99) | 18.26 (20.28) | 0.706 [0.097;0.902] | 8.47% [−25.23;42.17] | 112.28% |
| Madgwick fusion filter | |||||||
| IoC | 0.95 (<0.01 ) | 2 | 1.17 (0.13) | 1.80 (0.40) | −0.024 [−0.233;0.250] | 55.23% [41.21;69.25] | 48.53% |
| nSUB (n) | 0.27 (0.15) | 1 | 4.06 (5.64) | 6.55 (6.14) | 0.929 [0.457;0.979] | 2.18 [1.41;2.95] | 3.99 (98.28%) |
| TC (%) | 0.36 (0.19) | 0 | 16.10 (9.97) | 6.31 (7.44) | 0.557 [−0.258;0.858] | −9.79% [−14.07;−5.52] | 15.13% (93.98%) |
| No ZUPT | |||||||
| IoC | 0.08 (0.67) | 1 | 1.13 (0.05) | 1.06 (0.05) | 0.377 [−0.227;0.716] | −0.07 [−0.09;−0.05] | 0.10 (8.85%) |
| nSUB (n) | 0.74 (<0.01) | 1 | 3.16 (1.61) | 2.62 (1.28) | 0.941 [0.449;0.983] | −15.51% [−20.15;−10.86] | 27.64% |
| TC (%) | 0.19 (0.50) | 1 | 18.71 (10.64) | 17.24 (9.42) | 0.981 [0.943;0.994] | −0.97% [−2.54;0.61] | 5.35% (28.59%) |
| Task | MT (s) | IoC | nSUB (n) | TC (%) | |
|---|---|---|---|---|---|
| IMU vs. MoCap | DRINK or LIGHT | 10.83% (0.13 s) a | 3.62% (0.04) a | 39.62% (1.2) a | 25.11% (3.64%) a |
| Patterson et al. [37] | Reach to grasp can (4 times) | 0.42 s | 0.08 | – | – b |
| Wagner et al. [36] | Point various targets (2 times each) | 38.9–70.0% | 7.4–28.9% | 24.4–67.6% | – |
| Engdahl et al. [38] | Reach to grasp can (10 times) | – | 0.15 | 0.50 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merlau, B.; Cormier, C.; Alaux, A.; Morin, M.; Montané, E.; Amarantini, D.; Gasq, D. Assessing Spatiotemporal and Quality Alterations in Paretic Upper Limb Movements after Stroke in Routine Care: Proposal and Validation of a Protocol Using IMUs versus MoCap. Sensors 2023, 23, 7427. https://doi.org/10.3390/s23177427
Merlau B, Cormier C, Alaux A, Morin M, Montané E, Amarantini D, Gasq D. Assessing Spatiotemporal and Quality Alterations in Paretic Upper Limb Movements after Stroke in Routine Care: Proposal and Validation of a Protocol Using IMUs versus MoCap. Sensors. 2023; 23(17):7427. https://doi.org/10.3390/s23177427
Chicago/Turabian StyleMerlau, Baptiste, Camille Cormier, Alexia Alaux, Margot Morin, Emmeline Montané, David Amarantini, and David Gasq. 2023. "Assessing Spatiotemporal and Quality Alterations in Paretic Upper Limb Movements after Stroke in Routine Care: Proposal and Validation of a Protocol Using IMUs versus MoCap" Sensors 23, no. 17: 7427. https://doi.org/10.3390/s23177427
APA StyleMerlau, B., Cormier, C., Alaux, A., Morin, M., Montané, E., Amarantini, D., & Gasq, D. (2023). Assessing Spatiotemporal and Quality Alterations in Paretic Upper Limb Movements after Stroke in Routine Care: Proposal and Validation of a Protocol Using IMUs versus MoCap. Sensors, 23(17), 7427. https://doi.org/10.3390/s23177427






