Electrochemical and Optical Sensors for Real-Time Detection of Nitrate in Water
Abstract
1. Introduction
2. Electrochemical Methods
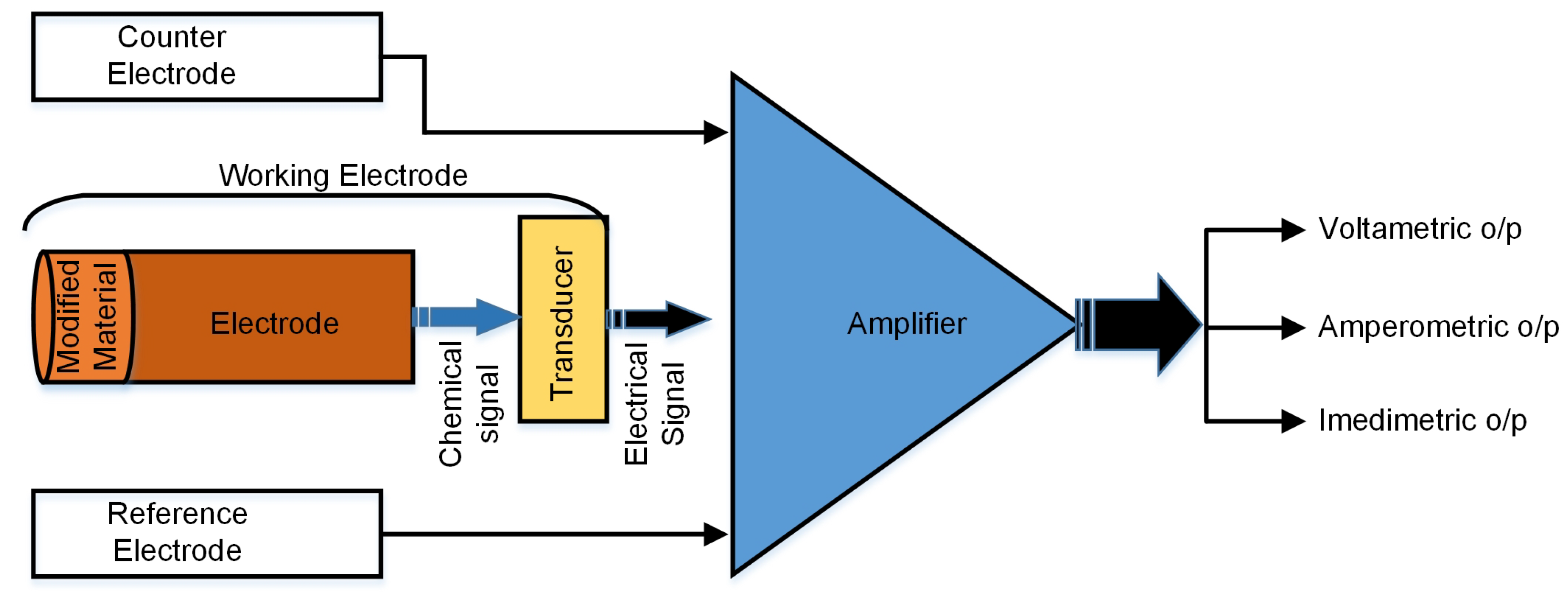
2.1. Working Principle
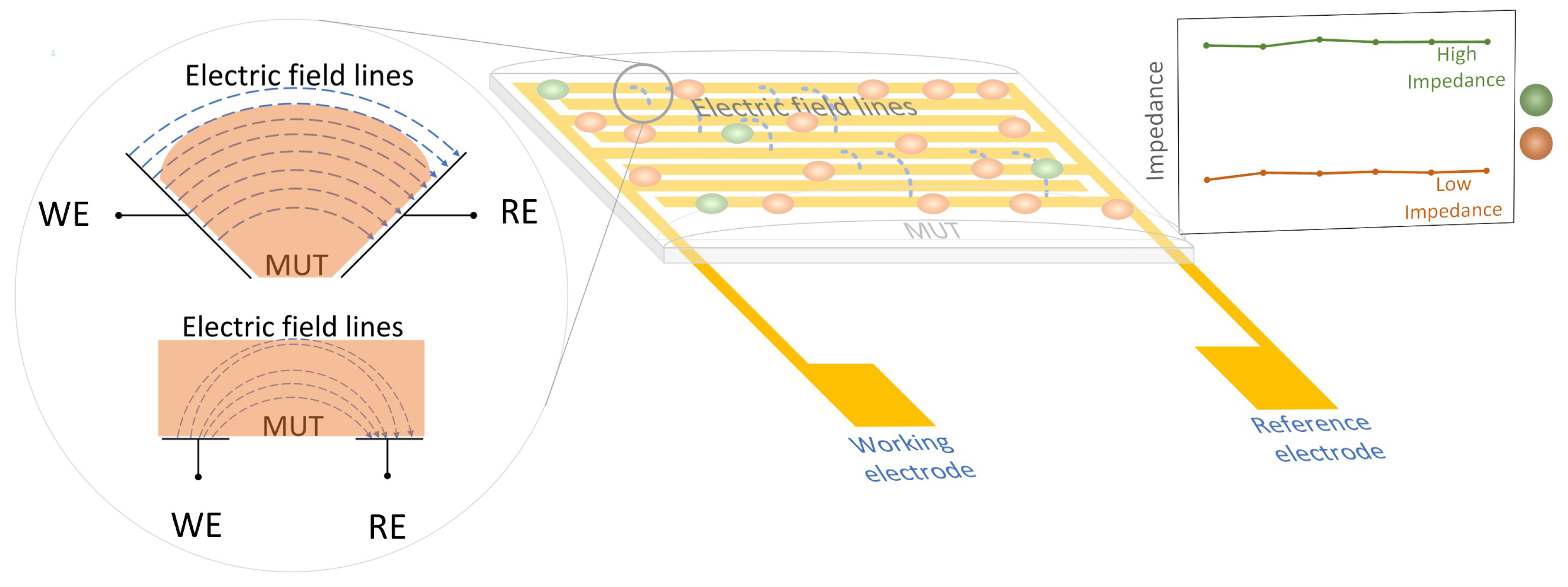
2.2. Electrode Design
2.3. Electrochemical Measurement Techniques
| Advantages | Disadvantages | Ref. | |
|---|---|---|---|
| Two-electrode | Suitable for portable or handheld sensors Provides a cost-effective design Simple design and implementation Easy to operate | Limited dynamic range Prone to drift | [50,51] |
| Three-electrode | Accurate and stable results Wider dynamic range of detection Reduced drift and improved reliability | Expensive and larger sensor size Increased complexity of measurement setup Requires regular maintenance and calibration | [52,53] |
3. Optical Methods
3.1. Working Principle
3.2. Wavelength Selection
3.3. Measurement Methods
4. Real-Time Nitrate Sensors and Recent Developments
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilliam, J.; Skaggs, R.; Weed, S. Drainage Control to Diminish Nitrate Loss from Agricultural Fields; Technical Report; Wiley Online Library: Hoboken, NJ, USA, 1979. [Google Scholar]
- Jaywant, S.A.; Arif, K.M. A comprehensive review of microfluidic water quality monitoring sensors. Sensors 2019, 19, 4781. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Mukhopadhyay, S.C. Detection methods of nitrate in water: A review. Sens. Actuators A Phys. 2018, 280, 210–221. [Google Scholar] [CrossRef]
- Wierzbicka, E. Novel methods of nitrate and nitrite determination—A review. J. Elem. 2020, 25, 97–106. [Google Scholar] [CrossRef]
- Azmi, A.; Azman, A.A.; Ibrahim, S.; Yunus, M.A.M. Techniques in advancing the capabilities of various nitrate detection methods: A review. Int. J. Smart Sens. Intell. Syst. 2017, 10, 1–39. [Google Scholar] [CrossRef]
- Amali, R.; Lim, H.; Ibrahim, I.; Huang, N.; Zainal, Z.; Ahmad, S. Significance of nanomaterials in electrochemical sensors for nitrate detection: A review. Trends Environ. Anal. Chem. 2021, 31, e00135. [Google Scholar] [CrossRef]
- Jakszyn, P.; González, C.A. Nitrosamine and related food intake and gastric and oesophageal cancer risk: A systematic review of the epidemiological evidence. World J. Gastroenterol. WJG 2006, 12, 4296. [Google Scholar] [CrossRef] [PubMed]
- Brender, J.D.; Werler, M.M.; Kelley, K.E.; Vuong, A.M.; Shinde, M.U.; Zheng, Q.; Huber, J.C., Jr.; Sharkey, J.R.; Griesenbeck, J.S.; Romitti, P.A.; et al. Nitrosatable drug exposure during early pregnancy and neural tube defects in offspring: National Birth Defects Prevention Study. Am. J. Epidemiol. 2011, 174, 1286–1295. [Google Scholar] [CrossRef]
- Korostynska, O.; Mason, A.; Al-Shamma’a, A. Monitoring of nitrates and phosphates in wastewater: Current technologies and further challenges. Int. J. Smart Sens. Intell. Syst. 2012, 5, 149–176. [Google Scholar] [CrossRef]
- Stortini, A.; Moretto, L.; Mardegan, A.; Ongaro, M.; Ugo, P. Arrays of copper nanowire electrodes: Preparation, characterization and application as nitrate sensor. Sens. Actuators B Chem. 2015, 207, 186–192. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004; Volume 1. [Google Scholar]
- Ali, M.A.; Jiang, H.; Mahal, N.K.; Weber, R.J.; Kumar, R.; Castellano, M.J.; Dong, L. Microfluidic impedimetric sensor for soil nitrate detection using graphene oxide and conductive nanofibers enabled sensing interface. Sens. Actuators B Chem. 2017, 239, 1289–1299. [Google Scholar] [CrossRef]
- Jiang, C.; He, Y.; Liu, Y. Recent advances in sensors for electrochemical analysis of nitrate in food and environmental matrices. Analyst 2020, 145, 5400–5413. [Google Scholar] [CrossRef] [PubMed]
- An’amt, M.N.; Yusoff, N.; Sagadevan, S.; Wahab, Y.A.; Johan, M.R. Recent progress in nitrates and nitrites sensor with graphene-based nanocomposites as electrocatalysts. Trends Environ. Anal. Chem. 2022, 34, e00162. [Google Scholar]
