Potential of a Miniature Spectral Analyzer for District-Scale Monitoring of Multiple Gaseous Air Pollutants
Abstract
1. Introduction
2. Materials and Methods
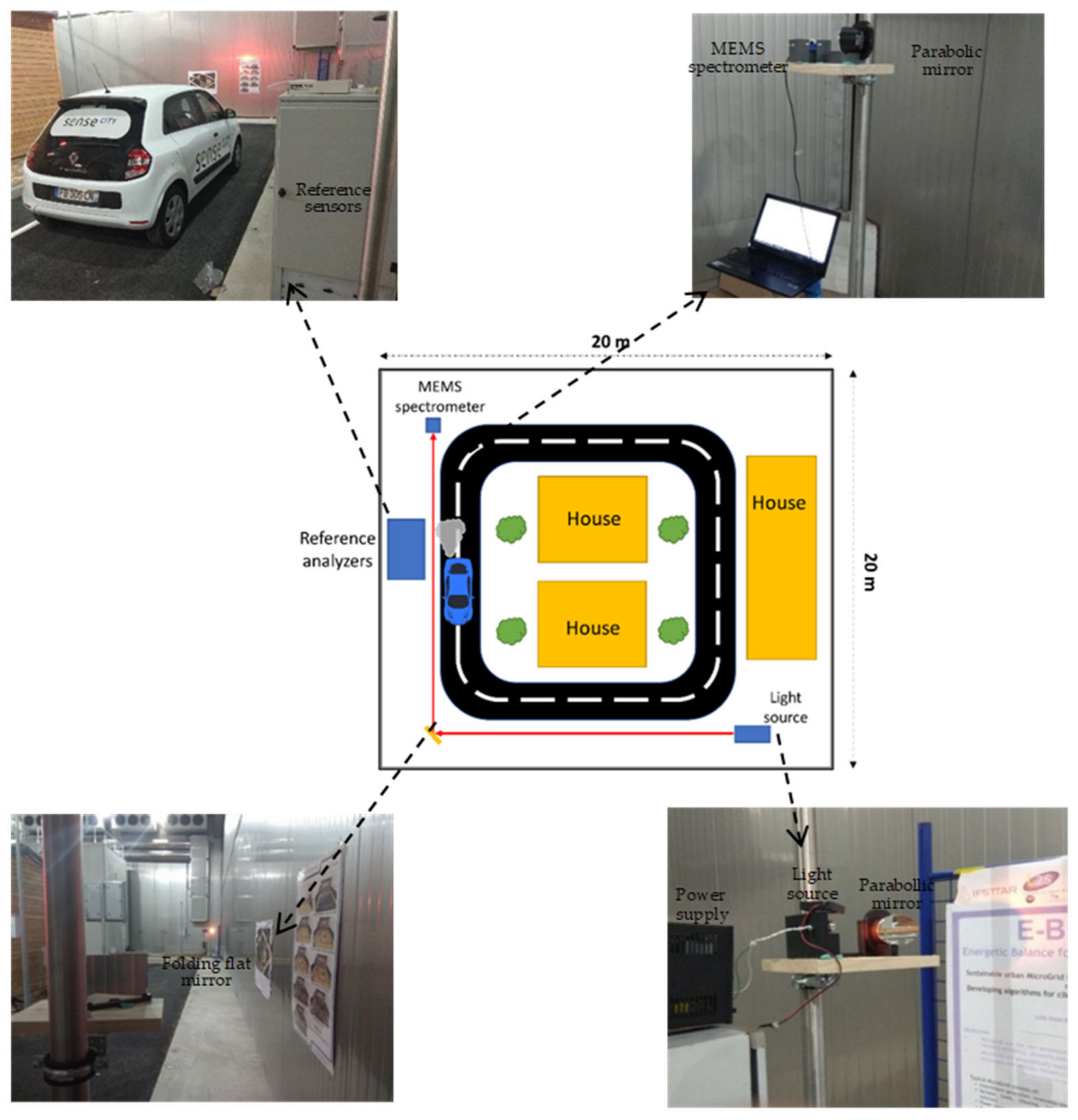
2.1. Sense City: A District-Scale Climatic Chamber
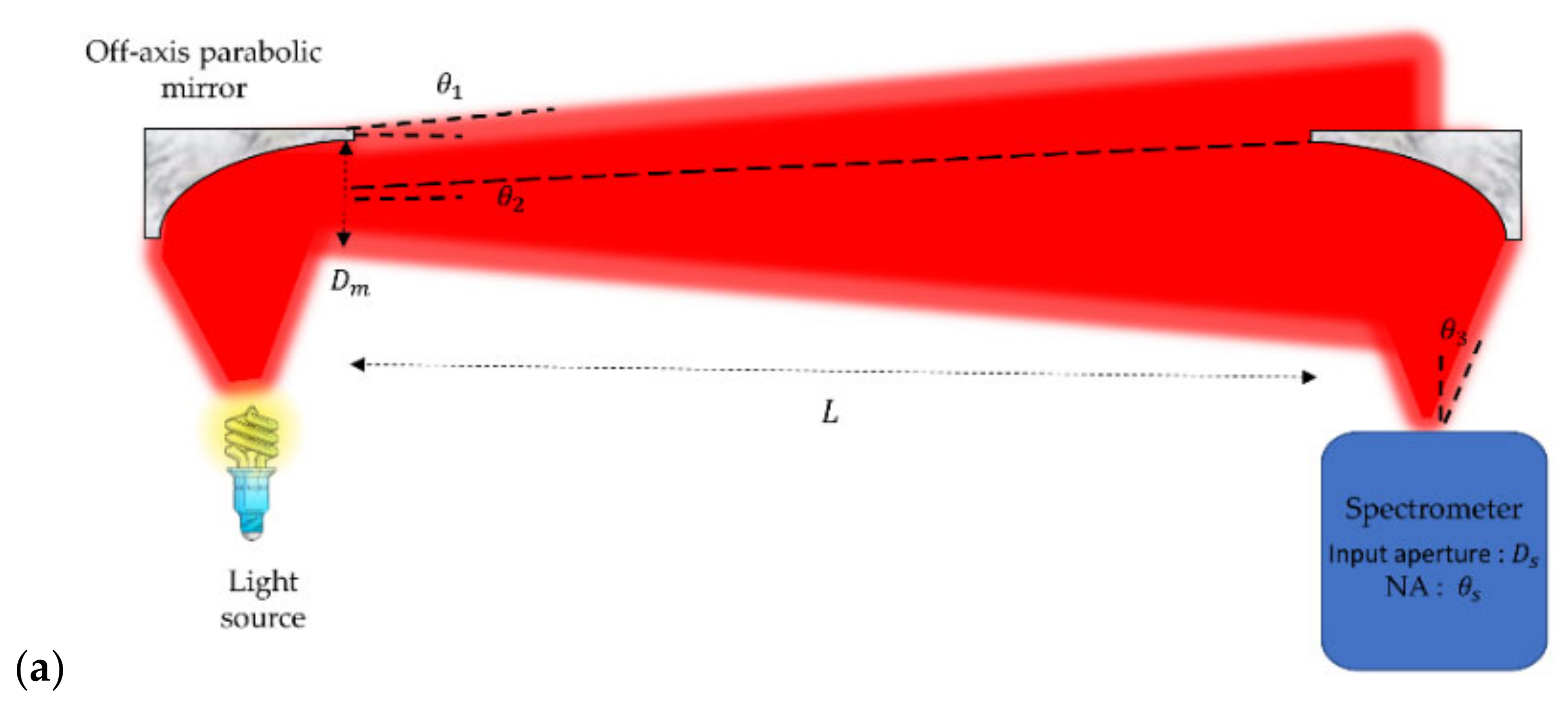
