Abstract
Microfluidic technology is a powerful tool to enable the rapid, accurate, and on-site analysis of forensically relevant evidence on a crime scene. This review paper provides a summary on the application of this technology in various forensic investigation fields spanning from forensic serology and human identification to discriminating and analyzing diverse classes of drugs and explosives. Each aspect is further explained by providing a short summary on general forensic workflow and investigations for body fluid identification as well as through the analysis of drugs and explosives. Microfluidic technology, including fabrication methodologies, materials, and working modules, are touched upon. Finally, the current shortcomings on the implementation of the microfluidic technology in the forensic field are discussed along with the future perspectives.
1. Introduction
Forensic investigations cover a wide range of diagnosis spanning from identification and analysis of body fluids to drugs of abuse and explosive residues. For each category, well-developed presumptive and confirmatory tests have already been established, which enable forensic investigators and police forces to develop a chain of events and, more importantly, identify the engaged individuals. To find and apprehend any suspects, time may be of the essence. According to Addington, “murders are solved quickly or not at all” [1]. The chance of solving a case normally drops significantly after one week. This further clarifies the need for prompt on-scene identification combined with a qualitative and quantitative analysis of samples during the first hours of investigation, i.e., “golden hours” [2]. Microfluidic technology can address these needs due to distinctive properties, namely portability (small footprint), requirement of small volumes of precious sample, and flexible design providing instant sample analysis. This technology further provides the possibility of (1) integrating multiple analysis steps and (2) analysis of multiple analytes (multiplexing) in a single platform contributing to effective case scenario developments. There are multiple review articles in which the application of microfluidics in specific areas of forensic diagnosis, namely DNA analysis and on-scene human identification [3,4] as well as drug analysis [5], are detailed. In a review paper by Musile et al., applications of a subclass of microfluidic platforms (paper-based microfluidic devices) are discussed for DNA, drug, and explosive analyses with a specific focus on fabrication strategies [6].
This review paper aims to provide a comprehensive overview on “forensic investigations (Section 2)”, “microfluidic technology (Section 3)”, and “microfluidics in forensic applications (Section 4)”. In Section 2, an abridged overview on the current state-of-the-art presumptive and confirmatory methods for forensic serology (detection and identification of body fluids, namely blood, semen, and saliva) is given. In this section, the most encountered classes of drugs and explosives along with the corresponding presumptive and confirmatory methods are further introduced. In Section 3, a general summary on the microfluidic technology, applications, fabrication methods, materials, and various modules which exist in a microfluidic platform are provided. To bridge these two parts, in Section 4 the application of microfluidic technology in various categories of forensic investigations are outlined. These categories include forensic serology, genetic profiling and human identification, screening and analysis of drugs (seized drugs and drugs in biological samples), and analysis of explosives. Finally, the shortcomings of the microfluidic technology for forensic applications, future considerations, and perspectives are provided in Section 5.
2. Forensic Investigations
2.1. Forensic Serology
Body fluids are among the most vital pieces of evidence found at a crime scene. In serological analysis, evidence is sampled and tested for the presence of body fluids (BFs). The detection and identification of body fluids recovered from a crime scene provide contextual information for forensic casework such as event timeline, scene reconstruction, and involvement of possible individuals [4]. The detection (determining the presence or absence of body fluids) and identification of BFs (determining the source to conclusively identify an individual via further laboratory testing including DNA analysis) are vital in a wide range of investigations [7].
Identifying the nature of a fluid is not always straightforward as many BFs share the same appearance and are invisible to the naked eye [8]. Further absolute confirmation is always necessary for the evidence to be court-proof. The most common BFs discovered at crime scenes are blood, semen, and saliva. Other BFs such as vaginal fluid, sweat, and urine are also essential evidence since they contain valuable genetic information (e.g., DNA). Traditional techniques that are currently used for forensic identification of BFs are categorized into presumptive and confirmatory tests [7]. Presumptive tests are preliminary indicators which can establish the likelihood of the presence and/or absence of a certain BF. Confirmatory tests are utilized to conclusively specify a certain biological material/substance in the BF. Both tests are intended to save time and money via prioritizing samples for further DNA analysis [9]. In review papers by Virkler et al. and An et al., the current and emerging methods for the identification of all BFs are extensively discussed [8,10]. The possible results of the tests can be categorized into (a) true positive (or negative) where the species of interest are (or are not) present and the test outcome indicates a positive (or negative) result; (b) false positive (or negative) wherein the species of interest are not (or are) present, but the test outcome indicates a positive (or negative) result [11]. The advantages of presumptive tests include simplicity, ease of interpretation, narrowing the options for the subsequent test, possibility of utilization on larger areas, and locating evidence not visible to the naked eye. The risk of false positive/negative results, being body-fluid-specific, destructive to genetic evidence (DNA), and not being label-free are among the main disadvantages of most of the presumptive tests [7]. The results of presumptive tests must be supported by confirmatory tests which have a reduced risk of false positive/negative results and can conclusively identify a substance. However, they are costly, time-consuming, non-universal, require additional equipment, and intense sample preparation. Emerging vibrational spectroscopic techniques such as Raman spectroscopy have the potential to provide a universal, non-destructive, and label-free technique for body fluid identification (BFID) at crime scenes [12,13,14]. Raman spectroscopy is intrinsically a very selective and non-destructive method which can preserve the DNA evidence of the tested BF. It has thus gained increasing attention among forensic scientists over the last decade. For instance, coupling Raman spectroscopy with advanced chemometric analysis showed the possibility of determining a donor’s sex as well as race from saliva and blood stains, respectively [15,16]. Using this coupled technique, a differentiation between human and animal blood is also possible [17,18]. This method, based on pairing Raman spectroscopy with chemometrics, can identify and discriminate six forensically relevant BFs, namely blood, semen, saliva, sweat, vaginal fluid, and urine [7,15]. Via Raman spectroscopic mapping on different BFs, a spectral data sheet library is generated. Subsequently a classification model known as “support vector machine discriminant analysis” is developed during the analysis which can achieve a 100% accuracy in the validation step. For more information on this method, the reader is referred to the published works of the Lednev research group at the University of Albany, USA [7,15].
2.1.1. Blood
Presumptive Tests
A good presumptive test for blood should be sensitive, quick, simple, safe, and specific [19]. Blood presumptive tests should detect a certain blood component which cannot be commonly found in everyday environment/chemicals [20]. Most presumptive tests for blood achieve this goal based on the peroxidase-like activity of hemoglobin [20]. Blood presumptive tests can be classified into two general categories, i.e., catalytic color tests and forensic (or alternative) light source. Catalytic tests are the largest group of blood-presumptive tests.
All catalytic tests share a similar mechanism at the early step, namely the oxidation of the reagent by a peroxide (e.g., hydrogen peroxide) in the presence of a catalyst (e.g., peroxidase). As stated before, hemoglobin triggers the oxidation of the reagent due to its peroxidase-like activity. These tests are based on peroxidase-catalyzed oxidation in which the oxidation state of a reagent is changed, leading to a color change in the substrate (reagent) [21]. A subcategory of catalytic color tests is chemiluminescence, in which, upon reaction of the reagent with hemoglobin, a light is emitted. In this case, the reagent is electronically excited leading to a subsequent emission of light instead of a color change [21,22]. The production of light is through luminescence and does not require an alternate light source (ALS) [23]. Fluorescein is another common presumptive test which, upon reaction with hemoglobin, produces light. A light source with a wavelength of 415–480 nm (blue light in the visible spectrum) is required to excite fluorescein [24]. In forensic light source or alternate light source (ALS), no chemical reaction is happening and thus it is the safest technique for the investigators. An example is Polilight®, in which the suspected area is simply illuminated via a bright light with an adjustable wavelength. The reflected light or the emitted fluorescence (when ultraviolet (UV) wavelengths are used) are an indication of the blood stains [21]. Table 1 summarizes the widely used presumptive tests for blood.

Table 1.
Most commonly used presumptive blood tests based on chemical reaction (catalytic color change) along with corresponding pros and cons (inputs from references [20,21,22]).
Confirmatory Tests
After a positive presumptive test, confirmatory tests should be performed to identify the substance with the lowest likelihood of a false positive [24]. The confirmatory tests can be categorized as follows:
I- Crystal tests (e.g., Takayama and Teichman tests)
The working principle is based on the formation of crystals from heme (an iron-containing prosthetic group which helps the hemoglobin protein function properly). Since other proteins also use heme (such as catalases and peroxidases), they could lead to false positives.
II- Immunochromatographic tests (e.g., HemaTrace®, RSID, ELISA)
The working mechanism is based on liquid chromatography in which molecules are separated according to their speed of transport through a liquid. In these tests, a solid phase is also present which uses antibodies. Antibodies are proteins that recognize the shape and characteristic of a biological substance. The immunoassay technique is based on a specific antibody which selectively binds the molecule of interest (antigen). Subsequently, an antibody–antigen complex is formed, which includes a label and can be detected using florescence, for instance. The binding between antibody and antigen is a resemblance of immune system response in which antibodies are generated and bind to antigens (invaders) to remove them [25]. Upon mixing an antibody and antigen in the right ratio, a lattice called precipitin is formed. Cross-reactivity (antibody binds to two or more antigens) can be problematic, leading to false positives. A selective antibody is the one which reacts with very few antigens. In rapid stain identification for blood (RSID-blood), an antibody which recognizes glycophorin (a protein found in erythrocytes (red blood cells)) is used. HemaTrace® uses an antibody which recognizes hemoglobin. Enzyme-linked immunosorbent assay (ELISA) identifies blood based on a similar approach using different antibodies [8]. Immunochromatographic tests generally require specific buffer solutions for the elution of the biological sample. If the BF consists of various materials (e.g., saliva or semen), the application of different tests can be troublesome. Recently, Basset et al. developed a protocol for performing three immunochromatographic tests using the same buffer [9]. RSID is a lateral flow test strip composed of (1) a membrane component enclosed in a plastic cassette, (2) a sample well, and (3) a visualization window. It provides qualitative results as positive or negative depending on the presence or absence of a red or blue line upon adding the sample to the sample well. RSID reader systems have been developed to record and report the results [26].
III- Microscopic tests (e.g., scanning electron microscopy (SEM))
This method concerns the direct visualization of blood cells under a microscope. The visual identification of blood components, such as red and white cells as well as fibrin, is a conclusive proof establishing the presence of blood [8]. SEM imaging of non-conductive materials generally requires the coating of the material with a conductive layer. Coated bloodstains can be imaged at high vacuum mode and high accelerating voltage, leading to high resolution and thus a high level of surface details. When a high level of surface details is not needed, high vacuum and low accelerating voltage can be used for imaging non-coated samples [27,28,29]. Environmental (or variable-pressure) SEM, which works at low vacuum levels with high accelerating voltage, is usually used for examining non-coated bloodstains [30,31]. Advanced light microscopy techniques, such as confocal laser scanning microscope (CLSM), have been recently utilized for imaging bloodstains [32]. CLSM images can provide similar surface details compared to the SEM images of non-coated bloodstains taken at high vacuum and low accelerating voltage. Examining bloodstains on relatively large objects, e.g., household items, using conventional SEM is not practical due to the size constraints of the sample chamber. Light microscopy, e.g., CLSM, does not have the sample size constraint to the same extent as the SEM imaging does. In the case of CLSM, the sample size is dependent on the XYZ range of the motorized stage, which is larger than the conventional SEM chamber [33].
IV- Spectroscopic tests (e.g., ultraviolet-visible (UV–Vis) spectroscopy)
UV–Vis absorption is a reliable technique for confirming the presence of blood. The working mechanism is based on the characteristic absorbance band of various derivatives of hemoglobin, which makes it possible to distinguish between these different derivatives. This absorbance band which is around 400 nm is called Soret band [34]. This method is useful for the identification of older stains which show negative results via presumptive or crystal tests [8]. Environmental conditions, e.g., exposure of the bloodstain to sunlight, heat, or rust interferes with the UV–Vis spectral results. Fluorescence spectroscopy is another method which is based on fluorescence of hematoporphyrin upon excitation with UV light. This method is not affected by the exposure of the bloodstain to environmental conditions and is useful for the detection of old bloodstains on oxidized surfaces [8].
V- Chromatographic tests (e.g., LC–MS/GC–MS)
Examples of these methods include liquid chromatography–mass spectrometry (LC–MS) and/or gas chromatography–mass spectrometry (GC–MS), which are based on the separation of hemoglobin and its derivatives to identify blood. These methods are normally time-consuming since they involve multiple steps and require sample preparation. For more detailed information, the reader is referred to the review paper by Virkler et al. [8].