- Rosenberg, D.; Reynoldson, T.; Resh, V. Establishing Reference Conditions in the Fraser River Catchment, British Columbia, Canada, Using the BEAST (BEnthic Assessment of SedimenT) Predictive Model. Assessing the Biological Quality of Rresh Waters: RIVPACS and Other Techniques. In Proceedings of the International Workshop, Oxford, UK, 16–18 September 1997; Freshwater Biological Association (FBA): Cumbria, UK, 2000; pp. 181–194. [Google Scholar]
- Beaton, A.D.; Wadham, J.L.; Hawkings, J.; Bagshaw, E.A.; Lamarche-Gagnon, G.; Mowlem, M.C.; Tranter, M. High-resolution in situ measurement of nitrate in runoff from the Greenland ice sheet. Environ. Sci. Technol. 2017, 51, 12518–12527. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Thakur, D.; Chowdhury, D. DNA carbon-nanodots based electrochemical biosensor for detection of mutagenic nitrosamines. ACS Appl. Bio Mater. 2020, 3, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Alahi, M.E.E.; Xie, L.; Mukhopadhyay, S.; Burkitt, L. A temperature compensated smart nitrate-sensor for agricultural industry. IEEE Trans. Ind. Electron. 2017, 64, 7333–7341. [Google Scholar] [CrossRef]
- Rehmani, M.A.A.; Lal, K.; Shaukat, A.; Arif, K.M. Laser ablation assisted micropattern screen printed transduction electrodes for sensing applications. Sci. Rep. 2022, 12, 6928. [Google Scholar] [CrossRef]
- Ludeña-Choez, J.; Choquehuanca-Zevallos, J.J.; Yasmany-Juarez, A.; Mayhua-López, E.; Zea, J.; Talavera-Núñez, M.E.; Magallanes-Magallanes, J.L.; Pérez-Montaño, H.S. Capacitance sensitivity study of interdigital capacitive sensor based on graphene for monitoring Nitrates concentrations. Comput. Electron. Agric. 2022, 202, 107361. [Google Scholar] [CrossRef]
- Mazlan, N.; Ramli, M.; Abdullah, M.M.A.B.; Halin, D.C.; Isa, S.M.; Talip, L.; Danial, N.; Murad, S.Z. Interdigitated electrodes as impedance and capacitance biosensors: A review. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2017; Volume 1885, p. 020276. [Google Scholar]
- Liang, J.; Zheng, Y.; Liu, Z. Nanowire-based Cu electrode as electrochemical sensor for detection of nitrate in water. Sens. Actuators B Chem. 2016, 232, 336–344. [Google Scholar] [CrossRef]
- Tan, J.F.; Anastasi, A.; Chandra, S. Electrochemical detection of nitrate, nitrite and ammonium for on-site water quality monitoring. Curr. Opin. Electrochem. 2022, 32, 100926. [Google Scholar] [CrossRef]
- Bagheri, H.; Hajian, A.; Rezaei, M.; Shirzadmehr, A. Composite of Cu metal nanoparticles-multiwall carbon nanotubes-reduced graphene oxide as a novel and high performance platform of the electrochemical sensor for simultaneous determination of nitrite and nitrate. J. Hazard. Mater. 2017, 324, 762–772. [Google Scholar] [CrossRef]
- Amini, N.; Maleki, A.; Maleki, P. Electrochemical detection of nitrate ions via reduction of NO2− and oxidation of NO reactions based on Cu@ TiO2 coreshell/nafion/polyalizarin immobilized electrode. Mater. Chem. Phys. 2021, 264, 124384. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, Y.; Linthicum, W.; Liu, F.; Beringhs, A.O.; Dang, Y.; Xu, Z.; Chang, S.Y.; Ling, J.; Huey, B.D. Toward long-term accurate and continuous monitoring of nitrate in wastewater using poly (tetrafluoroethylene)(PTFE)–solid-state ion-selective electrodes (S-ISEs). ACS Sens. 2020, 5, 3182–3193. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Bian, C.; Tong, J.; Dong, H.; Zhang, H.; Xia, S. Copper nano-clusters prepared by one-step electrodeposition and its application on nitrate sensing. AIP Adv. 2015, 5, 041312. [Google Scholar] [CrossRef]
- Pan, D.; Lu, W.; Zhang, H.; Zhang, L.; Zhuang, J. Voltammetric determination of nitrate in water samples at copper modified bismuth bulk electrode. Int. J. Environ. Anal. Chem. 2013, 93, 935–945. [Google Scholar] [CrossRef]
- Hafezi, B.; Majidi, M.R. A sensitive and fast electrochemical sensor based on copper nanostructures for nitrate determination in foodstuffs and mineral waters. Anal. Methods 2013, 5, 3552–3556. [Google Scholar] [CrossRef]