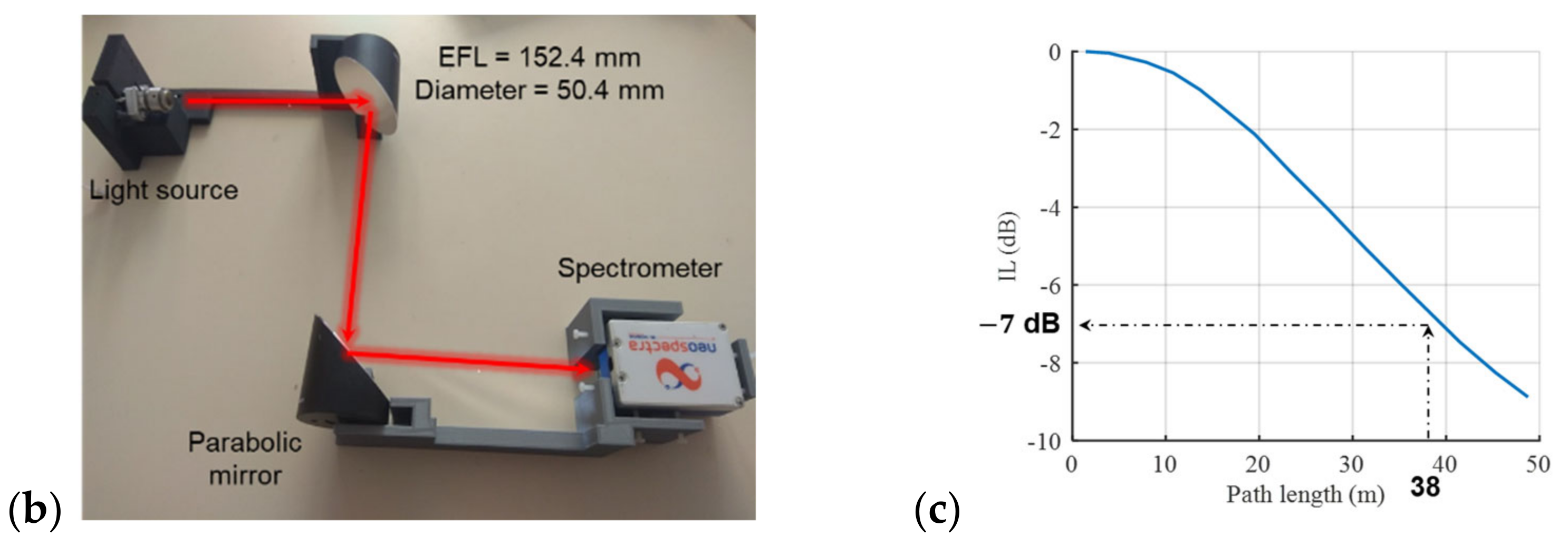
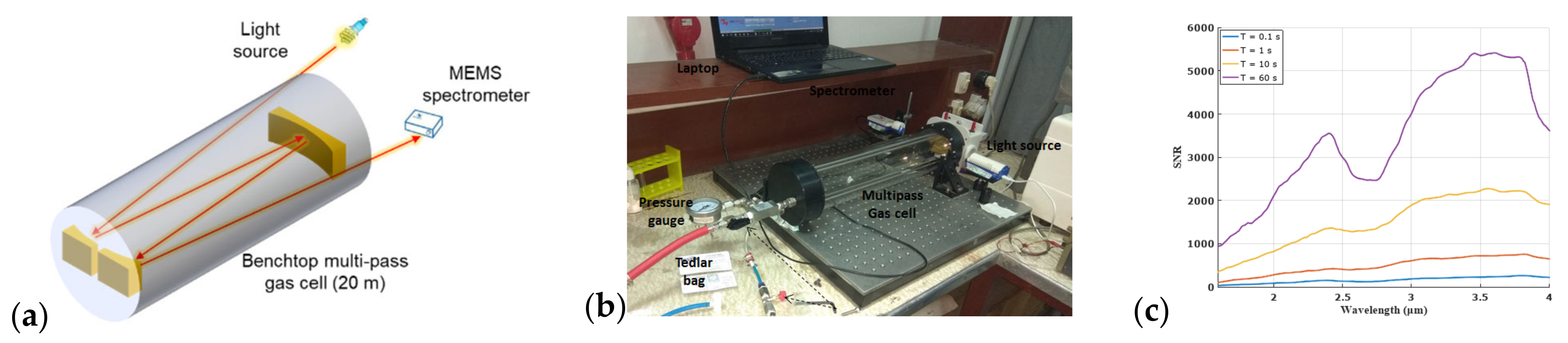
2.2. Experimental Setup
3. Results and Discussion
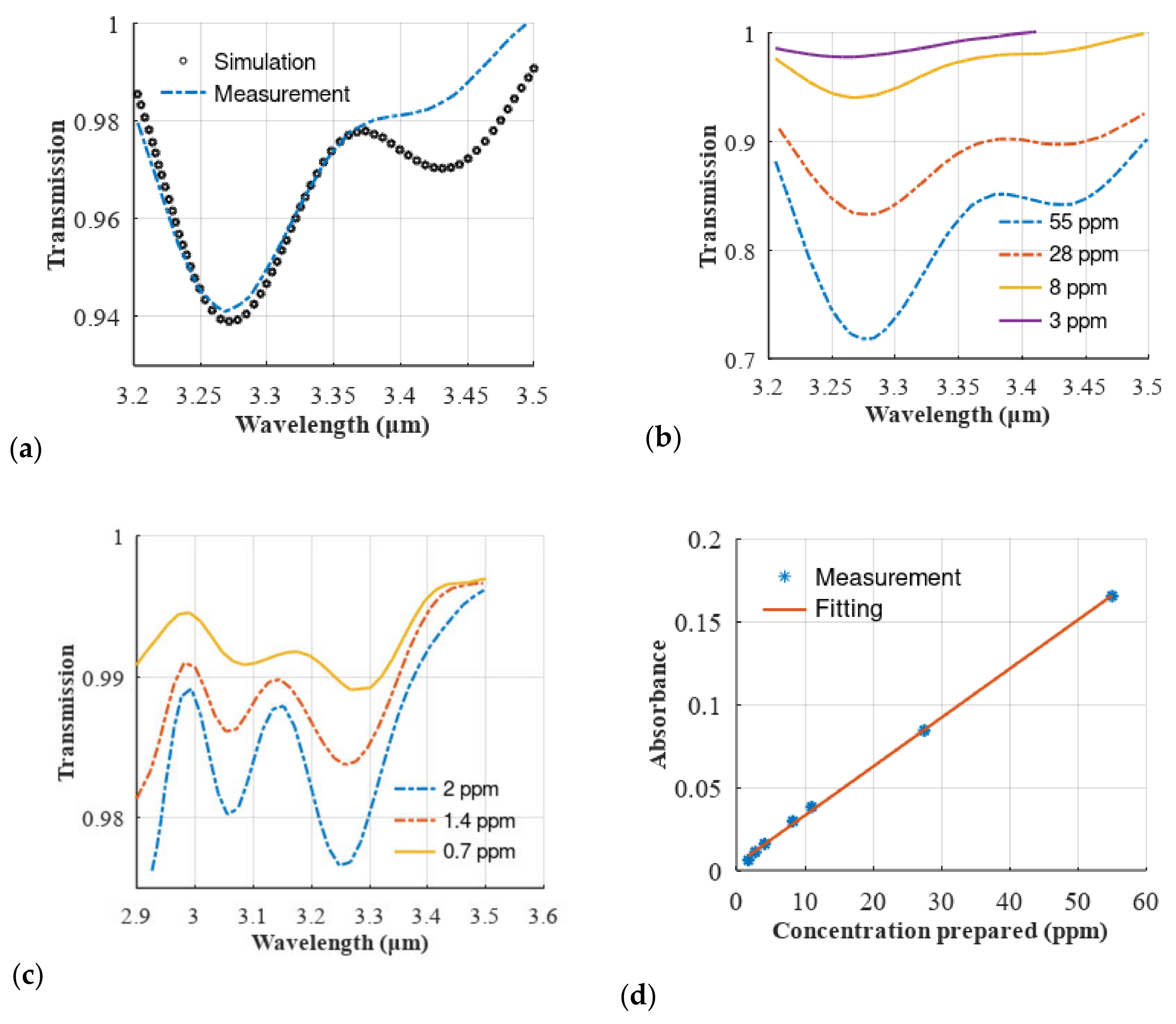