VI- Methods based on mRNA
These confirmatory tests for blood utilize messenger RNA (mRNA) as, in many proteins, specific sequences of mRNA can be found in high quantity [8,24]. The first step in these methods is the fabrication of a DNA copy of the RNA in the bloodstain using reverse transcriptase [24]. Subsequently analogous to the common DNA profiling, PCR (polymerase chain reaction) is used to amplify the DNA copy. Due to the functional differences between cells and tissues, mRNA markers can be used to identify the most forensically relevant body fluids [35]. In the case of blood, a reliable differentiation between menstrual and non-menstrual blood can be obtained [36]. This method should be used with caution as it can lead to false negative results. Defining cut-off values for the control markers and quantifying corresponding PCR results can be implemented to address the shortcomings [35]. One of the major challenges of this method is RNA degradation and fragmentation after death, leading to a reduction in overall RNA. The results by Bauer et al. revealed that suitable mRNA for reverse transcription PCR (RT-PCR) can still be obtained after post-mortem intervals of around 96 h [37]. mRNA can also be extracted from dried stains as old as 15 years [38]. The condition of storage is important as unfavorable conditions affect RNA more than DNA [35]. The size of the sample is also a crucial parameter; while small-sized samples can lead to results in DNA testing, they may not contain enough RNA for analysis. For more detailed information on the principles, techniques, and applications of RNA analysis in forensic science for BFID, the reader is referred to the review paper by Bauer [35].
2.1.2. Semen
Presumptive Tests
I- Alternate light source (ALS) (UV light and Polilight®)
It is a non-destructive method for the identification of semen among other BFs. Some light sources such as Wood’s lamp is not very specific and can lead to false positives if ointments or creams are also present [8]. Blue-maxxTM is another commercial light source which has more sensitivity to semen stains [39].
II- Seminal acid phosphatase test (SAP or Aka Walker test)
It is the most popular and accepted presumptive test for semen. Acid phosphatase is an enzyme secreted by the prostate gland, which exists in large amounts in semen [10]. This enzyme can catalyze the hydrolysis of organic phosphates [40]. The product of this reaction reacts with diazonium salt chromogen causing a color change [41]. There can be false positives with the presence of vaginal acid phosphatase. To avoid this, the color change occurring between 5 and 30 s should be considered, as SAP does not give fast results [8]. Currently, Phosphatesmo KM rapid test strips are used for the presumptive testing of acid phosphates. These stripes contain the necessary components which do not require the addition of chemical reagents [42].
III- Microscopy (SEM)
The microscopy technique is not as popular as SAP tests for semen identification. Equipped SEM with an energy dispersive X-ray analyzer (EDX) can be used to detect solidum, chlorine, phosphorous, or metal elements in semen. The proportion of these elements vary among various BFs, leading to the identification of unknown stains. This method is considered as a presumptive test for semen due to the interference of the substrate spectrum which can dominate that of the fluid [8].
Confirmatory Tests
I- Microscopic identification of sperm cells using Christmas tree stain
It is a widely accepted confirmatory test for semen since semen is the only BF containing sperm cells. In this method, the sperm cells are made visible via treating the heads (which contain large amounts of DNA) with a stain. The Christmas tree stain is the most popular one, which makes the heads red and the tails green [8].
II- Prostate-specific antigen (PSA) detection
PSA is a glycoprotein produced by prostatic epithelial cells [9]. This antigen is present in seminal plasma and its concentration in other BFs (e.g., urine and breast milk) is very low, eliminating false positives [43]. Immunoelectrophoresis, which involves the combination of diffusion and electrophoresis (movement of charged particles in an electric field), or ELISA are among the original techniques for detecting PSA [8,24]. A commercial kit which works based on antibody–antigen reactions is the Biosign® PSA test, which is cheaper and easier to operate than ELISA [44].
III- Immunochromatographic stripes (e.g., RSIDTM-semen)
Similar to RSID-blood, RSIDTM-semen is a lateral flow immunochromatographic test which utilizes two human semenogelin monoclonal antibodies to detect the presence of semenogelin (a protein produced by seminal vesicles) [45]. Compared to other methods, RSIDTM-semen is faster and has higher sensitivity and specificity as it is specific to human semen only.
IV- mRNA markers
The same method which has already been mentioned for bloodstains based on mRNA can be used for the detection of semen as well [8,35]. Semen-specific markers, e.g., Protamine 1 (PRM1), have been investigated by Alvarez et al., which can be used to detect semen based on RNA and DNA co-isolation [46].
2.1.3. Saliva
Presumptive Tests
I- Alternate light source (ALS)
Similar to blood and semen, ALS can also be used to locate saliva stains. The lack of solid particles in saliva makes this technique less straightforward for the identification of saliva compared to the other two BFs [41]. Under UV light, saliva stains show a blue/white color. It is, however, not easy to be distinguished from other BFs. Laser light sources are other examples which have been investigated by Auvdel in 1988 [47]. The Lumatec® superlite 400 (with an emitted light wavelength between 320 and 700 nm) was used to detect human saliva samples on different substrates with different colors. The saliva stains could be detected in darkness or in the presence of daylight [48].
II- Starch-iodide test
This test, as well as the phadebas test, are based on the activity of amylase (the most characteristic enzyme) in saliva regardless of the source of the saliva. In general, the reaction of iodine with starch develops a dark blue color. However, the components of starch (monosaccharides or disaccharides) do not react with iodine and no color will be developed upon the reaction. Amylase in saliva can break down starch into monosaccharides or disaccharides, leading to a color change [8]. This test can give false positives with the presence of other proteins such as albumin and globulin in blood and semen since they can also break down iodine.
III- Phadebas test
The Phadebas reagent consists of a cross-linked starch with a dye (procion red amylopectin). Since amylase can digest starch, the presence of saliva leads to the digestion of starch and thus the release of the dye. The solution turns blue and the intensity of the color can be used for the qualitative and quantitative confirmation of saliva [49]. This test can lead to false positive results in the presence of washing powder, urine, and hand creams [50].
Confirmatory Tests
Many methods that have been discussed for blood and semen can also be applied for saliva. Immunological methods such as ELISA have been tested to detect the activity of amylase in saliva stains [51]. Like semen, microscopy techniques, such as SEM-EDX, have also been studied for measuring the relative concentration of potassium, sulfur, phosphorous, sodium, and metal trace elements in saliva samples. Potassium gives the largest peak, which forms the basis of the saliva identification [52]. Fluorescence spectroscopy has also been utilized to detect dried saliva after dissolving the content in potassium chloride (KCl) solution [53]. Emission spectral measured in the range of 345-355 nm showed good intensity.
I- RSID-saliva
A lateral flow immunochromatographic test kit is based on two antisalivary amylase monoclonal antibodies, which can specifically identify human salivary amylase rather than detecting enzyme activity [54]. Like RSID-blood and semen, the method is based on the principles of antibody–antigen interactions. With the presence of saliva, a pink line appears in the observation window [49]. This method has high sensitivity and specificity to human saliva with no cross-reactivity with other BFs, e.g., semen, urine, or vaginal fluid [54]. The extraction buffer can effectively extract amylase from the stain and can detect saliva as low as 1 L.
II- mRNA markers
Saliva contains specific protein coding genes (statherin (STATH) and histatin 3 (HTN3)) that can be exclusively identified. mRNA profiling is a useful method for saliva stain detection [10]. In the review by Bauer, the use of RNA for the detection of saliva has been discussed [35]. The same co-isolation method for DNA and RNA described by Alvarez et al. is also applicable to saliva stains to detect HTN3 [46]. Saliva-specific genes were also detected by RT-PCR [55]. Similar sensitivity and less specificity, compared to the same method for blood and semen, were obtained.
2.2. Forensic Analysis of Drugs and Explosives
2.2.1. Drug Analysis
Forensic drug analysis includes the detection and identification of a suspected controlled substance, e.g., illicit drugs or drugs of abuse. Table 2 shows the classes of commonly used drugs according to certain common effects on the user [56].

Table 2.
Commonly used drugs and the corresponding effect on the user.
Presumptive Tests
Presumptive tests for forensic drug analysis identify the presence or absence of certain substance(s) or classes of drugs. These tests include color/spot tests, microcrystalline tests, ultraviolet spectroscopy, thin layer chromatography (TLC), immunoassays, and urine dipstick test [25]. In the following subsections, some of these techniques are explained.
I- Color/spot tests
This presumptive colorimetric test is based on a chemical reaction between a substance (analyte) and an indicator (reagent), which creates a color stain depending on the tested substance. The color spots are visually inspected (by the human eye or color-identification smartphone applications [57]) and compared to a standard color chart (Munsell color chart) [25]. There are many indicators, e.g., nitric acid, marquis, Duquenois–Levine, cobalt thiocyanate, ferric chloride, Dille–Koppanyi, para-dimethylaminobenzaldehyde, potassium permanganate, and silver nitrate [58]; while this method destroys the sample, it is rather sensitive (with a sensitivity limit in the g range) and specific provided that the proper standards are used. Most drugs of abuse, including analgesics (e.g., opioids), stimulants (e.g., amphetamines, cocaine), plant-based narcotics (e.g., heroin), and psychotomimetic (e.g., lysergic acid diethylamide (LSD)) can be detected with colorimetric tests. For most novel psychoactive substances, associated tests are not there yet [25].
II- Microcrystalline tests
In these tests, upon reaction of a specific reagent with an analyte, unique microcrystals are formed. The evaluation of the formed crystal under normal optical microscope and the comparison with the reference standards are used to identify and detect the substance [59]. Common drugs of abuse, e.g., heroin, methadone, cocaine, methamphetamines, and amphetamines can be identified with this method. It is a specific technique due to the unique choice of reagent and analytes. However, contaminant or dilutents can impede the formation of distinctive microcrystals, limiting this method to pure or purified samples; while this method is relatively cheap, user-friendly, sensitive (g range), and requires only small amounts of reagents, it destroys the sample and is not quantitative [25].
III- UV–Vis spectroscopy
In this method, UV light is shined through the sample, leading to a rise in the energy level of electrons. A characteristic UV absorption spectrum is then obtained according to the electronic structure of the molecules. UV–Vis can be used for quantitative and qualitative analysis, which can yield structural information as well [25]. It can be used to detect depressants (e.g., diazepam and barbiturates (phenobarbital)), ketamine, and cocaine hydrochlorides. Li et al. used this technique to accurately discriminate various compounds in a mixture [60]. It is a relatively easy method to use and, in combination with chromatographic techniques, higher specificity and selectivity can be obtained.
IV- Thin layer chromatography (TLC)
In this method, there are two phases, namely the planar stationary and liquid mobile phases. The sample is administrated onto the stationary phase and the mobile phase is passed through due to capillary action. The analyte of interest is absorbed in either of these phases and the corresponding retention time is measured [61]. Various components of a sample travel at different paces depending on the size and affinity to a phase. The components are thus separated, leaving the so-called “plate of spots” [25]. TLC can be used to detect various classes of frequently encountered drugs, including depressants (e.g., barbiturates, benzodiazepines, oxycodone), stimulants (cocaine, methylenedioxymethamphetamine (MDMA) or ecstasy, LSD, marijuana), narcotic analgesics (e.g., opium, heroin, morphine), and psychotomimetic (e.g., marijuana, synthetic cannabinoids). The separation and detection of novel psychoactive substances are difficult to achieve with this method [62]; while the TLC method has sensitivity in the microgram range, and is relatively low-cost and easy to operate, it is not specific to a single compound and should be used in conjunction with other methods (e.g., Raman spectroscopy or colorimetric testing) to increase the specificity [61].
Confirmatory Tests
Most confirmatory tests for forensic drug analysis are spectrometry-based methods, namely, mass spectrometry (MS), ion mobility spectrometry (IMS), and infrared (IR) spectroscopy. Techniques such as Raman spectroscopy (an optical method) and X-ray diffractometry (XRD) are other widely used methods. In the following subsections, some techniques and the corresponding pros and cons are summarized. The reader is referred to the review paper by Harper et al. for more detailed information on these methods, corresponding working mechanisms, detectable substances, and operational considerations [25].
I- Mass spectrometry combined with chromatographic techniques
This technique is currently the gold standard in forensic drug analysis [63]. In MS, three steps of separation, ionization, and final detection are performed to determine the exact mass to charge ratio (m/z) of ions. In the review by Harper et al., these techniques are explained in detail [25]. In summary, separation techniques include gas chromatography (GC), liquid chromatography (LC), or capillary electrophoresis (CE). Ionization methods can be categorized into soft or hard methods. The commonly used ionization methods in forensic drug analysis are electron ionization, fast atom bombardment, and direct analysis in real time. Using MS combined with chromatographic techniques, any substance with a concentration as low as attomolar range (10) can be detected and identified [64]. It requires small amounts of sample and provides unique properties, e.g., high resolution, specificity, and sensitivity. Major drawbacks of this technique are sample destruction, operational costs, requirement of poisonous/hazardous chemicals, non-portability, and requirement of trained personnel [25].
II- Infrared (IR) spectroscopy
This method is based on the amount of absorbed or emitted IR by a sample versus the wavelength. The corresponding spectrum reveals the molecular functional groups [65]. The IR spectra of pure compounds are distinctive fingerprints that can be used to discriminate compounds from each other. Since all compounds have IR-active vibrational modes, this method can be used for the quantitative and qualitative investigation of almost all compounds using reference spectra. Portable infrared spectrometers exist, which can be used at the point-of-need (PON). However, the interpretation of the results requires expert knowledge of the technician/personnel. The quantification of unknown substances is technically possible but can pose a problem due to a laborious procedure and the requirement of a knowledgeable user with expertise in spectroscopy [25].