- da Silva, I.S.; de Araujo, W.R.; Paixão, T.R.; Angnes, L. Direct nitrate sensing in water using an array of copper-microelectrodes from flat flexible cables. Sens. Actuators B Chem. 2013, 188, 94–98. [Google Scholar] [CrossRef]
- Li, G.; Yuan, H.; Mou, J.; Dai, E.; Zhang, H.; Li, Z.; Zhao, Y.; Dai, Y.; Zhang, X. Electrochemical detection of nitrate with carbon nanofibers and copper co-modified carbon fiber electrodes. Compos. Commun. 2022, 29, 101043. [Google Scholar] [CrossRef]
- Su, P.G.; Shieh, H.C. Flexible NO2 sensors fabricated by layer-by-layer covalent anchoring and in situ reduction of graphene oxide. Sens. Actuators B Chem. 2014, 190, 865–872. [Google Scholar] [CrossRef]
- Riahifar, V.; Haghnazari, N.; Keshavarzi, F.; Nasri, F. Design a high sensitive electrochemical sensor based on immobilized cysteine on Fe3O4@ Au core-shell nanoparticles and reduced graphene oxide nanocomposite for nitrite monitoring. Microchem. J. 2021, 166, 106217. [Google Scholar] [CrossRef]
- Ali, M.A.; Jiao, Y.; Tabassum, S.; Wang, Y.; Jiang, H.; Dong, L. Electrochemical detection of nitrate ions in soil water using graphene foam modified by TiO2 nanofibers and enzyme molecules. In Proceedings of the 2017 19th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Kaohsiung, Taiwan, 18–22 June 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 238–241. [Google Scholar]
- Can, F.; Ozoner, S.K.; Ergenekon, P.; Erhan, E. Amperometric nitrate biosensor based on Carbon nanotube/Polypyrrole/Nitrate reductase biofilm electrode. Mater. Sci. Eng. C 2012, 32, 18–23. [Google Scholar] [CrossRef]
- Li, X.; Ping, J.; Ying, Y. Recent developments in carbon nanomaterial-enabled electrochemical sensors for nitrite detection. TrAC Trends Anal. Chem. 2019, 113, 1–12. [Google Scholar] [CrossRef]
- Rajapaksha, R.; Hashim, U.; Gopinath, S.; Fernando, C. Sensitive pH detection on gold interdigitated electrodes as an electrochemical sensor. Microsyst. Technol. 2018, 24, 1965–1974. [Google Scholar] [CrossRef]
- Wasiewska, L.A.; Seymour, I.; Patella, B.; Inguanta, R.; Burgess, C.M.; Duffy, G.; O’Riordan, A. Reagent free electrochemical-based detection of silver ions at interdigitated microelectrodes using in-situ pH control. Sens. Actuators B Chem. 2021, 333, 129531. [Google Scholar] [CrossRef]
- Lal, K.; Thomas, T.; Arif, K. Performance Evaluation of Interdigitated Electrodes for Electrochemical Detection of Nitrates in Water. In International Conference on Sensing Technology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 407–413. [Google Scholar]
- Seymour, I.; O’Sullivan, B.; Lovera, P.; Rohan, J.F.; O’Riordan, A. Electrochemical detection of free-chlorine in Water samples facilitated by in-situ pH control using interdigitated microelectrodes. Sens. Actuators B Chem. 2020, 325, 128774. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Patella, B.; Daly, R.; Seymour, I.; Robinson, C.; Lovera, P.; Rohan, J.; Inguanta, R.; O’Riordan, A. A simulation and experimental study of electrochemical pH control at gold interdigitated electrode arrays. Electrochim. Acta 2021, 395, 139113. [Google Scholar] [CrossRef]
- Baghayeri, M.; Alinezhad, H.; Fayazi, M.; Tarahomi, M.; Ghanei-Motlagh, R.; Maleki, B. A novel electrochemical sensor based on a glassy carbon electrode modified with dendrimer functionalized magnetic graphene oxide for simultaneous determination of trace Pb (II) and Cd (II). Electrochim. Acta 2019, 312, 80–88. [Google Scholar] [CrossRef]
- Fu, L.; Wang, A.; Xie, K.; Zhu, J.; Chen, F.; Wang, H.; Zhang, H.; Su, W.; Wang, Z.; Zhou, C.; et al. Electrochemical detection of silver ions by using sulfur quantum dots modified gold electrode. Sens. Actuators B Chem. 2020, 304, 127390. [Google Scholar] [CrossRef]
- Okpara, E.C.; Fayemi, O.E.; Sherif, E.S.M.; Ganesh, P.S.; Swamy, B.K.; Ebenso, E.E. Electrochemical evaluation of Cd2+ and Hg2+ ions in water using ZnO/Cu2ONPs/PANI modified SPCE electrode. Sens. Bio-Sens. Res. 2022, 35, 100476. [Google Scholar] [CrossRef]