3.1. Design Considerations on the Setup for Open-Path Gas Absorption Spectroscopy
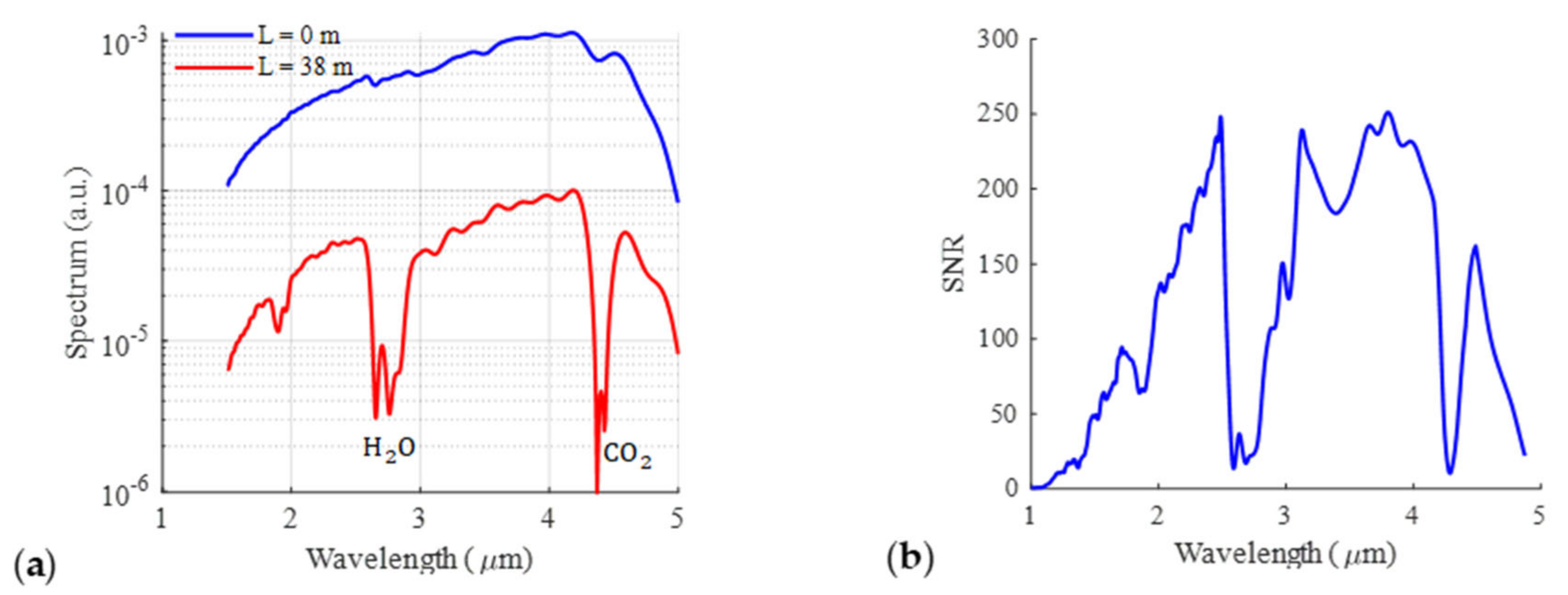