III- Raman spectroscopy
In Raman spectroscopy, the radiated laser light is scattered by the sample molecules providing spectral vibrational information based on the plot of shifted light intensity as a function of frequency [66]. By determining the active pharmaceutical ingredients as well as polymorphs (molecules with the same chemical formula but different molecular arrangement),any drug can be identified with this technique [25]. Portable Raman spectrometers have been developed (e.g., TruNarcTM by Thermo Fisher Scientific, Waltham, MA, USA), which can be used at the point-of-need (PON) [67]. Raman spectroscopy is a fast and non-destructive method without the requirement of chemical reagents. It can be used to detect multiple substances (both organic and inorganic) in a mixture without any interference form the surrounding water or moisture medium [25]. This method is capable of quantitative and qualitative analysis, but similar to IR, quantitative analysis is an extensive procedure and requires user expertise. The identification and detection of plant-based narcotics, e.g., heroin, can be difficult and requires proper sample preparation since this substance exhibits strong fluorescence.
IV- X-ray diffractometry (XRD)
In XRD, high-energy X-ray radiation is used to bombard the drug sample. The scattering of the X-ray radiation by the crystalline lattice structure of the sample reveals the spatial structure of the molecules. The angle along with the intensity of the diffracted X-ray are used to obtain the crystalline structure and chemical bonds in the sample. Any solid crystalline or partially crystalline substances can be detected with this technique (powder or pills, e.g., cocaine, ketamine, methamphetamines) [68]. XRD has high sensitivity, due to its sensitivity to the polymorphs and contaminants, and high specificity due to its distinctive diffraction lines (X-ray fingerprint) of substances; while it is limited to solid substances and cannot be used out of laboratory, it is non-destructive and requires small sample amounts without any sample preparation [25]. Since, in this method, highly radioactive X-rays are used, high levels of expertise and training are required for the user.
2.2.2. Explosives
Explosives are a mixture of an oxidizer and a fuel in which the oxidizer provides a source of oxygen to induce a combustion-like reaction in the fuel [69]. Oxidizers may present in either of the following forms: (1) heterogeneous mixture, e.g., ammonium nitrate and fuel oil; or (2) in the same molecule, e.g., trinitrotoluene (TNT) [70]. Fuel sources can be categorized into hydrocarbons (e.g., charcoal, sugar, diesel), elemental fuels (e.g., sulfur, aluminum, magnesium), and energetic hydrocarbons (e.g., nitrocellulose, nitrobenzene) [69]. Stimulants, such as heat, shock, friction, etc., are needed to trigger the explosion without any influence on the energy of the explosion. Table 3 summarizes the three classes of explosives [71].

Table 3.
Three classes of commonly used explosives, the corresponding requirements, and the subsequent effects.
The detection of explosives concerns two general classes, namely homemade (improvised) and military (commercial) explosives. Improvised explosives can be classified into the following categories:
1- Low explosives such as black powder: They contain inorganic salts in a mixture of oxidizers (e.g., perchlorate or nitrate) and fuel (e.g., sugar, sulfur). A mixture of potassium chlorate (known as flash powder) and metal fuels such as Ba, Sr, or Cu, and nitrate salts creates colored flames/fireworks [72].
2- Fertilizer-based explosives: These explosives consist of AN and UN which can be obtained from fertilizers [73]. Mixture of AN and a fuel (e.g., kerosene or diesel) generates a blasting agent [70].
3- Peroxides: These are dangerous primary explosives which can be initiated by impact, heat, or shock [74,75]. These explosives are based on organic and inorganic peroxides which can be easily synthesized using obtainable products [76]. For instance, a mixture of concentrated hydrogen peroxide and a fuel (e.g., flour or pepper) can be used as an explosive. Cyclic organic compounds such as TATP contain peroxides in their functional groups [77]. Organic peroxide does not show fluorescent characteristics nor UV light absorbance, which makes its detection and analysis troublesome [78,79].
Commercial explosives such as Semtex (consisting of pentaerythritol tetranitrate, plasticizers, cyclotrimethylenetrinitramene (RDX)) and C4 (consisting of RDX, stabilizers and plasticizers) have been used in terrorist attacks [80]. In the following sections, the presumptive and confirmatory detection methods for explosives are summarized.
Presumptive Tests
These detection methods are simple, rapid, user-friendly, and inexpensive techniques used for on-site detection and identification of the explosive materials.
I- Explosive detection canines
It is the most common method in which dogs are trained to react to a specific scent (or combination of scents) released by the explosives or narcotics. This method has low specificity as the dogs cannot determine which explosive material is present. In addition, it has high maintenance costs and requires a skilled trainer [81].
II- Analytical instruments
Methods such as ion mobility spectrometry (IMS), Raman spectroscopy, and Fourier transform infrared (FT-IR) spectroscopy are examples of analytical methods which rely on the detection of volatile compounds [82,83,84]; while these methods can be portable, they can be costly and bulky in some cases [85].
III- Colorimetric and immunoassay-based tests
Three categories of colorimetric test kits are available commercially.
1- ETK Five: It relies on liquid reagent to detect explosives. The liquid colorimetric reagents are kept in glass ampules which, upon breakage, supply the reagent to an absorbent paper [86].
2- EXPRAY: In this kit, the chemical reagent is sprayed (e.g., aerosol sprays) onto an absorbent pad which is used for swipe sampling [87]. This kit can only detect the family of nitrate compounds leaving out peroxides, chlorates, or perchlorates.
3- XCAT: This kit consists of a portable colorimetric detector and swipe analysis (e.g., optical inks on detection cards). The cards are inserted into XCAT after swiping the sample area. A Software is used to detect and identify the explosive material [88].
While these techniques provide on-site detection, they are not multiplexed, and multiple tests must be performed to analyze an unknown explosive. Other drawbacks include inability to detect perchlorate, requirement of liquid reagents, and possibility of spilling before use [89].
Confirmatory Tests
A common inorganic compound of most explosive mixtures, namely nitrates (NO), can be used to detect explosives using analytical methods. The detection of explosives containing oxidizers, e.g., perchlorates (ClO), chlorates (ClO) or peroxides (O), is a more complicated procedure [89]. Since these materials possess a wide range of properties (e.g., various composition, volatility, and polarity properties), a variety of analytical methods can used to identify the associated explosives.
I- LC–MS, GC–MS, and high-performance liquid chromatography (HPLC)–MS
GC–MS and LC–MS are used for the detection of organic compounds (e.g., TNT) [77,90,91]. HPLC–MS has been proven useful for the detection of nitrate ions, chlorite, and perchlorate [92]. Mass spectrometry combined with chromatographic methods can also be used for the detection of inorganic ions, namely AN and UN. Since ammonium and nitrates are commonly encountered ions in the environment, these ions should exist in ion pairs to prove the presence of explosives. To achieve this goal, non-aqueous mobile phases (e.g., crown ethers) should be used to ensure that ions do not dissociate [73]. Crown ethers allow for the detection of both organic and inorganic explosives due to lack of interaction with organic explosives [89]. Combined MS with an electrochemical detector can be used for the simultaneous detection of hydrogen peroxide (HO) and organic/inorganic ions. The electrochemical detector is placed prior to the MS [93].
II- Ion chromatography (IC) and capillary electrophoresis (CE)
These two methods have been used to detect inorganic compounds (e.g., AN) [78,79,94]. IC equipped with conductivity detection (to measure electrical conductivity between two electrodes) has been developed to detect ionic species such as chlorate, perchlorate, and inorganic nitrate [89]. Gradients in ion chromatography have been shown to be the best procedure to detect inorganic explosives [95]. In CE, contactless conductivity, or indirect UV detection are used to separate ionic species [78,96].
III- FT-IR and Raman spectroscopy
These methods can be used for the simultaneous detection of organic and inorganic explosives, especially at trace levels [89]. They have been utilized for screening peroxide-based explosives [91,97].
IV- SEM and XRD
Metals are identified and detected using the XRD technique or with SEM coupled with energy dispersive spectroscopy (SEM–EDS) [98,99]. These techniques require extensive instrumentation, which is only available in the laboratory, making them non-portable.
3. A Short Summary on Microfluidics
Microfluidic technology is characterized by the precise manipulation of a small volume of fluids (mililiter (10 L) to picoliter (10 L)) in channels with dimensions ranging from 10 to 100 m. Two distinctive characteristics of microfluidics are (1) small size and (2) manipulating fluids in laminar flow regime [100]. Owing to the small sizes, higher surface-to-volume ratio, greater surface tension, and improved capillary action can be achieved in microfluidic platforms which can provide an enhancement in the conventional separation, detection, and/or analysis methods [101]. The technology in which laboratory-based methods are integrated in a microfluidic chip is known as lab-on-chip (LOC). Microfluidic technology has attracted increasing attention in various fields due to its unique properties, as summarized below [102].
1- Effectiveness: In point-of-care (POC) devices, the possibility of miniaturization leads to a reduction in the sample size as well as the required reagents. Multiplexing capability makes it possible to perform multiple analyses simultaneously within a single device which contains various microchannels. The channel geometry and overall architecture can be readily adjusted leading to the increased efficiency of the analysis. Compared to conventional diagnostic methods, POC devices can dramatically reduce the processing time form hours to minutes.
2- Easy handling: due to the small footprint (smaller than palm size), microfluidics provide prominent advantages, e.g., portability, accessibility, and ease-of-use. This further leads to much simpler devices which do not require trained/expert users.
3- Cost-effective: Compared to conventional devices, microfluidic-based diagnostic devices offer a reduced cost of the final product due to the diverse range of materials available for fabrication. Not only silicon or glass-based wafers, but also a diverse range of polymeric materials such as poly dimethylsiloxane (PDMS), polymethylmethacrylate (PMMA), polystyrene (PS), polyurethane (PU), cyclic olefin copolymer (COC), and polycarbonate (PC) can be utilized to fabricate microfluidic devices. These polymers are cost-effective and can be processed easily. One of the most cost-effective materials which has been used recently is paper. It is lightweight, biocompatible, and disposable.
A diverse application of microfluidics and various materials that can be used to fabricate microfluidic devices along with the fabrication techniques are summarized in Table 4 and Table 5, respectively.

Table 4.
A brief overview on the applications of microfluidic devices (inputs from reference [102]).
3.1. Portable Microfluidic-Based Devices (PMDs)
In various fields spanning from healthcare and clinical studies to forensic diagnostics, there is a great need for a portable device to achieve in situ (real time) qualitative and quantitative analysis of the sample with minimum intervention. Over the last decade, there has been significant research in developing PMDs via combination with smartphones. The current generation of smartphones are equipped with components such as powerful processors, cameras, and a variety of sensors which, along with features such as high data storage capacity, real-time location tracking (GPS), and wireless connectivity, serve as a powerful digital platform for developing PMDs. To improve the field of view, optical components such as customized lenses can be added to smartphones, making them an optical microscope with various imaging modes [103]. They can be then used as readers to analyze results such as colorimetric, chemiluminescent, or fluorescent data. Smartphone-based devices for monitoring blood pressure/pulse rate, diabetic, and weight management have been already commercialized. In a recent review paper by Beduk et al., emerging applications such as electrochemical and optical sensing (smartphone-based multiplexed sensors) for POC devices have been detailed [104]. A smartphone-based acoustofluidic platform has been developed for enhanced colorimetric detection and evaluation of hemoglobin (Hb) in blood [105]. Red and green fluorescence nanoparticles are used as probes for visual testing and measurement of blood Hb levels.

Table 5.
Overview of the materials and techniques used for fabrication of microfluidic devices (inputs from reference [102]).
Table 5.
Overview of the materials and techniques used for fabrication of microfluidic devices (inputs from reference [102]).
| Material Type | Subcategory | Example | Fabrication Techniques 1 | Pros and Cons |
|---|---|---|---|---|
| Inorganic | Silicon | Silicon wafer | LIGA (X-ray lithography, micro-molding, electroplating)
Anodic/fusion bonding (post processing to close open channels) | Resistant to organic solvent Excellent physical properties Need for clean room Expensive Non-flexible Use of toxic chemicals Limited opacity |
| Glass | Glass capillary | Photo lithography Wet/dry etching Anodic/fusion bonding (post processing to close open channels) | Optical transparency Chemical inertness Electrical insulation Biocompatible Cumbersome assembly of capillary-based micro reactors Brittle Need for clean room | |
| Organic (polymers) | Elastomer | PDMS | Soft micromachining (e.g., laser ablation) [106,107] Computer numerical control (CNC) micromachining [108,109] Optical/X-ray/photo lithography | Low cost Optical transparency Biocompatible |
| Thermoplastic | PC, PMMA, PU, PS | Soft lithography Hot embossing [110,111,112] Injection molding [113] | Disposable Design flexibility | |
| Cyclic olefin polymers (COPs) | Cyclic olefin copolymers (COCs) | Micromilling CNC machining Hot embossing [114,115] Injection molding 3D printing | Low water absorptivity Electrical insulating Optical transparency High rigidity Inert to acids/alkalines/solvents | |
| Paper | Pressed cellulosic fibers | Pure cotton-based | Inkjet printing Wax patterning Lithography [116] Plasma/laser treatment Paper origami and stacking (for 3D paper-based microfluidics) | Flexible Biocompatible Cost-effective Disposable Special requirements and chemical treatment to avoid fast degradation |
1 Fabrication techniques, e.g., CNC, lithography, and hot embossing mentioned for polymer-based microfluidics, are mainly used for fabrication of the required master mold to create negative replicas in the corresponding polymer, e.g., PDMS. In the case of paper-based microfluidics, photolithography is used for initial patterning of the paper. For more detailed information, the reader is referred to the corresponding references.