- Akhter, F.; Siddiquei, H.; Alahi, M.E.E.; Mukhopadhyay, S. Design and development of an IoT-enabled portable phosphate detection system in water for smart agriculture. Sens. Actuators A Phys. 2021, 330, 112861. [Google Scholar] [CrossRef]
- Parsaei, M.; Asadi, Z.; Khodadoust, S. A sensitive electrochemical sensor for rapid and selective determination of nitrite ion in water samples using modified carbon paste electrode with a newly synthesized cobalt (II)-Schiff base complex and magnetite nanospheres. Sens. Actuators B Chem. 2015, 220, 1131–1138. [Google Scholar] [CrossRef]
- Akhter, F.; Siddiquei, H.; Alahi, M.E.E.; Mukhopadhyay, S.C. An IoT-enabled portable sensing system with MWCNTs/PDMS sensor for nitrate detection in water. Measurement 2021, 178, 109424. [Google Scholar] [CrossRef]
- Scott, K. Electrochemical principles and characterization of bioelectrochemical systems. In Microbial Electrochemical and Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2016; pp. 29–66. [Google Scholar]
- Bui, M.P.N.; Brockgreitens, J.; Ahmed, S.; Abbas, A. Dual detection of nitrate and mercury in water using disposable electrochemical sensors. Biosens. Bioelectron. 2016, 85, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Capitán-Vallvey, L.F.; Arroyo-Guerrero, E.; Fernández-Ramos, M.D.; Santoyo-Gonzalez, F. Disposable receptor-based optical sensor for nitrate. Anal. Chem. 2005, 77, 4459–4466. [Google Scholar] [CrossRef] [PubMed]
- Brett, C.M. Electrochemical sensors for environmental monitoring. Strategy and examples. Pure Appl. Chem. 2001, 73, 1969–1977. [Google Scholar] [CrossRef]
- Alam, A.U.; Clyne, D.; Jin, H.; Hu, N.X.; Deen, M.J. Fully integrated, simple, and low-cost electrochemical sensor array for in situ water quality monitoring. ACS Sens. 2020, 5, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Baghayeri, M.; Ghanei-Motlagh, M.; Tayebee, R.; Fayazi, M.; Narenji, F. Application of graphene/zinc-based metal-organic framework nanocomposite for electrochemical sensing of As (III) in water resources. Anal. Chim. Acta 2020, 1099, 60–67. [Google Scholar] [CrossRef]
- Zia, A.I.; Syaifudin, A.M.; Mukhopadhyay, S.; Yu, P.; Al-Bahadly, I.H.; Gooneratne, C.P.; Kosel, J.; Liao, T.S. Electrochemical impedance spectroscopy based MEMS sensors for phthalates detection in water and juices. J. Phys. Conf. Ser. 2013, 439, 012026. [Google Scholar] [CrossRef]
- MacKay, S.; Abdelrasoul, G.N.; Tamura, M.; Lin, D.; Yan, Z.; Chen, J. Using impedance measurements to characterize surface modified with gold nanoparticles. Sensors 2017, 17, 2141. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Afsarimanesh, N.; Mukhopadhyay, S.C.; Burkitt, L. Development of the selectivity of nitrate sensors based on ion imprinted polymerization technique. In Proceedings of the 2017 Eleventh International Conference on Sensing Technology (ICST), Sydney, NSW, Australia, 4–6 December 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–6. [Google Scholar]
- Mumtarin, Z.; Rahman, M.M.; Marwani, H.M.; Hasnat, M.A. Electro-kinetics of conversion of NO3− into NO2− and sensing of nitrate ions via reduction reactions at copper immobilized platinum surface in the neutral medium. Electrochim. Acta 2020, 346, 135994. [Google Scholar] [CrossRef]
- Patella, B.; Russo, R.; O’Riordan, A.; Aiello, G.; Sunseri, C.; Inguanta, R. Copper nanowire array as highly selective electrochemical sensor of nitrate ions in water. Talanta 2021, 221, 121643. [Google Scholar] [CrossRef]
- Inam, A.S.; Costa Angeli, M.A.; Shkodra, B.; Douaki, A.; Avancini, E.; Magagnin, L.; Petti, L.; Lugli, P. Flexible screen-printed electrochemical sensors functionalized with electrodeposited copper for nitrate detection in water. ACS Omega 2021, 6, 33523–33532. [Google Scholar] [CrossRef] [PubMed]
- Alahi, M.E.E.; Pereira-Ishak, N.; Mukhopadhyay, S.C.; Burkitt, L. An internet-of-things enabled smart sensing system for nitrate monitoring. IEEE Internet Things J. 2018, 5, 4409–4417. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Mukhopadhyay, S.C.; Burkitt, L. Imprinted polymer coated impedimetric nitrate sensor for real-time water quality monitoring. Sens. Actuators B Chem. 2018, 259, 753–761. [Google Scholar] [CrossRef]