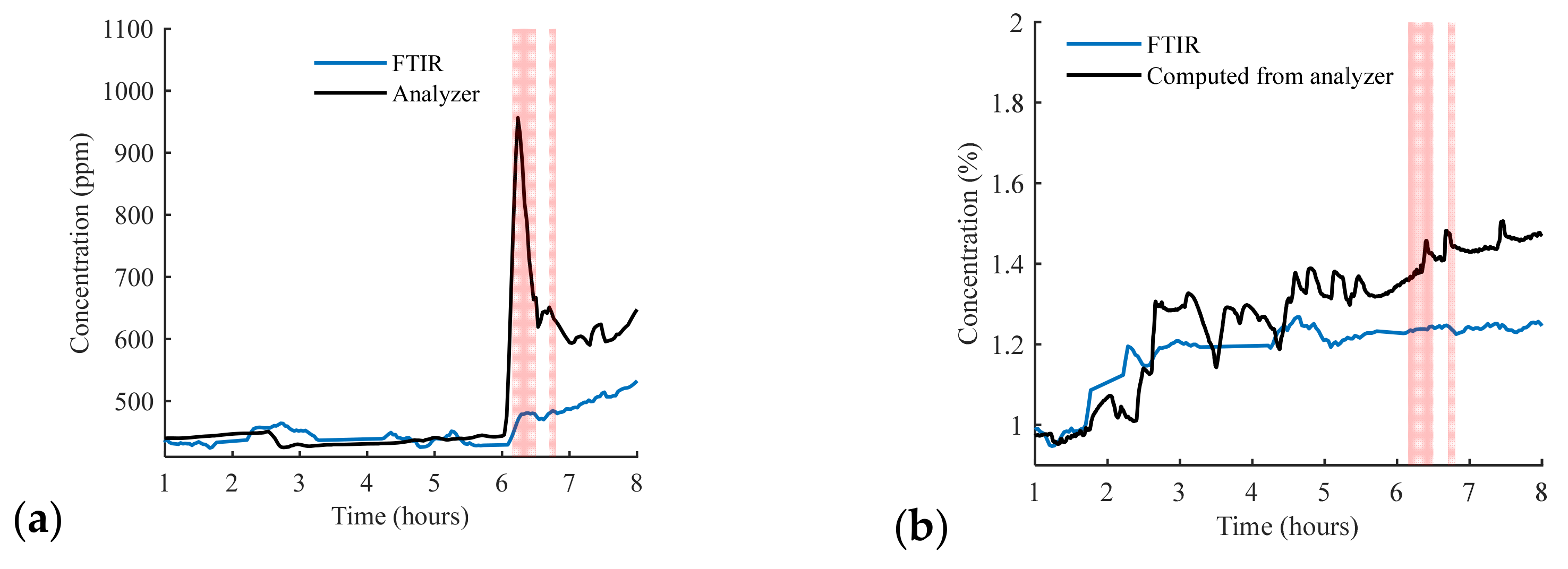
3.2. District-Scale Experimental Study in Sense City
3.3. Laboratory-Scale Study on Volatile Organic Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Air Pollution. Available online: https://www.who.int/health-topics/air-pollution#tab=tab_1 (accessed on 16 August 2020).