3.2. General Components of Microfluidic-Based Point-of-Need Devices (PON)
An integrated PON microfluidic device consists of three main modules: (I) control and pumping; (II) sample preparation and processing; and (III) detection and analysis. Various microfluidic techniques have been developed to achieve each of these modules [117]. Figure 1 shows an overview of the modules and the corresponding elements which are explained below.

Figure 1.
Overview of various modules constituting integrated PON devices.
Module (I): Control and pumping
Precise flow control of the sample and reagents is the key to achieve an efficient PON. Pumping can be categorized into two general classes of active and passive pumping.
1- Active pumping: External forces are applied to drive the flow and control the sample flow rate. In addition to the commonly used syringe and peristaltic pumps, electro-osmotic pumping is another well-known method in which ion drag is established upon application of a tangential electric field leading to a pressure gradient and thus fluid flow [118]. In another method, the digital manipulation of targeted reagents or droplets can be achieved via the utilization of forces such as acoustic [119], magnetic [120], or optical [121].
2- Passive pumping: A driving force inside the microchannels is used to drive the fluid flow without the need for external peripherals. A chemical gradient on the surface, osmotic pressure, capillary gradient, or permeation in PDMS can be used to achieve pumping [122]. The most common passive pumping, which is used in paper-based microfluidics (e.g., lateral flow assays), is the capillary-driven flow [123]. It is a low-cost and simple design without the need for external instruments/power in which the wetting properties of the substrate material are used to drive the flow. The control of the flow rate in capillary-driven flows is achieved through the controlled evaporation of the liquid, or the use of asymmetric micropillars [124]. The major drawback of the capillary-driven flow is the change in wetting properties of the material throughout time, poor reproducibility, and lack of standardization.
Module (II): Sample preparation and processing
In PON devices, a sample of blood, urine, or saliva should be treated first, i.e., the target analyte must be separated. In some cases, pre-concentration is needed to increase the concentration of the analyte of choice [122]. Similar to pumping, active and passive forces can be used to obtain the desired sorting and separation. An elaborate overview of different approaches for separation/isolation in microfluidic channels can be found in the review by Dalili et al. [125].
1- Active forces: Acoustic, electric, or magnetic forces can be applied in continuous or batch-wise processes for trapping, washing, or enrichment of the cells/analytes. This requires the addition of external or integrated components in the chip. For example, Lenshof et.al. used acoustophoresis to obtain high-quality plasma with low cellular content from the whole blood [126]. They further combined this technique with a silicon-based antibody microarray chip for the detection of a prostate-specific antigen (PSA) via fluorescence readout. Not only silicon, but also PS-based microfluidic channels have been used to implement acoustophoretic-based separation [127]. Techniques based on dielectrophoresis exploit differences in dielectric properties of analytes in a sample to achieve separation [128]. In magnetophoresis, the labeling of analytes with magnetic beads, which have been functionalized with specific antibodies to the target, is required.
2- Passive forces: Inertial effects, altering or modification of geometries within the microfluidic device, incorporating micro pillar arrays or filter membranes can all be used to achieve passive separation [125]. These approaches are more cost-effective as no additional external/integrated peripheral is needed.
Module (III): Detection and analysis of the target
The detection and analysis of the target require the conversion of the biochemical recognition in the analytes into the electrical or optical signals. Dungchai et al. demonstrated label-free electrochemical detection in paper-based microfluidic devices to detect electrically active targets, namely glucose, lactate, and uric acid in biological samples [129]. Optical-based detection methods are mainly based on fluorescence, chemiluminescence, or colorimetric techniques in which the analyte is labeled by attaching a fluorophore or chromophore to an antibody or nucleic acid strand [123]. Colorimetric detection has been widely used in lateral flow assays due to its simplicity and ease of use. In colorimetric detection, a reaction between the molecular probe and the analyte leads to a color change which is visible to the naked eye. The detection of glucose in blood is one of the examples of colorimetric detection [130]. Fluorescence-based techniques make use of fluorescent nanoparticles or quantum dots, demonstrating an increase in sensitivity compared to colorimetric methods. Recently, developments in microfabrication along with advancements in nanotechnology have led to the so-called “nanomaterial-assisted microfluidics” [131]. In these platforms, the multiplexed detection of various biomarkers is possible due to the unique coupling between the microfluidic-based analytical methods and nanomaterial-based biochemistry analysis [131]. Various nanomaterials such as quantum dots, carbon nanotubes, and metal nanoparticles have been implemented to enhance the performance of microfluidic analytical devices, e.g., paper-based and slip-driven microfluidics known as SlipChip [132]. Paper-based analytical microfluidic devices based on SlipChip have also been developed, which are composed of two wax-patterned chromatography paper layers [133]. In a review paper by Wang et al., these highly integrated systems and potential applications in clinical diagnostics are detailed [131]. A combination of nanocatalysis (e.g., enzyme-based nanocatalysts) and microfluidics has also gained attention recently since both components can enable efficient bioanalysis [134]. In a review paper by Gao et al., the recent developments in this emerging field, including widely studied nanocatalysts and microfluidic platforms, detection methods, and unique advantages, are explained in detail [134]. Microfluidic devices with integrated LEDs and microscopes have been developed significantly to detect fluorescence signals.
3.3. Microfluidic Paper-Based Analytical Device (PAD)
To make the microfluidic-based technology for PON analysis widely available, cost optimization is the key element. Paper-based microfluidics can bring down the final product cost due to simplicity, low-cost materials (compared to silicon or glass-based devices), and limited need of external peripherals, e.g., pumps/valves [117]. These devices can be used by non-trained personnel in remote areas wherein resources are scarce and/or laboratories are not available, satisfying the ASSURED criteria: any analytical device must be Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment-free, and Deliverable to enable analysis outside of well-equipped laboratories [135]. PAD, which was first developed in 2007 by Whitesides group [116], are a subclass of the so-called wicking microfluidic devices in which capillary action is responsible for the transport of the sample and reagent without the need for external peripherals (power source, mechanical components) [136]. Other examples of wicking microfluidic devices exploit membranes (polymeric or glass fiber-based) or cotton threads.
The unique advantages of PAD such as requirement of low sample volumes, fast results, comparable specificity, and sensitivity to those of immunological assays make them an interesting candidate for diagnosis applications ranging from biomedical to forensics (see Section 4). Such microfluidic devices have been used for blood and urine analysis in medical research [137,138]. Papers used for making microfluidic-based diagnostic devices have special requirements in terms of paper material, porosity, pore size, and wetting behavior [139]:
Paper material: While everyday papers are made of cellulosic fibers obtained from wood, bamboo, or cotton, paper-based microfluidics are made from pure cotton to avoid fast degradation in diagnostic applications.
Porosity: It is the void fraction of the 3D porous structure of the paper which determines the flow rate of the analytes through capillary action.
Pore size: It is defined as the largest diameter of the substances that can pass through the paper.
Wetting behavior: Hydrophilic papers are preferred over hydrophobic ones since most reagents and samples are aqueous-based and thus have more affinity toward hydrophilic substrates. However, patterning the paper into hydrophilic and hydrophobic regions enables multiple assays in a single device since samples can be distributed into several locations [116].
Detection of analytes in paper-based microfluidic devices is based on a reaction between the target analyte in the sample and the reagents (e.g., enzymes, dyes, indicators) which have been already immobilized. Sensing mechanisms that can be implemented are as follows.
1- Colorimetric sensing: Martinez et al. have developed a patterned paper-based microfluidic platform for multiplexed bioassays. Glucose and protein were simultaneously detected in artificial urine samples [116]. The distribution of the sample to various zones led to a color change depending on the type of the analyte. A quantitative measurement of the analyte level was performed by comparing the color to a calibration chart. Colorimetric sensing is the widely used method for the detection of hemoglobin in human blood. Yang et al. developed a PAD using chromatography paper for Hb detection in blood based on scanning of bloodstains and subsequent digital analysis [140]. A mixture of blood and reagent led to a visible brownish color change which was quantified using a portable scanner. For more detailed information on quantitative colorimetric analysis via PADs for on-site chemical analysis, the reader is referred to reference [135].
2- Electrochemical detection: A three-electrode paper-based microfluidic platform has been developed by Dungchai et al. for electrochemical detection of analytes in biological samples [129]. Oxidase enzyme reactions in distinct reaction zones were used to detect glucose, lactate, and uric acid. In another study, using commercial handheld glucometer, electrochemical PADs were developed for quantitative electrochemical analysis of vital biomarkers, e.g., glucose, cholesterol, lactate, and alcohol in human blood [141].
3- Chemiluminescence (CL): It is based on the emission of light upon a chemical reaction. Yu et al. used CL in PAD to simultaneously detect uric acid and glucose in artificial urine samples [142]. A rhodamine derivative is used to induce CL reaction with the generated hydrogen peroxide. Electrochemiluminescence (ECL) has also been utilized in which luminescence is generated due to an electrochemical reaction. Delaney et al. combined an inkjet-printed paper-based microfluidic substrate with screen-printed electrodes to obtain a sensor to detect 2-(dibutylamino)- ethanol without the need of a photodetector [143]. ECL reaction between Ru(bpy) and the analyte of choice was used to obtain orange luminescence. Using a mobile camera, the intensity of the sensor luminescence was detected and used to obtain a calibration curve.
4. Microfluidics in Forensic Applications
As discussed in detail in Section 2, presumptive and confirmatory tests have some common disadvantages. Most presumptive tests suffer from being (1) body-fluid-specific, (2) prone to false positive/negative results, (3) destructive to valuable DNA evidence, (4) not label-free, and (5) susceptible to sample contamination by chemical reagents. Confirmatory tests, on the other hand, are (1) time-consuming, (2) have a costly procedure, (3) require intense sample preparation, (4) can be destructive, and (5) are non-universal [7,144]. Microfluidic devices and LOC technology (see Section 3) can overcome some of these shortcomings due to distinctive characteristics, namely rapid analysis, decreased volume of reagents/samples, small footprint, portability, reduced risk of (cross-)contamination, and safe storage of sample for further analysis. In the following sections, the application of microfluidics in various categories of forensic analysis is detailed.
4.1. Forensic Serology: Body Fluid Screening (BFS) and Identification (BFID)
Body fluid screening (BFS): The determination of the presence of BFs at a crime scene (forensic serology) involves a two-step process in which the potential presence of a BF is first determined via presumptive tests followed by more accurate BFID using confirmatory tests in the second step [145]. In some cases, these multiple tests lack specificity, are time-consuming, can waste the sample, and be destructive to the valuable DNA; while methods based on immunoassay and spectroscopy [8,17,146] as well as advanced ones such as proteomic and epigenetic techniques [147] have been developed with enhanced specificity, they are not portable and require complex devices.
Microfluidic lab-on-chip devices, especially PADs, can address these shortcomings. Cromartie et al. developed a portable multiplexed PAD with the colorimetric detection method for presumptive testing of various BFs at a crime scene [148]. This PAD, which was made of chromatography paper, could simultaneously detect four BFs, namely blood, semen, saliva, and urine. Melted wax was utilized to define hydrophilic channels to guide the sample toward arrays of colorimetric sensors. The biocompatible chromatography paper substrate allowed for the transport of BFs to test wells via capillary wicking. In this PAD, a single channel branches off into multiple detection pads, creating a branched structure which enabled a multiplex analysis of samples in 10–15 min (Figure 2). The device showed a shelf life of two weeks when stored in a dry and dark location. Traditional colorimetric methods, namely, Kastle–Meyer, starch-iodine, Nesslers reagent, and acid phosphatase were used to detect blood, saliva, urine, and semen, respectively.

Figure 2.
Multiplexed PAD before and after use in forensic serology for simultaneous detection of urine, blood, saliva, and semen. Sodium perborate tetrahydrate and phenolphthalein are, respectively, placed in areas labeled “A” and “B” [148] (Used with permission of the Royal Society of Chemistry, from (Development of a microfluidic device (PADs) for forensic serological analysis, Cromartie et al., 11, 5, ©2019); permission conveyed through Copyright Clearance Center, Inc.).