- Albanese, D.; Di Matteo, M.; Alessio, C. Screen printed biosensors for detection of nitrates in drinking water. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2010; Volume 28, pp. 283–288. [Google Scholar]
- Sabrina, W.N.; Hidayah, A.N.; Faridah, S.; Rashid, M.; Adibah, Y.N.; Zamri, I. Electroimmobilization of nitrate reductase into polypyrrole films on screen printed carbon electrode (SPCE) for amperometric detection of nitrate. Procedia Chem. 2016, 20, 69–72. [Google Scholar] [CrossRef][Green Version]
- Van Anh, T.D.; Zevenbergen, M.A. Low Cost Nitrate Sensor for Agricultural Applications. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin/Heidelberg, Germany, 23–27 June 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1285–1288. [Google Scholar]
- Wan, H.; Sun, Q.; Li, H.; Sun, F.; Hu, N.; Wang, P. Screen-printed gold electrode with gold nanoparticles modification for simultaneous electrochemical determination of lead and copper. Sens. Actuators B Chem. 2015, 209, 336–342. [Google Scholar] [CrossRef]
- Wan, H.; Sun, Q.; Li, H.; Sun, F.; Hu, N.; Wang, P. Design of a miniaturized multisensor chip with nanoband electrode array and light addressable potentiometric sensor for ion sensing. Anal. Methods 2015, 7, 9190–9197. [Google Scholar] [CrossRef]
- Essousi, H.; Barhoumi, H.; Bibani, M.; Ktari, N.; Wendler, F.; Al-Hamry, A.; Kanoun, O. Ion-imprinted electrochemical sensor based on copper nanoparticles-polyaniline matrix for nitrate detection. J. Sens. 2019, 2019, 4257125. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, M.; Li, S.; Ren, Y.; Qin, G. Copper wires with seamless 1D nanostructures: Preparation and electrochemical sensing performance. Mater. Lett. 2018, 211, 247–249. [Google Scholar] [CrossRef]
- Capella, J.V.; Bonastre, A.; Ors, R.; Peris, M. In line river monitoring of nitrate concentration by means of a Wireless Sensor Network with energy harvesting. Sens. Actuators B Chem. 2013, 177, 419–427. [Google Scholar] [CrossRef]
- Sagan, V.; Peterson, K.T.; Maimaitijiang, M.; Sidike, P.; Sloan, J.; Greeling, B.A.; Maalouf, S.; Adams, C. Monitoring inland water quality using remote sensing: Potential and limitations of spectral indices, bio-optical simulations, machine learning, and cloud computing. Earth-Sci. Rev. 2020, 205, 103187. [Google Scholar] [CrossRef]
- Singh, P.; Singh, M.K.; Beg, Y.R.; Nishad, G.R. A review on spectroscopic methods for determination of nitrite and nitrate in environmental samples. Talanta 2019, 191, 364–381. [Google Scholar] [CrossRef]
- Wang, Q.H.; Yu, L.J.; Liu, Y.; Lin, L.; Lu, R.g.; Zhu, J.p.; He, L.; Lu, Z.L. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Catalan-Carrio, R.; Saez, J.; Cuadrado, L.Á.F.; Arana, G.; Basabe-Desmonts, L.; Benito-Lopez, F. Ionogel-based hybrid polymer-paper handheld platform for nitrite and nitrate determination in water samples. Anal. Chim. Acta 2022, 1205, 339753. [Google Scholar] [CrossRef]
- Beaton, A.D.; Cardwell, C.L.; Thomas, R.S.; Sieben, V.J.; Legiret, F.E.; Waugh, E.M.; Statham, P.J.; Mowlem, M.C.; Morgan, H. Lab-on-chip measurement of nitrate and nitrite for in situ analysis of natural waters. Environ. Sci. Technol. 2012, 46, 9548–9556. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.Y.; Jafri, M.Z.M.; San, L.H.; Ho, T.C. Detection of nitrate ions in water by optical fiber. In Proceedings of the 2012 International Conference on Computer and Communication Engineering (ICCCE), Kuala Lumpur, Malaysia, 3–5 July 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 271–273. [Google Scholar]
- Camas-Anzueto, J.; Aguilar-Castillejos, A.; Castañón-González, J.; Lujpán-Hidalgo, M.; De Leon, H.H.; Grajales, R.M. Fiber sensor based on Lophine sensitive layer for nitrate detection in drinking water. Opt. Lasers Eng. 2014, 60, 38–43. [Google Scholar] [CrossRef]