- Gallego, E.; Folch, J.; Teixidor, P.; Roca, F.J.; Perales, J.F. Outdoor air monitoring: Performance evaluation of a gas sensor to assess episodic nuisance/odorous events using active multi-sorbent bed tube sampling coupled to TD-GC/MS analysis. Sci. Total Environ. 2019, 694, 133752. [Google Scholar] [CrossRef] [PubMed]
- Connolly, R.E.; Yu, Q.; Wang, Z.; Chen, Y.-H.; Liu, J.Z.; Collier-Oxandale, A.; Papapostolou, V.; Polidori, A.; Zhu, Y. Long-term evaluation of a low-cost air sensor network for monitoring indoor and outdoor air quality at the community scale. Sci. Total Environ. 2022, 807, 150797. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Du, W.; Lei, Y.; Chen, Y.; Wang, Z.; Mao, K.; Tao, S.; Pan, B. Quantifying the dynamic characteristics of indoor air pollution using real-time sensors: Current status and future implication. Environ. Int. 2023, 175, 107934. [Google Scholar] [CrossRef] [PubMed]
- Palomeque-Mangut, S.; Meléndez, F.; Gómez-Suárez, J.; Frutos-Puerto, S.; Arroyo, P.; Pinilla-Gil, E.; Lozano, J. Wearable system for outdoor air quality monitoring in a WSN with cloud computing: Design, validation and deployment. Chemosphere 2022, 307, 135948. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.C.; Kumar, P.; Pilla, F.; Skouloudis, A.N.; Di Sabatino, S.; Ratti, C.; Yasar, A.; Rickerby, D. End-user perspective of low-cost sensors for outdoor air pollution monitoring. Sci. Total Environ. 2017, 607–608, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Baraton, M.-I.; Merhari, L. Nanoparticles-based chemical gas sensors for outdoor air quality monitoring microstations. Mater. Sci. Eng. B 2004, 112, 206–213. [Google Scholar] [CrossRef]
- Griffith DW, T.; Pöhler, D.; Schmitt, S.; Hammer, S.; Vardag, S.N.; Platt, U. Long open-path measurements of greenhouse gases in air using near-infrared Fourier transform spectroscopy. Atmos. Meas. Tech. 2018, 11, 1549–1563. [Google Scholar] [CrossRef]
- Deutscher, N.; Griffith, D.; Naylor, T.; Caldow, C.; McDougall, H.; Rayner, P.; Silver, J. Improved open path FTIR detection of fugitive CO2, CH4 and other trace gases in the atmosphere. Geophys. Res. Abstr. 2019, 21, 1. [Google Scholar]
- You, Y.; Moussa, S.G.; Zhang, L.; Fu, L.; Beck, J.; Staebler, R.M. Quantifying fugitive gas emissions from an oil sands tailings pond with open-path Fourier transform infrared measurements. Atmos. Meas. Tech. 2021, 14, 945–959. [Google Scholar] [CrossRef]
- Todd, L.A.; Ramanathan, M.; Mottus, K.; Katz, R.; Dodson, A.; Mihlan, G. Measuring chemical emissions using open-path Fourier transform infrared (OP-FTIR) spectroscopy and computer-assisted tomography. Atmos. Environ. 2001, 35, 1937–1947. [Google Scholar] [CrossRef]
- Kirchgessner, D.A.; Piccot, S.D.; Chadha, A. Estimation of methane emissions from a surface coal mine using open-path FTIR spectroscopy and modeling techniques. Chemosphere 1993, 26, 23–44. [Google Scholar] [CrossRef]
- Hong, D.W.; Heo, G.S.; Han, J.S.; Cho, S.Y. Application of the open path FTIR with COL1SB to measurements of ozone and VOCs in the urban area. Atmos. Environ. 2004, 38, 5567–5576. [Google Scholar] [CrossRef]
- Richter, D.; Erdelyi, M.; Curl, R.F.; Tittel, F.K.; Oppenheimer, C.; Duffell, H.J.; Burton, M. Field measurements of volcanic gases using tunable diode laser based mid-infrared and Fourier transform infrared spectrometers. Opt. Lasers Eng. 2002, 37, 171–186. [Google Scholar] [CrossRef]
- Sense-City, Marne-la-Vallée, France. Available online: https://sense-city.ifsttar.fr/en/ (accessed on 14 May 2023).