PADs were also used for blood detection and blood typing assays. Ansari et al. developed patterned PADs using a laser printer and wax for on-site, rapid blood detection and typing [149]. A flower pattern was developed on Whatman paper using wax printing in which the sample deposition zone was connected to five other detection zones (Figure 3a). Routine presumptive and confirmatory tests based on colorimetric reagents, namely TMB, LMG, phenolphthalein (PHP), Takayama (TAK), and Teichman’s (TEI) were used for blood detection on the PAD (Figure 3b). Dried blood stains (up to 48 days) could be detected with this PAD.

Figure 3.
(a) PAD designed using wax printing with a flower pattern containing a single sample deposition and five detection zones. (b) Colorimetric detection of blood using different reagents [149] (Reprinted by permission of Taylor & Francis Ltd. (http://www.tandfonline.com (accessed on 12 May 2023)), A portable microfluidic paper-based analytical device for blood detection and typing assay, Ansari et al., Australian Journal of Forensic Sciences, 2021, Taylor & Francis).
Body fluid identification (BFID): current methods for BFID, e.g., spectroscopic-based techniques (Raman or FT-IR), require trained personnel, expensive instruments, and are time-consuming [150]. Emerging methods based on molecular biology, such as transcriptomics, are a promising confirmatory method for BFID since RNA profiling and recovery can be performed on body fluid stains without compromising or consuming the valuable DNA (see confirmatory tests for blood (Section 2.1.1), number 6) [151]. Various types of RNA, e.g., mRNA [152], microRNA [153,154], and circular RNA [155,156] have been investigated. RNA-based BFID involves RT-PCR, which can detect low concentrations of mRNA in small-sized samples [157]. Recently, Layne et al. developed a microfluidic platform (centrifugal microelectrophoresis Disc (EDisc)) to separate mRNA amplicons in BFs using electrophoresis. A low-cost prototyping technique, namely “print-cut-laminate” [158], was used to fabricate the layered microfluidic platform using various polymers (Figure 4) [151]. PCR-amplified fragments were detected using laser-induced fluorescence. mRNA targets were electrokinetically separated into various BFs, e.g., saliva, blood (menstrual and venous), semen, vaginal fluid, and seminal fluid in pure and mixed forms. Comparable results to those from conventional CE were obtained, but at a four-fold decrease in the analysis time of the electrophoresis.
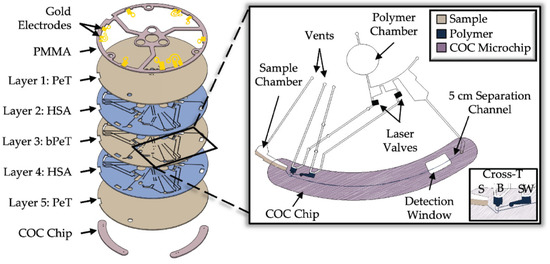
Figure 4.
Schematic illustration of the centrifugal microelectrophoresis Disc (EDisc) for electrophoretic separation of mRNA in various BFs. Microfluidic disc is composed of 5 layers (layers 1–5) made from polyethylene terephthalate (PeT), heat-sensitive adhesive (HSA), black PeT, and PMMA. The separation chip is made from cyclic olefin copolymer (COC) [151] (Copyright ©2022 Layne et al. Published by MDPI. Distributed under the terms of the Creative Commons CC BY 4.0 license, http://creativecommons.org/licenses/by/4.0/ (accessed on 12 May 2023).
4.2. Genetic Profiling and Human Identification (DNA Typing)
In recent decades, microfluidic technology has been proposed to expedite the current laborious human identification (HID) procedures including DNA typing and short tandem repeat (STR) analysis [159]. Forensic DNA analysis in microfluidics is one of the widely studied applications of microfluidics in forensic diagnosis. This is mainly due to the “Rapid DNA Initiative” proposed by the Federal Bureau of Investigation (FBI) in 2010, which laid the foundation for the integration of microfluidics into existing forensic genetic workflows for HID to obtain an automated and portable device [160]. Over the last decade, multiple reviews have been published in which all aspects of forensic DNA analysis in microfluidics, spanning from methodologies for individual steps, advantages and disadvantages, potential shortcomings, and perspectives have been detailed [3,161]. In a recent review by Bruijns et al., three integrated systems for forensic DNA analysis, which are commercially available (ParaDNA (Laboratory of the Government Chemist, Teddington, UK), RapidHIT(developed by IntegenX which is part of Thermo Fisher Scientific, South San Francisco, CA, USA), and ANDE (developed by NetBio (Waltham, MA, USA))), have been systematically reviewed [162]. Various aspects of these systems, e.g., ease-of-operation, associated costs, time of analysis, and portability are discussed; while the advantages of these systems are prominent, further improvements regarding the possibility of analyzing a wider range of forensic samples and regarding the cost of the cartridges are needed [162]. In another review by Turiello et al., the fully automated microfluidic-based systems for DNA analysis, the so-called, “swab-in-profile-out” have been investigated critically [4]. Despite the tremendous investments and research works which have been performed so far, only a few automated systems are available commercially. The authors further elaborated on the contributing factors as well as technical and contextual reasons for this outcome. There are trade-offs with these automated systems to compete with conventional methods in terms of cost per sample, sensitivity, reproducibility, and multiplexing capability. Here, the process of forensic DNA analysis and application of microfluidics in each step are touched upon. For more detailed information, the reader is referred to individual papers. The five steps of DNA analysis procedure include (1) trace sampling, (2) sample work-up, (3) amplification reaction, (4) detection, and (5) secure storage [3]. The applications of microfluidics in steps (2)–(4) are summarized below.
4.2.1. Microfluidic in DNA Sample Work-Up
DNA sample work-up consists of (1) cell lysis and (2) DNA extraction and purification. The applications of microfluidics in these two steps are summarized below.
1- Cell lysis-on-a-chip: It has been studied based on various lysis mechanisms, e.g., chemical, thermal, electrical/electrochemical, and mechanical [163]. The main advantage of mechanical and electrical lysis over the chemical and thermal one is the absences of reagents and heating elements [164,165]. As an example, Di Carlo et al. introduced the so-called “nano-knives” in the microfluidic channel for mechanical cell lysis [164]. Since only mechanical forces, such as shear and friction, are not sufficient to induce cell lysis, they integrated sharp nanostructures into the channel which could rupture the cell membrane. Other convectional lysis methods including osmotic, optical, acoustic, or ultrasonic have been translated into microfluidic platforms. In a book chapter by Le Gac et al., various cell lysis methods on a chip have been extensively reviewed [166].
2- DNA extraction and purification-on-a-chip: They have been mainly performed by solid phase extraction (SPE) to effectively bind the DNA [167,168,169]. Silica beads are the most widely studied binding agent in microfluidic-based SPE, which can be used in the ng range to achieve efficient DNA adsorption–desorption [170]. Durate et al. introduced the “dynamic SPE” method, in which magnetic silica beads are used on a chip for DNA extraction [171]. Using a small amount of blood sample (0.6 L), they could recover more than 65% of DNA with concentrations above 3 ng/L.
Most of these methods have already been implemented in clinical applications but the application in the forensic field imposes complications due to a wide variety of raw samples which are, in most cases, contaminated by chemical, biological, or radiological agents [172]. A more complicated extraction procedure involves sexual assault cases, in which the sample contains a mixture of cells (epithelial and sperm) from at least two donors [173]. Differential extraction (DE) methodologies have been developed to enable the separation of DNA fractions from both male and female samples. Ultrasound and sonication have been used for successful DE for forensic analysis of sexual assault cases [174,175]. In a short period of 15 min, Voorhees Norris et al. used ultrasound to selectively capture sperm cells from a female epithelia cell lysate [174]. In a patent by Belgrader et al., sonication and filtration were used to lyse epithelial cells and separate them from sperm cells, respectively, [175]. In review papers by Clark et al. and Chong et al., the corresponding dominant methodologies and emerging approaches are discussed in detail [172,173]. Microfluidic technology has shown promising results in terms of analysis time, reliability, accuracy, and ease-of-use for DE applications. Inci et al. implemented a carbohydrate ligand for binding egg and sperm (oligosaccharide sequence (SLeX)) on-chip to selectively capture sperm cells followed by sperm lysis on-chip for further DNA genomic analysis [176] (Figure 5). The method has been validated using forensic mock samples from a decade ago showing 70–92% capture efficiency. It could further reduce the DE analysis time from 8 h to 80 min.
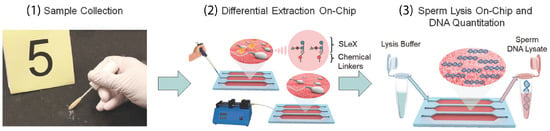
Figure 5.
The workflow for on-chip differential extraction used in forensic assault cases to separate sperm and epithelial cells. (2) Single-step pipetting and incubation have been used for sample introduction into the microfluidic chip. Sperm cells are selectively captured in the channels, while epithelial cells are removed due to their lack of adhesion to the channel wall and their large size. (3) Lysis-on-chip using a buffer is utilized for sperm cell lysis and DNA is collected for further genomic analysis [176] (Copyright ©2018 Inci et al. Published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. Distributed under the terms of the Creative Commons CC BY license, https://creativecommons.org/licenses/(accessed on 12 May 2023).
4.2.2. Microfluidics in DNA Amplification and Detection
DNA amplification is an essential step in forensic DNA typing since generally forensic samples have a low amount of DNA. The amplification reaction must be performed to increase the amount of DNA for the subsequent step of detection and STR profiling [3]. Polymerase chain reaction (PCR) is a widely used amplification method which is also one of the most time-consuming steps of DNA analysis. The first application of microfluidics for PCR amplification was introduced by Kopp et al. in 1998 [177]. A wide variety of microfluidic-based PCR methods have been investigated since. Two main types of PCR chips, namely “well-based” and “continuous flow”, have been developed [178]. In the well-based PCR chips, the entire chip goes through thermal cycling, while in the continuous flow PCR chips, the sample is heated/cooled locally via fixed temperature zones.
Microfluidic-based PCR in droplets is an emerging technique in which each droplet acts as an individual pL-nL sized reactor [179]. Monodisperse water-in-oil droplets can be formed in microfluidic channels via different techniques [180,181]. Droplet-based PCR has unique advantages, namely (1) faster mass transfer and better mixing due to increased surface-to-volume ratio and (2) prevention of (cross-)contamination since droplets are isolated and act as separate reactors [179]. In review papers by Ahrberg et al. and Bruijns et al., a detailed overview on various (real-time) PCR chips including droplet-based, isothermal, and digital ones is provided [3,182]. Multiplexed PCR in which multiple DNA loci are amplified simultaneously has also been studied on microfluidic platforms [183,184]. Estes et al. significantly improved the on-chip PCR via enhancing control systems, valves, software, and amplification chemistry [183]. Via an optimized solid phase encapsulating assay mix integrated within the microfluidic platform, they encapsulated the reagent for PCR into a solid phase with long shelf life, enabling multiplexed PCR on-field. DuVall et al. studied chip-based multiplexed PCR on various substates, namely transparent and black PeT, and obtained STR profiles in 10–15 min (depending on the size of the multiplex) [184]. Their microdevice showed a great potential for integration within upstream DNA extraction as well as downstream electrophoresis. Cornelis et al. developed a novel forensic DNA fingerprinting by combining PCR amplification and HyBeacon melting assays in a single microfluidic chip with integrated heating elements [185]. Four STR loci and amelogenin gender markers could be analyzed simultaneously, showing a step forward for mass-producing portable devices for on-site forensic DNA analysis (Figure 6).

Figure 6.
Microfluidic chip for DNA fingerprinting based on combination of PCR amplification and HyBeacon melting assays developed by Cornelis et al. (a) Top view of the complete chip with integrated heaters, 24 inlets, and printed circuit boards. (b) Back side of the chip showing reaction chambers and access holes connected via microfluidic channels [185] (Copyright ©2019, Cornelis et al. Published by Springer Nature. Distributed under the terms of the Creative Commons Attribution 4.0 Generic License, http://creativecommons.org/licenses/by/4.0/(accessed on 12 May 2023).
DNA detection techniques on a chip are mainly based on fluorescence sometimes combined with capillary electrophoresis (CE). In conventional CE, a long (circa 30–60 cm) circular silica capillary is used in which amplicons (tagged with fluorescent dye) are injected. Upon application of voltage, the amplicons are separated (based on size) and move across a detection window wherein they are excited by a laser. The detection system and further analysis produce the corresponding electropherogram to accurately size DNA fragments and obtain STR profiles [186]. Due to the simplicity of the capillaries and the associated mechanisms, CE was successfully translated into microfluidics. Microchip electrophoresis (ME) was developed via optically clear materials (glass and transparent polymers), which consisted of circular channels as a reminiscence of silica capillaries [159,187]. Due to reduced lengths in channels in ME, a 10–100 times faster separation could be achieved compared to conventional CE [159]. The integration of detection on-chip is not an easy task and still most of the developed MEs use off-chip detection methods [3]. SYBR Green I or EvaGreen are widely used fluorescent dyes for this application due to their simplicity and fast results [188]. However, they are non-specific and multiplexing is not possible. To achieve specific detection, fluorescent primers or probes should be utilized at the cost of more complicated chip design. Hopwood et al. developed an integrated microfluidic system in which three steps of DNA purification, PCR-based amplification, as well as separation and detection using CE were performed in separate reaction chambers in a single device [189]. A micro CE with a resolution of 1.2 base pairs was used to separate fluorescently labelled STR fragments. The produced DNA profile, which was achieved in <4 h, was compatible with the UK DNA database. A detailed overview on various chip-based detection methods, DNA biosensing, and profiling via STR analysis is given in two reviews by Bruijns et al. [3,190].