- Mahmud, M.P.; Ejeian, F.; Azadi, S.; Myers, M.; Pejcic, B.; Abbassi, R.; Razmjou, A.; Asadnia, M. Recent progress in sensing nitrate, nitrite, phosphate, and ammonium in aquatic environment. Chemosphere 2020, 259, 127492. [Google Scholar] [CrossRef]
- Su, S.G.; Cheng, H.Y.; Zhu, T.T.; Wang, H.C.; Wang, A.J. A novel bioelectrochemical method for real-time nitrate monitoring. Bioelectrochemistry 2019, 125, 33–37. [Google Scholar] [CrossRef]
- Bluett, S.; O’Callaghan, P.; Paull, B.; Murray, E. Robust off-grid analyser for autonomous remote in-situ monitoring of nitrate and nitrite in water. Talanta Open 2023, 7, 100173. [Google Scholar] [CrossRef]
- Chen, X.; Pu, H.; Fu, Z.; Sui, X.; Chang, J.; Chen, J.; Mao, S. Real-time and selective detection of nitrates in water using graphene-based field-effect transistor sensors. Environ. Sci. Nano 2018, 5, 1990–1999. [Google Scholar] [CrossRef]
- Lee, H.C.; Banerjee, A.; Fang, Y.M.; Lee, B.J.; King, C.T. Design of a multifunctional wireless sensor for in-situ monitoring of debris flows. IEEE Trans. Instrum. Meas. 2010, 59, 2958–2967. [Google Scholar] [CrossRef]
- Jiang, P.; Xia, H.; He, Z.; Wang, Z. Design of a water environment monitoring system based on wireless sensor networks. Sensors 2009, 9, 6411–6434. [Google Scholar] [CrossRef]
- Lambrou, T.P.; Anastasiou, C.C.; Panayiotou, C.G.; Polycarpou, M.M. A low-cost sensor network for real-time monitoring and contamination detection in drinking water distribution systems. IEEE Sens. J. 2014, 14, 2765–2772. [Google Scholar] [CrossRef]
- Corke, P.; Wark, T.; Jurdak, R.; Hu, W.; Valencia, P.; Moore, D. Environmental wireless sensor networks. Proc. IEEE 2010, 98, 1903–1917. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, F. Application of wireless sensor network in water quality monitoring. In Proceedings of the 2017 IEEE International Conference on Computational Science and Engineering (CSE) and IEEE International Conference on Embedded and Ubiquitous Computing (EUC), Guangzhou, China, 21–24 July 2017; IEEE: Piscataway, NJ, USA, 2017; Volume 2, pp. 368–371. [Google Scholar]
- Adu-Manu, K.S.; Tapparello, C.; Heinzelman, W.; Katsriku, F.A.; Abdulai, J.D. Water quality monitoring using wireless sensor networks: Current trends and future research directions. ACM Trans. Sens. Netw. (TOSN) 2017, 13, 1–41. [Google Scholar] [CrossRef]
- Bonastre, A.; Capella, J.; Ors, R.; Peris, M. In-line monitoring of chemical-analysis processes using Wireless Sensor Networks. TrAC Trends Anal. Chem. 2012, 34, 111–125. [Google Scholar] [CrossRef]
- Xu, K.; Chen, Q.; Zhao, Y.; Ge, C.; Lin, S.; Liao, J. Cost-effective, wireless, and portable smartphone-based electrochemical system for on-site monitoring and spatial mapping of the nitrite contamination in water. Sens. Actuators B Chem. 2020, 319, 128221. [Google Scholar] [CrossRef]
- Di Gennaro, P.; Lofú, D.; Vitanio, D.; Tedeschi, P.; Boccadoro, P. WaterS: A Sigfox-compliant prototype for water monitoring. Internet Technol. Lett. 2019, 2, e74. [Google Scholar]
- Gartia, M.R.; Braunschweig, B.; Chang, T.W.; Moinzadeh, P.; Minsker, B.S.; Agha, G.; Wieckowski, A.; Keefer, L.L.; Liu, G.L. The microelectronic wireless nitrate sensor network for environmental water monitoring. J. Environ. Monit. 2012, 14, 3068–3075. [Google Scholar] [CrossRef]
- Tuna, G.; Nefzi, B.; Arkoc, O.; Potirakis, S.M. Wireless sensor network-based water quality monitoring system. In Key Engineering Materials; Trans Tech Publications: Stafa-Zurich, Switzerland, 2014; Volume 605, pp. 47–50. [Google Scholar]
- Wang, X.; Ma, L.; Yang, H. Online water monitoring system based on ZigBee and GPRS. Procedia Eng. 2011, 15, 2680–2684. [Google Scholar] [CrossRef]
- Nasser, N.; Ali, A.; Karim, L.; Belhaouari, S. An efficient Wireless Sensor Network-based water quality monitoring system. In Proceedings of the 2013 ACS International Conference on Computer Systems and Applications (AICCSA), Ifrane, Morocco, 27–30 May 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 1–4. [Google Scholar]