- Wolfe, W.L. Introduction to Radiometry; SPIE Press: Bellingham, WA, USA, 1998. [Google Scholar]
- Lieber, C.A.; Mahadevan-Jansen, A. Automated method for subtraction of fluorescence from biological Raman spectra. Appl. Spectrosc. 2003, 57, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-M.; Chen, S.; Liang, Y.-Z. Baseline correction using adaptive iteratively reweighted penalized least squares. Analyst 2010, 135, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Griffiths, P.R. Automatic baseline correction by wavelet transform for quantitative open-path Fourier transform infrared spectroscopy. Environ. Sci. Technol. 2007, 41, 7054–7059. [Google Scholar] [CrossRef] [PubMed]
- Magnus, G. Versuche über die Spannkräfte des Wasserdampfs. Ann. Phys. 1844, 137, 225–247. [Google Scholar] [CrossRef]
- Childers, J.W.; Thompson, E.L., Jr.; Harris, D.B.; Kirchgessner, D.A.; Clayton, M.; Natschke, D.F.; Phillips, W.J. Multi-pollutant concentration measurements around a concentrated swine production facility using open-path FTIR spectrometry. Atmos. Environ. 2001, 35, 1923–1936. [Google Scholar] [CrossRef]
- Griffiths, P.R.; Shao, L.; Leytem, A.B. Completely automated open-path FT-IR spectrometry. Anal. Bioanal. Chem. 2009, 393, 45–50. [Google Scholar] [CrossRef] [PubMed]
- SBIR. SBIR Phase II: An Improved Open-Path FTIR Spectrometer for Remote Monitoring of Atmospheric Gases. Available online: https://www.sbir.gov/sbirsearch/detail/392679 (accessed on 14 May 2023).
- Fathy, A.; Sabry, Y.M.; Amr, M.; Gnambodoe-Capo-chichi, M.; Anwar, M.; Ghoname, A.O.; Amr, A.; Saeed, A.; Gad, M.; Al Haron, M.; et al. MEMS FTIR optical spectrometer enables detection of volatile organic compounds (VOCs) in part-per-billion (ppb) range for air quality monitoring. In Proceedings of the MOEMS and Miniaturized Systems XVIII, Photonics West Conference, San Francisco, CA, USA, 2–7 February 2019; SPIE: Bellingham, WA, USA, 2019; Volume 10931, p. 1093109. [Google Scholar]


| Gas | Open-Path FTIR | Reference Analyzer | Outdoor | |
|---|---|---|---|---|
| Wavelength (nm) | Detection Limit (1) | Detection Limit (1) | Typical Values | |
| Relative humidity (RH) | 2700 | 0.07% | - | 30–50% |
| 4596 | 800 ppb | 25 ppb | 50–120 ppb | |
| 4256 | 40 ppb | - | 250–400 ppm | |
| 2677 | 50 ppm | 0.2 ppb | 0.1–20 ppb | |
| 3446 | 1 ppm | 0.2 ppb | 40–500 ppb | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathy, A.; Gnambodoe-Capochichi, M.; Sabry, Y.M.; Anwar, M.; Ghoname, A.O.; Saeed, A.; Leprince-Wang, Y.; Khalil, D.; Bourouina, T. Potential of a Miniature Spectral Analyzer for District-Scale Monitoring of Multiple Gaseous Air Pollutants. Sensors 2023, 23, 6343. https://doi.org/10.3390/s23146343
Fathy A, Gnambodoe-Capochichi M, Sabry YM, Anwar M, Ghoname AO, Saeed A, Leprince-Wang Y, Khalil D, Bourouina T. Potential of a Miniature Spectral Analyzer for District-Scale Monitoring of Multiple Gaseous Air Pollutants. Sensors. 2023; 23(14):6343. https://doi.org/10.3390/s23146343
Chicago/Turabian StyleFathy, Alaa, Martine Gnambodoe-Capochichi, Yasser M. Sabry, Momen Anwar, Amr O. Ghoname, Ahmed Saeed, Yamin Leprince-Wang, Diaa Khalil, and Tarik Bourouina. 2023. "Potential of a Miniature Spectral Analyzer for District-Scale Monitoring of Multiple Gaseous Air Pollutants" Sensors 23, no. 14: 6343. https://doi.org/10.3390/s23146343
APA StyleFathy, A., Gnambodoe-Capochichi, M., Sabry, Y. M., Anwar, M., Ghoname, A. O., Saeed, A., Leprince-Wang, Y., Khalil, D., & Bourouina, T. (2023). Potential of a Miniature Spectral Analyzer for District-Scale Monitoring of Multiple Gaseous Air Pollutants. Sensors, 23(14), 6343. https://doi.org/10.3390/s23146343