4.3. Illicit Drugs and Drugs of Abuse
The largest application field of microfluidics in forensic drug analysis is the determination of illicit drugs. It provides accurate quantitative and qualitative data for on-site detection and analysis for cases ranging from drug analysis in sports to driving under the influence of drugs [191]. Various analytical drug detection and analysis techniques, namely screening via immunoassays (e.g., ELISA), extraction (e.g., filtration, SPE), separation (e.g., CE, micellar electrokinetic chromatography (MEKC)), and detection (e.g., CL, electrochemical) (see Section 2.2.1) have been translated into microfluidic platforms and are detailed in a review by Al-Hetlani [5]. The first application of microfluidics for analysis of drugs of abuse dated back to 2000 and was developed by Greenway et al. [192]. In this platform, Ru(bipy) chemiluminescence (CL) was used for the detection of cocaine.
Drug analysis can be categorized into seized drugs and drugs of abuse in biological samples. The analysis of possible adulteration in seized drugs as well as food and beverages (using psychoactive drugs) is an important forensic investigation topic [6]. In the following subsections, the applications of microfluidics in these two categories are summarized.
4.3.1. Seized Drugs
The presumptive identification of seized drugs is classified into (1) colorimetric and (2) electrochemical analysis [6]. An example of electrochemical analysis includes square wave voltammetry used by Riberio et al. to detect LSD after dissolution in LiClO in a paper-based microfluidic device [193]. The colorimetric detection methods are summarized below.
In 2015, Musile et al. introduced the first PAD platform for multiplexed colorimetric detection of various drug compounds, namely cocaine, opiates, ketamine, and phenylamines [194]. Six hydrophilic channels were created on chromatographic paper utilizing wax printing and were connected to a single stem. Various colorimetric reagents were placed in each channel, enabling multiplexing (Figure 7a). Krauss et al. developed a centrifugal microfluidic platform (made from inexpensive polyester toner) in which the tested material was placed into multiple reaction chambers for fast screening [195]. An enhanced objective image analysis method (based on hue and saturation analysis) using a smartphone was developed for more accurate colorimetric detection and decreased analysis time (compared to scanner-based methods). As a proof-of-concept, cocaine and methamphetamine were tested with a limit of detection (LOD) in the mg range. The device could identify 30 unknown samples.
To further improve the specificity of presumptive illegal drug testing and overcome the inherent subjective interpretation, Bruijns et al. developed a microfluidic platform using cyclic olefin copolymer (COC) for presumptive testing [196]. A portable UV–Vis spectrometer was combined with the microchip, providing more analytical information about the compound based on the accurate absorption wavelength. Lockwood et al. developed a twelve-lane paper-based analytical device (idPAD) for the detection of a more complex drug mixture (illicit drugs and cutting agents) in seized drugs with 95% sensitivity, 100% specificity, and LOD in the g range [197] (Figure 7b). The idPAD was printed with wax followed by baking at 100 °C to form a hydrophobic barrier and deposition of reagents into the 12 lanes. After the addition of the sample in the dry form on the “swipe line”, the card was placed for 3 min in water to rehydrate the reagents and bring the chemicals into contact with each other via capillary action. The developed unique color was analyzed and compared with a standard library. The idPAD demonstrates an inexpensive and user-friendly platform for on-field detection of illicit substances (e.g., cocaine HCl, heroin, crack, and methamphetamine) which does not require samples in the solution form.
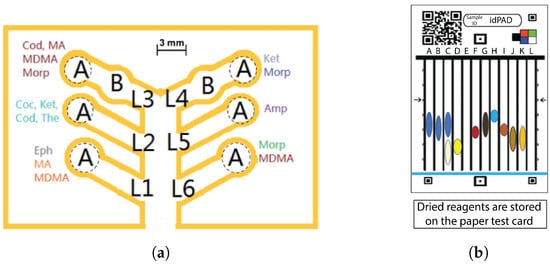
Figure 7.
(a) Developed PAD by Musile et al. for multiplexed screening of seized drugs based on colorimetric detection. In each lane, one or two zones are realized for placing of reagents (A, B). Through the middle stem, the sample (dissolved in a solvent) is introduced and travels toward each reaction zone via capillary action. Abbreviation of drug names: Eph (ephedrine), MA and MDMA (methamphetamine), Coc (cocaine), Cod (codeine), Ket (ketamine), The (thebaine), Morp (morphine), Amp (amphetamine) [194] (Used with permission of the Royal Society of Chemistry, from (The development of paper microfluidic devices for presumptive drug detection, Musile et al., 7, 19, ©2015); permission conveyed through Copyright Clearance Center, Inc.). (b) The idPAD developed by Lockwood et al. for simultaneous detection of twelve drug compounds [197] (Permission is granted for Lockwood et al., idPAD: Paper analytical device for presumptive identification of illicit drugs, Wiley-Blackwell, ©2020 American Academy of Forensic Sciences).
More selective colorimetric-based detection methods can be achieved using aptamers/ antibody recognition. Aptamers are engineered nucleic acids with specific recognition characteristics for small molecules. Based on ligand binding, the conformation of the aptamer changes, enabling molecular recognition [198]. Cocaine detection using a DNA aptamer has been investigated based on fluorescence [199,200] and electrochemical detection methods [201,202]. Wang et al. developed a PAD on which gold nanoparticles and anti-cocaine aptamers were coupled for the detection of seized cocaine samples [203]. Gold nanoparticles on the microfluidic paper were aggregated in the presence of salt and cocaine, leading to a color change (black) detectable by the naked eye or a camera (Figure 8). This aptamer-based PAD provides high specificity, sensitivity, and can detect cocaine in <5 min with LOD in the g range. With the development of new aptamers, this technique can be used to detect other illicit drugs.
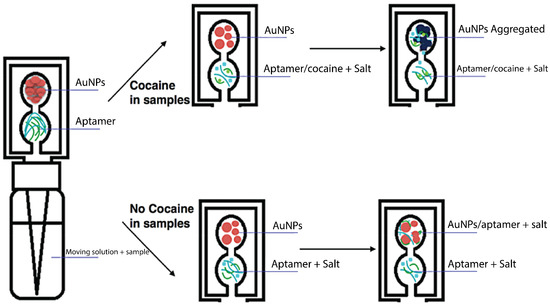
Figure 8.
Aptamer-based PAD coupled with gold nanoparticles (AuNPs) developed by Wang et al. for detection of cocaine in seized drugs. Aptamers and salts are placed in the middle of the channel, while AuNPs are placed at the end. The presence of cocaine and salt leads to the aggregation of AuNPs and thus a color change [203] (Permission is granted for Wang et al., An aptamer-based paper microfluidic device for the colorimetric determination of cocaine, Wiley-VCH, ©2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim).
In another study, Kawano et al. developed a highly selective method to detect cocaine in a microchip using a biological nanopore (transmembrane toxin R-hemolysin (HL)) combined with a DNA aptamer (cocaine-binding aptamer (CBA)) [204]. In the absence of cocaine, the single-stranded CBA with a diameter around 1 nm can pass through the HL pore which has a larger diameter (1.5 nm). In the presence of cocaine, the ligan-bound aptamer cannot pass through and is captured by the nanopore. The change in the channel currents was used to detect the presence of ligand-bound aptamers and thus cocaine. The device could detect cocaine in <1 min with LOD in the ng range (cutoff limit of the drug test).
4.3.2. Drugs in Biological Samples
Single compound: Microchip-based ELISA has been used for the determination of amphetamine in plasma and urine samples using CL detection [205] and D-methamphetamine in hair samples [206]. Mobioni Far et al. developed a disposable microfluidic chip which consisted of a serpentine channel coated with antibodies [205]. The sample (containing amphetamine) was mixed with labeled amphetamine (with horseradish peroxidase) and pumped through the channel. The amphetamine (unbound and horseradish peroxidase-labeled) was removed from the channel and CL was triggered using luminol and hydrogen peroxide. The concentration of the drug was inversely proportional to the detected signal. A colorimetric hydrogel-PAD was developed by Tian et al. based on highly selective aptamer-based recognition to detect cocaine in urine samples [207]. Crosslinked hydrogels with glucoamylase-trapped aptamers were used as the molecular recognition unit showing amplified signals upon cascade enzymatic reaction; while the platform showed lower sensitivity with cocaine metabolite (present in urine after cocaine ingestion), it showed high sensitivity to cocaine itself, showing a great potential in detecting cocaine as the major compound in blood/saliva. For more examples, the reader is referred to the review paper by Musile et al. [6].
Multiple compounds: Detecting various drug compounds in a biological sample first requires extraction and preconcentration followed by separation and detection. Liquid–liquid extraction (LLE) was implemented on a microchip by Miyaguchi et al. for the extraction of amphetamine, methamphetamine, methoxyphenamine, and mephentermine in urine samples [206]. Monoliths as a new class of materials have been used for the extraction of pharmaceutical compounds [208]. Monoliths can be formed in situ in microfluidic chips providing a stationary phase for separation. Xu et al. used organic monoliths in a PDMS microchip for the extraction of promethazine in synthetic plasma samples [209]. The microchip consisted of a monolith channel and a detection channel to detect promethazine based on CL.
In forensic drug analysis, separation is normally conducted using electrokinetic-based methods, namely CE and MEKC [5]. Du et al. implemented MEKC separation combined with Ru(bipy) ECL detection on a chip to separate and detect codeine and heroin in urine samples [210]. Electrophoretic-based separation combined with the UV detection method has been used by Qiag et al. to detect eight illicit drug compounds (from a family of narcotics, depressants, and anti-depressants) in urine samples [211]. A PMMA microfluidic chip was coupled to a printed circuit board to apply the required voltage for separation. The UV detection was performed on-chip using a custom-built set-up. The extraction was performed off-chip prior to separation and detection via LLE. Other separation methods, e.g., HPLC, have also been performed on a chip. Bait et al. developed the first HPLC-on-a-chip using commercial polyimide-based platforms for the detection of flunitrazepam (rohypnol), a drug associated with rape cases, in urine [212]. The metabolite of rohypnol (7-aminoflunitrazepam) was detected in urine samples with LOD in the ng range. Comparison of the on-chip results with the routine GC–MS showed a difference of less than 20%.
4.4. Explosive Residues
There is a great demand for the rapid determination of explosives on the field, e.g., near military bases and areas under terrorist attacks. The latter is of utmost importance due to the tremendous increase in the use of improvised explosives by criminals since military explosives are more controlled and not easily accessible [213]. Microfluidics can offer small, portable, and user-friendly platforms for accurate presumptive explosive detection on-site. Over the last decade, colorimetric and electrochemical detection have been among the most widely used methods to detect high and low explosives.
Detection of high explosives: Due to the redox properties of the nitroaromatic explosives, they can be readily detected electrochemically. The inherent compact design of electrochemical detection (ECD) methods along with less signal loss and smaller detection volumes (compared to absorbance- or fluorescence-based methods) make these techniques a suitable candidate for miniaturization and integration on-chip [214]. The first applications of electrochemical detection of explosives on a chip (coupled with CE separation) dates back to 2000 [215,216]. Wang et al. developed a glass-based microchip electrophoresis combined with ECD to detect a mixture of high explosives including TNT [215,217]. They used amperometry [217] and square-wave voltammetry [215] methods as the ECD methods; while the amperometric detection demonstrated higher sensitivity with LOD in the g range for both TNT and DNB (1,3-dinitrobenzen) [217], the voltametric detection provided more information. In the integrated microfluidic chip (CE combined with ECD (CE–ECD)) developed by Hilmi et al., the working electrode (made from Au) was directly deposited into the separation channel of the CE for amperometric detection [216]. A mixture of four nitroaromatic-based explosives (family of TNT and dinitrotoluene (DNT)) were analyzed in 130 s using a sodium dodecyl sulfate (SDS)/borate buffer. They achieved higher sensitivity with LOD in the ng range owing to the highly active surface area provided by the gold nanoparticles. In the work by Piccin et al., the same integration strategy (CE–ECD)) was used to develop microchip protocols for the fast screening and detection of nitrate ester explosives (including low-temperature PETN (pentaerythritol tetranitrate) as one of the strongest high explosives) [79]. Four nitrate ester explosives could be separated using a 1500 V separation voltage in less than 3 min and amperometrically detected with high sensitivity (LOD in ppm range). The developed microchip showed great promise for onsite threat evaluation and security screening, namely testing people’s luggage, vehicle, or packages for nitrate ester explosives.