- Capella, J.; Bonastre, A.; Ors, R.; Peris, M. A Wireless Sensor Network approach for distributed in-line chemical analysis of water. Talanta 2010, 80, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Vergina, S.A.; Kayalvizhi, S.; Bhavadharini, R.; Kalpana Devi, S. A real time water quality monitoring using machine learning algorithm. Eur. J. Mol. Clin. Med 2020, 7, 2035–2041. [Google Scholar]
- Agir, I.; Yildirim, R.; Nigde, M.; Isildak, I. Internet of things implementation of nitrate and ammonium sensors for online water monitoring. Anal. Sci. 2021, 37, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Ramadhan, A.; Ali, A.; Kareem, H. Smart water-quality monitoring system based on enabled real-time internet of things. J. Eng. Sci. Technol. 2020, 15, 3514–3527. [Google Scholar]
- Alahi, M.E.E.; Nag, A.; Mukhopadhyay, S.C.; Burkitt, L. A temperature-compensated graphene sensor for nitrate monitoring in real-time application. Sens. Actuators A Phys. 2018, 269, 79–90. [Google Scholar] [CrossRef]
- Wong, Y.J.; Nakayama, R.; Shimizu, Y.; Kamiya, A.; Shen, S.; Rashid, I.Z.M.; Sulaiman, N.M.N. Toward industrial revolution 4.0: Development, validation, and application of 3D-printed IoT-based water quality monitoring system. J. Clean. Prod. 2021, 324, 129230. [Google Scholar] [CrossRef]
- Luna Juncal, M.J.; Skinner, T.; Bertone, E.; Stewart, R.A. Development of a real-time, mobile nitrate monitoring station for high-frequency data collection. Sustainability 2020, 12, 5780. [Google Scholar] [CrossRef]
- Chen, Y.T.; Crossman, J. The impacts of biofouling on automated phosphorus analysers during long-term deployment. Sci. Total Environ. 2021, 784, 147188. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Hassan, S.U.; Warren, B.M.; Makris, K.; Evans, G.W.; Papadopoulou, E.; Coleman, S.; Niu, X. A droplet microfluidic-based sensor for simultaneous in situ monitoring of nitrate and nitrite in natural waters. Environ. Sci. Technol. 2019, 53, 9677–9685. [Google Scholar] [CrossRef]
- Harnsoongnoen, S. Metamaterial-inspired microwave sensor for detecting the concentration of mixed phosphate and nitrate in water. IEEE Trans. Instrum. Meas. 2021, 70, 1–6. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A.; Charoen-In, U.; Siritaratiwat, A. Microwave sensor for nitrate and phosphate concentration sensing. IEEE Sens. J. 2019, 19, 2950–2955. [Google Scholar] [CrossRef]
- Korostynska, O.; Mason, A.; Ortoneda-Pedrola, M.; Al-Shamma’a, A. Electromagnetic wave sensing of NO3 and COD concentrations for real-time environmental and industrial monitoring. Sens. Actuators B Chem. 2014, 198, 49–54. [Google Scholar] [CrossRef]
- Mohammadi, S.; Nadaraja, A.V.; Roberts, D.J.; Zarifi, M.H. Real-time and hazard-free water quality monitoring based on microwave planar resonator sensor. Sens. Actuators A Phys. 2020, 303, 111663. [Google Scholar] [CrossRef]
- Rehmani, M.A.A.; Jaywant, S.A.; Arif, K.M. Study of microchannels fabricated using desktop fused deposition modeling systems. Micromachines 2020, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Clear Water Sensors. Clear Water Sensors—Nitrate + Nitrite Sensor. 2023. Available online: https://www.clearwatersensors.com/nitrate-sensor/ (accessed on 25 July 2023).
- Libelium. Waspmote Smart Water. 2023. Available online: https://www.libelium.com/libeliumworld/smart-water-ions-sensors-calcium-fluoride-chloride-nitrate-iodide-lead-bromide-cupric-silver/ (accessed on 25 July 2023).
- BOQU Instrument Co., Ltd. IoT Digital Multi-Parameter Water Quality Sensor. 2023. Available online: https://www.boquinstruments.com/iot-digital-multi-parameter-water-quality-sensor-product/ (accessed on 25 July 2023).



| Method | Electrode | LOD | Linear Range | Sensitivity | R2 | Ref. |
|---|---|---|---|---|---|---|
| DPV | PEG-SH/SeP/AuNP-modified carbon paper | 8.6 M | [49] | |||
| DPV | Cu nanowires | 1.35 M | 8 to 5860 M | 1.375 A/M | 0.999 | [22] |
| DPV | Cu-immobilized platinum surface | 0.159 M | 0.12 to 4.75 mM | 2.3782 A/M | [57] | |
| LSV | Cu nanowire array | 9.1 M | 10 to 50 | 0.0636 | [58] | |
| 50 to 1500 M | 0.73 A/M | |||||
| Amperometry | Screen-printed silver | 0.207 nM | 0.05 to 5 mM | 19.578 A/M | 0.987 | [59] |
| Amperometry | Screen-printed graphite | 100 nM | 0.1 to 10 mM | 0.12 A/M | 0.999 | [62] |
| Amperometry | Screen-printed carbon | 0.01 to 0.25 nM | 3.13 A/M | 0.97 | [63] | |
| Amperometry | Polypyrrole/carbon nanotube film | 0.17 mM | 0.44 to 1.45 mM | 300 | 0.97 nA/mM | [35] |
| Potentiometry | Screen-printed carbon | 100 mM | 0.1 to 100 mM | [64] | ||
| EIS | Interdigital capacitive | 1 to 10 mg/L | [61] | |||
| EIS | Graphene foam-based | 1 to 1000 M | 0.316 k/M/cm2 | [34] |
| Method | Absorbance Range | LOD | Dynamic Range | Sensitivity | Ref. |
|---|---|---|---|---|---|
| Spectrophotometry | 540 nm | 3 M | 3–334 M | 0.5 M | [73] |
| UV-Vis spectrometry | 365 nm | 2.3 mg/L | 2.3–3.4 mg/L | [74] | |
| Colorimetry | 0.025 M | 0.025–350 M | 0.025 M | [75] | |
| Fiber optic | 575 mn | 0–2.5 mg/L | [76] |
| Wireless | Merits | Shortcomings | Ref. |
|---|---|---|---|
| Transmission | |||
| Bluetooth | 1. Simple and convenient | 1. Less distance covered | [89] |
| communication | 2. Supports fewer nodes | ||
| 2. Data transfer rate can meet | 3. Transmission stability | ||
| the requirements for short-distance transmission | is not high | ||
| 3. Completely free | 4. Unreliable communication | ||
| Wi-Fi | 1. High bandwidth | 1. Short range | [18] |
| 2. Fast communication due | 2. High power consumption | ||
| to higher transmission speed | 3. Cost is involved | ||
| 3. A variety of large-scale | 4. Security issues | ||
| wireless device manufacturers | |||
| are confirmed | |||
| ZigBee | 1. Low power consumption | 1. Low data speed | [86] |
| 2. Easy to install | 2. Low transmission, as well as | ||
| 3. Supports a large number of nodes | low network stability | ||
| 4. High-reliability network | |||
| 5. Strong security | |||
| LoRa | 1. Low power consumption | 1. Limited bandwidth | [47] |
| 2. Long-range coverage | 2. Limited message size | ||
| 3. Supports a large number of nodes | 3. Complex network setup | ||
| Sigfox | 1. Low power consumption | 1. Limited bandwidth | [90] |
| 2. Long-range coverage | 2. Limited network availability | ||
| 3. Low cost | 3. Small data packet | ||
| 4. Easily scalable |
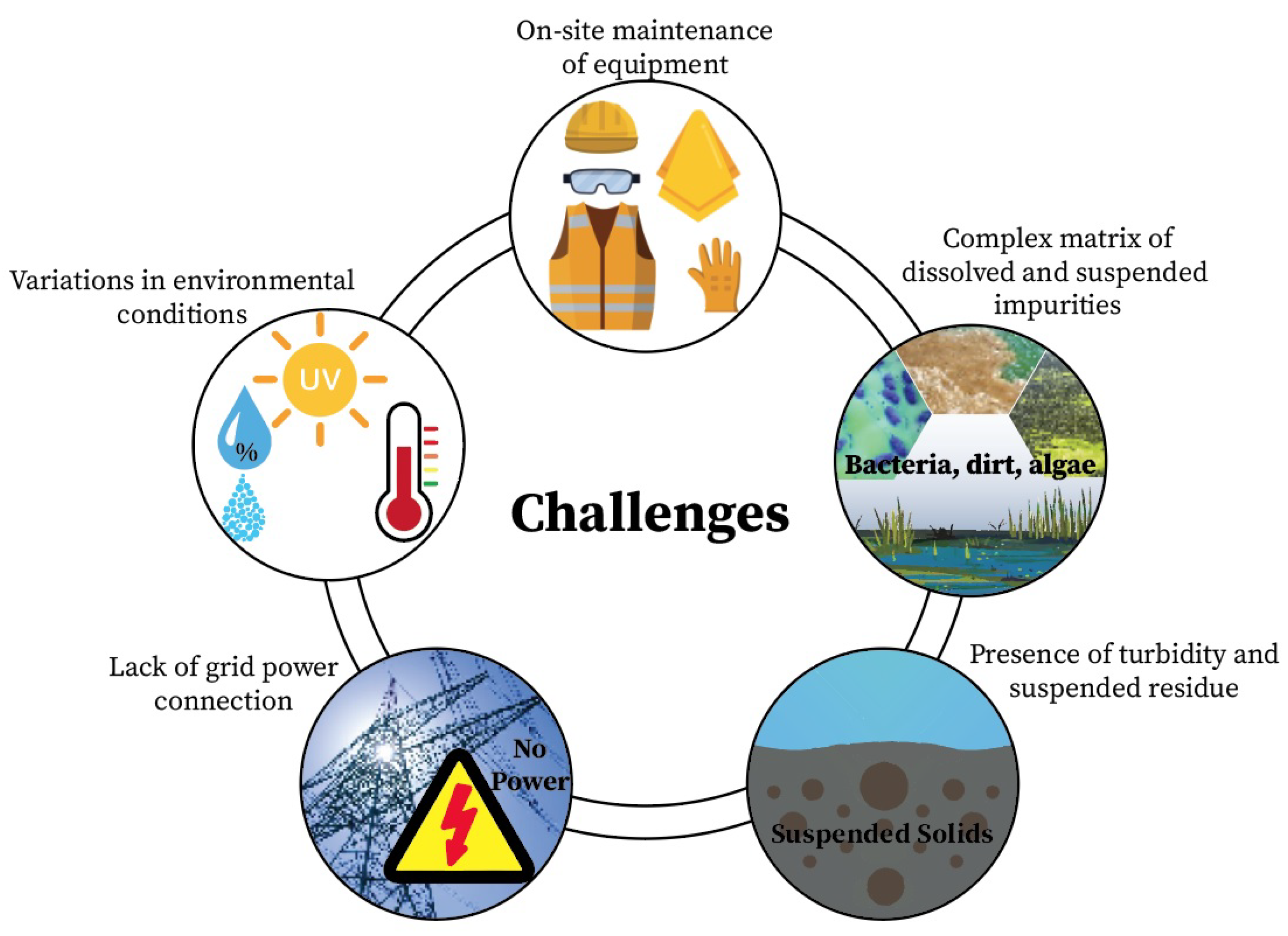
| Potential Methods | Strengths | Limitations |
|---|---|---|
| Colorimetry | Lower energy consumption Use of microfluidic system reduces reagent quantity | Influenced by temperature variation Needs regulator calibration Requires regular cleaning of sensor Reagents are often hazardous and toxic Turbidity variations affect accuracy |
| UV spectroscopy | High sensitivity Non-destructive technique Rapid measurements Wide wavelength range | Sensitive to turbidity Prone to inaccurate data due to sample matrix Variability in absorbance maxima |
| Electrochemistry | Quick response Affordable equipment Wide dynamic range Non-destructive technique Ability to perform on-site measurements | Suffers from drift overtime Sensitivity to temperature changes Prone to inaccurate data due to sample matrix |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lal, K.; Jaywant, S.A.; Arif, K.M. Electrochemical and Optical Sensors for Real-Time Detection of Nitrate in Water. Sensors 2023, 23, 7099. https://doi.org/10.3390/s23167099
Lal K, Jaywant SA, Arif KM. Electrochemical and Optical Sensors for Real-Time Detection of Nitrate in Water. Sensors. 2023; 23(16):7099. https://doi.org/10.3390/s23167099
Chicago/Turabian StyleLal, Kartikay, Swapna A. Jaywant, and Khalid Mahmood Arif. 2023. "Electrochemical and Optical Sensors for Real-Time Detection of Nitrate in Water" Sensors 23, no. 16: 7099. https://doi.org/10.3390/s23167099
APA StyleLal, K., Jaywant, S. A., & Arif, K. M. (2023). Electrochemical and Optical Sensors for Real-Time Detection of Nitrate in Water. Sensors, 23(16), 7099. https://doi.org/10.3390/s23167099