Colorimetric methods on microfluidic platforms have also been implemented for testing high and military explosives. In 2015, Peters et al., developed a PAD for the detection of high explosives, e.g., urea nitrate, TNT, and RDX [218]. Explosives including organic peroxide (TATP) and its precursor hydrogen peroxide could also be detected with LOD in the g range. The PAD consisted of five lanes, which were fabricated on a chromatography paper using wax printing. The corresponding colorimetric reagent was deposited on each lane showing a color change upon reaction with the explosive after 5 min. In all experiments, a mixture of acetone–water (50:50) was used as the solvent. This platform demonstrated an inexpensive, easy-to-use, and portable test for the rapid identification of explosive residues. Pesenti et al. developed a PAD consisting of two areas for presumptive colorimetric as well as confirmatory tests for the detection of trinitro aromatic explosives, e.g., TNT and trinitrobenzene (TNB) [219]. The device could detect TNT and TNB with LOD in the ng range with no interference from commercial products, such as perfumes, cleaning agents, oxidizers, etc. Another colorimetric-based PAD for the identification of high explosives, including TATP, was developed by Salles et al. [220]. Three reagents (KI, creatinine, and aniline) were used to discriminate five explosives based on the unique color pattern upon the reaction between the two, which was evaluated via a smartphone. Using unique analysis methods, namely hierarchical clustering analysis and principal component analysis, the analytes could be readily discriminated after 15 min (Figure 9).
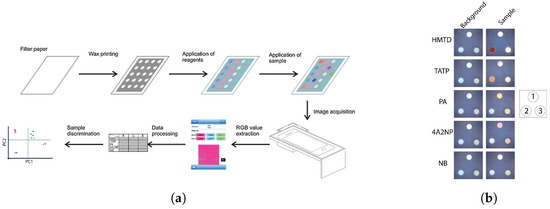
Figure 9.
(a) The fabrication and measurement process of the PAD developed by Salles et al. for colorimetric discrimination of (b) five high explosives upon reaction with each reagent (1: creatinine, 2: KI/H, and 3: aniline) (Used with permission of the Royal Society of Chemistry, from (Explosive colorimetric discrimination using a smartphone, paper device and chemometrical approach, Salles et al., 6, 7, ©2014); permission conveyed through Copyright Clearance Center, Inc.) [220].
Detection of low explosives and blasting agents: PADs were the most developed microfluidic platforms in the recent years for on-site colorimetric detection of low explosives and blasting agents. Chabaud et al. developed a PAD for the simultaneous detection of inorganic metallic salts present in primer residues and fireworks [221]. The PAD consisting of six hydrophobic channels was made by wax printing on chromatography paper. In each channel, a specific reagent was placed, demonstrating a color change upon reaction with the metal salt. The device could detect six metals (lead (Pb), iron (Fe), antimony (Sb), zinc (Zn), aluminum (Al), and barium (Ba)) in less than 10 min with LOD in the g range (Figure 10).
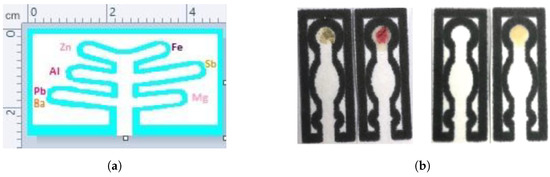
Figure 10.
(a) The multiplexed PAD design developed by Chabaud et al. for detection of various metal salts as inorganic residues of low explosives. (b) Results of colorimetric test on a single lane showing color change from tan to pink/purple for lead (left) and colorless to brown for antimony (right). In all experiments, water was used at the solvent [221] (Reprinted from publication Simultaneous colorimetric detection of metallic salts contained in low explosives residue using a microfluidic paper-based analytical device (PAD), 9, 35–41, Chabaud et al., Copyright ©2018, with permission from Elsevier).
A five-lane PAD was developed by Peters et al. for the detection of inorganic explosives, e.g., black powder, flash powder, and ammonium nitrate [218]. The fabrication strategy was similar to the previously described method (wax printing to create hydrophobic channels on chromatography paper). The explosives were dissolved in deionized water to enable capillary action. The PAD could detect inorganic explosives, e.g., ammonium, chlorate and perchlorate oxidizers, nitrate, and nitrite with LOD in the g range based on the color change upon reaction with the deposited reagent on each lane.
5. The Road Ahead for Microfluidic-Based Forensic Diagnosis
5.1. Shortcomings
Microfluidic platforms can serve as rapid and easy-to-use methodologies for on-scene forensic analysis. They are potentially cost-effective, non-destructive, and accurate, depending on the method and corresponding integrated analysis on the chip. Despite these advantages, they are not yet universally implemented in the field of forensic analysis. The possible reasons for this are as follows:
- Lack of standardization: Some developed microfluidic platforms, specifically paper-based ones, cannot withstand harsh environmental conditions, are sensitive to temperature and/or humidity, show limited stability of chemical reagents, and can have variations from batch to batch [117]. All these factors result in a lack of standardization which further impedes the acceptance of these platforms by the forensic authorities.
- Challenges in integration: An ideal microfluidic device for on-scene application should provide the so-called “sample-to-answer” and directly connect the forensic investigators to the results. The laboratory-based confirmatory tests (e.g., assays) normally involve multi-step procedures requiring sample collection and processing (e.g., pre-concentration), chemical/biological reactions and generation of signals, detection, analysis, and final reporting of the results. A successful microfluidic device which can provide a rapid and accurate alternative method on-site should have all these steps integrated and automated in a single platform. A vast majority of research on this field has mainly focused on developing proof-of-concept methodologies for individual steps as independent technologies. Undoubtedly, discretization is an imperative stage of developing any technology for resolving potential problems. To realize an end product, however, all these discrete technologies must be integrated. The transition from laboratory microfluidic prototypes to a commercial product is still challenging. Most of these platforms are mainly tested under controlled laboratory conditions, which makes them difficult to integrate with the other technologies under realistic conditions.
- Product cost: Material and manufacturing methods must be considered for mass production to enable a smooth transition of the technology to the forensic field. Most laboratory-based platforms are made of glass, silicon, or PDMS, which require cleanroom facilities and lithography techniques; while plastic and paper-based platforms are affordable alternatives for mass production, their universal applicability is questionable. The choice of material is highly constrained by the application, compatibility with the sample, and possible integration with detection elements.
- Associated trade-offs with sample-to-answer platforms: Up to this date, there are few commercial rapid DNA analysis platforms which can provide a sample-to-DNA profile. Compared to the conventional method, these platforms have some limitations and trade-offs including reduced sensitivity, higher costs than originally anticipated, speed, and throughput [4]. These trade-offs along with the cultural forensic landscape have further limited the use of such commercial sample-to-answer platforms, making the implementation of fluidic technology in the forensic field a complex task.
5.2. Future Perspectives
Considering the shortcomings and limitations explained above, the following strategies are proposed to further enhance the implementation of the microfluidic technology in the forensic field.
- Enhancing the existing capabilities: plastic and paper-based microfluidic platforms have grown tremendously over the last decade, mainly due to their low cost and ease-of use. These platforms offer multiplexing for simultaneous analysis of multiple compounds. At this stage, a focus change toward standardization and integration of these platforms with electronic devices (e.g., smartphones for detection and/or analysis steps) can further expand their applicability in different forensic fields.
- Empowering the current methodologies: as stated above, the integration of all analysis steps in a single platform is challenging, which in some cases makes the sensitivity/specificity of microfluidic technology questionable compared to the laboratory-based methods. In lieu of developing a competing technology with the current state-of-the-art, it is recommended to develop more innovative platforms which can empower the existing technologies and provide court-proof results. This can further help overburdened forensic laboratories to accelerate analysis and testing.
- Miniaturization of bulky peripherals: one of the other challenges which restricts the commercialization and final use of the microfluidic platforms for crime scene investigation is the need for bulky peripherals, e.g., pumps, optical detectors, power sources, etc. All the components must be miniaturized to achieve a fully portable platform. Research in this field has already been initiated to miniaturize peripheral set-ups and develop portable point-of-care (POC) devices [222]. It is suggested to consider a similar research direction to develop portable platforms for forensic applications.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript.
| ALS | Alternate light source |
| AN | Ammonium nitrate |
| BF | Body fluid |
| BFID | Body fluid identifictation |
| BFS | Body fluid screening |
| CBA | Cocaine-binding aptamer |
| CE | Capillary electrophoresis |
| CL | Chemiluminescence |
| CLSM | Confocal laser scanning microscope |
| CNC | Computer numerical control |
| CNS | Central nervous system |
| COC | Cyclic olefin copolymer |
| DE | Differential extraction |
| DNA | Deoxyribonucleic acid |
| DNB | 1,3-dinitrobenzene |
| DNT | Dinitrotoluene |
| ECD | Electrochemical detection |
| ECL | Electrochemiluminescence |
| EDS | Energy dispersive spectroscopy |
| EDX | Energy dispersive X-ray analyzer |
| ELISA | Enzyme-linked immunosorbent assay |
| FBI | Federal Bureau of Investigation |
| FT-IR | Fourier transform infrared |
| GC–MS | Gas chromatography–mass spectrometry |
| GHB | Gamma hydroxybutyrate |
| Hb | Hemoglobin |
| HID | Human identification |
| HPLC | High-performance liquid chromatography |
| HSA | Heat-sensitive adhesive |
| HTN3 | Histatin 3 |
| IC | Ion chromatography |
| IMS | Ion mobility spectrometry |
| IR | Infrared |
| KL | Kestle–Meyer |
| LC–MS | Liquid chromatography–mass spectrometry |
| LLE | Liquid–liquid extraction |
| LMG | Leuchomalachite green |
| LOC | Lab-on-chip |
| LOD | Limit of detection |
| LSD | Lysergic acid diethylamide |
| MDMA | Methylenedioxymethamphetamine |
| ME | Microchip electrophoresis |
| MEKC | Micellar electrokinetic chromatography |
| MS | Mass spectrometry |
| NG | Nitroglycerin |
| PC | Polycarbonate |
| PCR | Polymerase chain reaction |
| PDMS | Polydimethylsiloxane |
| PeT | Polyethylene terephthalate |
| PETN | Pentaerythritol tetranitrate |
| PHP | Phenolphthalein |
| PMD | Portable microfluidic-based device |
| PMMA | Polymethylmethacrylate |
| POC | Point-of-care |
| PON | Point-of-need |
| PRM1 | Protamine 1 |
| PS | Polystyrene |
| PSA | Prostate-specific antigen |
| PU | Polyurethane |
| RDX | Cyclotrimethylenetrinitramene |
| RNA | Ribonucleic acid |
| RSID | Rapid stain identification |
| RT-PCR | Reverse transcription polymerase chain reaction |
| SAP | Seminal acid phosphatase |
| SDS | Sodium dodecyl sulfate |
| SEM | Scanning electron microscopy |
| SPE | Solid phase extraction |
| STATH | Statherin |
| STR | Short tandem repeat |
| TAK | Takayama |
| TATP | Triacetone triperoxide |
| TEI | Teichman’s |
| TLC | Thin layer chromatography |
| TMB | Tetramethylbenzidine |
| TNB | Trinitrobenzene |
| TNT | Trinitrotoluene |
| UN | Urea nitrate |
| UV | Ultraviolet |
| UV–Vis | Ultraviolet-visible |
| XRD | X-ray diffractometry |
| PAD | Microfluidic paper-based analytical device |
| PON | Microfluidic-based point-of-need |
References
- Addington, L.A. Hot vs. Cold Cases: Examining Time to Clearance for Homicides Using NIBRS Data. Justice Res. Policy 2007, 9, 87–112. [Google Scholar] [CrossRef]
- Kloosterman, A.; Mapes, A.; Geradts, Z.; van Eijk, E.; Koper, C.; van den Berg, J.; Verheij, S.; van der Steen, M.; van Asten, A. The interface between forensic science and technology: How technology could cause a paradigm shift in the role of forensic institutes in the criminal justice system. Philos. Trans. R. Soc. Biol. Sci. 2015, 370, 1674. [Google Scholar] [CrossRef]
- Bruijns, B.; van Asten, A.; Tiggelaar, R.; Gardeniers, H. Microfluidic Devices for Forensic DNA Analysis: A Review. Biosensors 2016, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Turiello, R.; Nouwairi, R.L.; Landers, J.P. Taking the microfluidic approach to nucleic acid analysis in forensics: Review and perspectives. Forensic Sci. Int. Genet. 2023, 63, 102824. [Google Scholar] [CrossRef] [PubMed]
- Al-Hetlani, E. Forensic drug analysis and microfluidics. Electrophoresis 2013, 34, 1262–1272. [Google Scholar] [CrossRef]
- Musile, G.; Agard, Y.; Wang, L.; De Palo, E.F.; McCord, B.; Tagliaro, F. Paper-based microfluidic devices: On-site tools for crime scene investigation. TrAC Trends Anal. Chem. 2021, 143, 116406. [Google Scholar] [CrossRef]
- Vyas, B.; Halámková, L.; Lednev, I.K. A universal test for the forensic identification of all main body fluids including urine. Forensic Chem. 2020, 20, 100247. [Google Scholar] [CrossRef]
- Virkler, K.; Lednev, I.K. Analysis of body fluids for forensic purposes: From laboratory testing to non-destructive rapid confirmatory identification at a crime scene. Forensic Sci. Int. 2009, 188, 1–17. [Google Scholar] [CrossRef]
- Basset, P.; Blandin, P.; Grini, A.; Delemont, S.; Samie, L.; Castella, V. A simplified protocol for the detection of blood, saliva, and semen from a single biological trace using immunochromatographic tests. Forensic Sci. Med. Pathol. 2022, 18, 141–148. [Google Scholar] [CrossRef]
- An, J.H.; Shin, K.J.; Yang, W.I.; Lee, H.Y. Body fluid identification in forensics. BMB Rep. 2012, 45, 545–553. [Google Scholar] [CrossRef]
- Maskell, P.D.; Jackson, G. Presumptive drug testing—The importance of considering prior probabilities. WIREs Forensic Sci. 2020, 2, e1371. [Google Scholar] [CrossRef]
- Muro, C.K.; Doty, K.C.; Bueno, J.; Halámková, L.; Lednev, I.K. Vibrational Spectroscopy: Recent Developments to Revolutionize Forensic Science. Anal. Chem. 2015, 87, 306–327. [Google Scholar] [CrossRef] [PubMed]
- Virkler, K.; Lednev, I.K. Raman spectroscopic signature of blood and its potential application to forensic body fluid identification. Anal. Bioanal. Chem. 2010, 396, 525–534. [Google Scholar] [CrossRef]
- Virkler, K.; Lednev, I.K. Raman spectroscopic signature of semen and its potential application to forensic body fluid identification. Forensic Sci. Int. 2009, 193, 56–62. [Google Scholar] [CrossRef]
- Muro, C.K.; Doty, K.C.; de Souza Fernandes, L.; Lednev, I.K. Forensic body fluid identification and differentiation by Raman spectroscopy. Forensic Chem. 2016, 1, 31–38. [Google Scholar] [CrossRef]
- Mistek, E.; Halámková, L.; Doty, K.C.; Muro, C.K.; Lednev, I.K. Race Differentiation by Raman Spectroscopy of a Bloodstain for Forensic Purposes. Anal. Chem. 2016, 88, 7453–7456. [Google Scholar] [CrossRef]
- McLaughlin, G.; Doty, K.C.; Lednev, I.K. Discrimination of human and animal blood traces via Raman spectroscopy. Forensic Sci. Int. 2014, 238, 91–95. [Google Scholar] [CrossRef]
- Doty, K.C.; Lednev, I.K. Differentiation of human blood from animal blood using Raman spectroscopy: A survey of forensically relevant species. Forensic Sci. Int. 2018, 282, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Cox, M. A study of the sensitivity and specificity of four presumptive tests for blood. J. Forensic Sci. 1991, 36, 1503–1511. [Google Scholar] [CrossRef]
- Tobe, S.S.; Watson, N.; Daéid, N.N. Evaluation of Six Presumptive Tests for Blood, Their Specificity, Sensitivity, and Effect on High Molecular-Weight DNA. J. Forensic Sci. 2007, 52, 102–109. [Google Scholar] [CrossRef]
- Webb, J.L.; Creamer, J.I.; Quickenden, T.I. A comparison of the presumptive luminol test for blood with four non-chemiluminescent forensic techniques. Luminescence 2006, 21, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Colotelo, A.H.; Cooke, S.J.; Smokorowski, K.E. Application of forensic techniques to enhance fish conservation and management: Injury detection using presumptive tests for blood. Endanger. Species Res. 2009, 9, 169–178. [Google Scholar] [CrossRef]
- Barni, F.; Lewis, S.W.; Berti, A.; Miskelly, G.M.; Lago, G. Forensic application of the luminol reaction as a presumptive test for latent blood detection. Talanta 2007, 72, 896–913. [Google Scholar] [CrossRef] [PubMed]
- Chapter 8-Presumptive and Confirmatory Blood Testing. In Forensic Science Reform; Koen, W.J., Bowers, C.M., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 239–269. [Google Scholar]
- Harper, L.; Powell, J.; Pijl, E.M. An overview of forensic drug testing methods and their suitability for harm reduction point-of-care services. Harm Reduct. J. 2017, 14, 52. [Google Scholar] [CrossRef]
- Sinelnikov, A.; Kalinina, A.; Old, J.B.; Boonlayangoor, P.W.; Reich, K.A. Evaluation of Rapid Stain IDentification (RSID™) Reader System for Analysis and Documentation of RSID™ Tests. Appl. Sci. 2013, 3, 624–635. [Google Scholar] [CrossRef]
- Hortolà, P. SEM examination of human erythrocytes in uncoated bloodstains on stone: Use of conventional as environmental-like SEM in a soft biological tissue (and hard inorganic material). J. Microsc. 2005, 218, 94–103. [Google Scholar] [CrossRef]
- Hortolà, P. Secondary-electron SEM bioimaging of human erythrocytes in bloodstains on high-carbon steel substrate without specimen preparation. Micron 2008, 39, 53–55. [Google Scholar] [CrossRef]
- Hortolà, P. Human Bloodstains on Biological Materials: High-Vacuum Scanning Electron Microscope Examination Using Specimens without Previous Preparation. Microsc. Microanal. 2013, 19, 415–419. [Google Scholar] [CrossRef]
- Hortolà, P. MRT letter: Human bloodstains on antique aboriginal weapons: A guiding low-vacuum sem study of erythrocytes in experimental samples on ethnographically documented biological raw materials. Microsc. Res. Tech. 2012, 75, 1007–1011. [Google Scholar] [CrossRef]
- Hortolà, P. Human bloodstains on bone artefacts: An SEM intra- and inter-sample comparative study using ratite bird tibiotarsus. Micron 2016, 90, 108–113. [Google Scholar] [CrossRef]
- Hortolà, P. Microscopic imaging of human bloodstains: Testing the potential of a confocal laser scanning microscope as an alternative to SEMs. Micron 2020, 130, 102821. [Google Scholar] [CrossRef]
- Hovis, D.; Heuer, A. The use of laser scanning confocal microscopy (LSCM) in materials science. J. Microsc. 2010, 240, 173–180. [Google Scholar] [CrossRef]
- Eyring, M.B. Fundamentals of Visible Microspectrophotometry in Forensic Science. In Forensic Science Handbook, Volume I; CRC Press: Boca Raton, FL, USA, 2020; Chapter 5; p. 55. [Google Scholar]
- Bauer, M. RNA in forensic science. Forensic Sci. Int. Genet. 2007, 1, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Patzelt, D. Evaluation of mRNA markers for the identification of menstrual blood. J. Forensic Sci. 2002, 47, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Gramlich, I.; Polzin, S.; Patzelt, D. Quantification of mRNA degradation as possible indicator of postmortem interval—A pilot study. Leg. Med. 2003, 5, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Polzin, S.; Patzelt, D. Quantification of RNA degradation by semi-quantitative duplex and competitive RT-PCR: A possible indicator of the age of bloodstains? Forensic Sci. Int. 2003, 138, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.G.; Santucci, K.A. An Alternate Light Source to Detect Semen. Acad. Emerg. Med. 2002, 9, 1045–1048. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, U. Forensic Analysis of Semen: A Review. Int. J. Inf. Comput. Sci. 2018, 5, 81–86. [Google Scholar]
- Greenfield, A.; Sloan, M.M. Identification of Biological Fluids and Stains. In Forensic Science-An Introduction to Scientific and Investigative Techniques; CRC Press: Boca Raton, FL, USA, 2002; Chapter 12; p. 18. [Google Scholar]
- Idris, B.; Goodwin, W.H. Evaluating the sensitivity of presumptive and confirmatory tests for body fluids. Forensic Sci. Int. Genet. Suppl. Ser. 2022, 8, 276–278. [Google Scholar] [CrossRef]
- Laffan, Á.; Sawyer, I.; Quinones, I.; Daniel, B. Evaluation of semen presumptive tests for use at crime scenes. Med. Sci. Law 2011, 51, 11–17. [Google Scholar] [CrossRef]
- Maher, J.; Vintiner, S.; Elliot, D.; Melia, L. Evaluation of the BioSign PSA membrane test for the identification of semen stains in forensic casework. N. Z. Med. J. 2002, 115, 48–49. [Google Scholar] [PubMed]
- Old, J.; Schweers, B.A.; Boonlayangoor, P.W.; Fischer, B.; Miller, K.W.P.; Reich, K. Developmental Validation of RSIDTM-Semen: A Lateral Flow Immunochromatographic Strip Test for the Forensic Detection of Human Semen. J. Forensic Sci. 2012, 57, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.; Juusola, J.; Ballantyne, J. An mRNA and DNA co-isolation method for forensic casework samples. Anal. Biochem. 2004, 335, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Auvdel, M.J. Comparison of laser and high-intensity quartz arc tubes in the detection of body secretions. J. Forensic Sci. 1988, 33, 925–945. [Google Scholar] [CrossRef]
- Fiedler, A.; Rehdorf, J.; Hilbers, F.; Johrdan, L.; Stribl, C.; Benecke, M. Detection of Semen (Human and Boar) and Saliva on Fabrics by a Very High Powered UV-/VIS-Light Source. Open Forensic Sci. J. 2008, 1, 12–15. [Google Scholar] [CrossRef]
- Gupta, A.K. Forensic Biology and Serology- Definitions and Concepts. 2023. Available online: http://epgp.inflibnet.ac.in/epgpdata/uploads/epgp_content/S000016FS/P000699/M011528/ET/1516257136FSC_P12_M2_e-text.pdf (accessed on 8 May 2023).
- Martin, N.; Clayson, N.; Scrimger, D. The sensitivity and specificity of Red-Starch paper for the detection of saliva. Sci. Justice 2006, 46, 97–105. [Google Scholar] [CrossRef]
- Komuro, T.; Mukoyama, R.; Mukoyama, H. Application of enzyme-linked immunosorbent assay (ELISA) to the medico-legal identification. Nihon Rinsho. Jpn. J. Clin. Med. 1995, 53, 2322–2329. [Google Scholar]
- Seta, S. Application of scanning electron microscopy and energy dispersive x-ray microanalysis to the criminal identification of body fluid stains. Int. Crim. Police Rev. 1977, 307, 119–123. [Google Scholar]
- Soukos, N.S.; Crowley, K.; Bamberg, M.P.; Gillies, R.; Doukas, A.G.; Evans, R.; Kollias, N. A rapid method to detect dried saliva stains swabbed from human skin using fluorescence spectroscopy. Forensic Sci. Int. 2000, 114, 133–138. [Google Scholar] [CrossRef]
- Old, J.B.; Schweers, B.A.; Boonlayangoor, P.W.; Reich, K.A. Developmental validation of RSID™-saliva: A lateral flow immunochromatographic strip test for the forensic detection of saliva. J. Forensic Sci. 2009, 54, 866–873. [Google Scholar] [CrossRef]
- Juusola, J.; Ballantyne, J. Multiplex mRNA profiling for the identification of body fluids. Forensic Sci. Int. 2005, 152, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lledo-Fernandez, C.; Banks, C.E. An overview of quantifying and screening drugs of abuse in biological samples: Past and present. Anal. Methods 2011, 3, 1227–1245. [Google Scholar] [CrossRef]
- Elkins, K.M.; Weghorst, A.C.; Quinn, A.A.; Acharya, S. Colour quantitation for chemical spot tests for a controlled substances presumptive test database. Drug Test. Anal. 2017, 9, 306–310. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, C.L.; Crouch, D.J.; Fatah, A.A. Validation of twelve chemical spot tests for the detection of drugs of abuse. Forensic Sci. Int. 2000, 109, 189–201. [Google Scholar] [CrossRef]
- Elie, M.P.; Elie, L.E. Microcrystalline Tests in Forensic Drug Analysis. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Li, Q.; Qiu, T.; Hao, H.; Zhou, H.; Wang, T.; Zhang, Y.; Li, X.; Huang, G.; Cheng, J. Rapid and on-site analysis of illegal drugs on the nano–microscale using a deep ultraviolet-visible reflected optical fiber sensor. Analyst 2012, 137, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Cargill, K.; Kammrath, B.W. The identification of controlled substances by TLCSERS. In Proceedings of the 66th Annual Scientific Meeting of the American Academy of Forensic Sciences. Seattle: Forensic Sciences Foundation, Seattle, WA, USA, 17–22 February 2014. [Google Scholar]
- Kanai, K.; Takekawa, K.; Kumamoto, T.; Ishikawa, T.; Ohmori, T. Simultaneous analysis of six phenethylamine-type designer drugs by TLC, LC-MS, and GC-MS. Forensic Toxicol. 2008, 26, 6–12. [Google Scholar] [CrossRef]
- Tsai, J.S.C.; Lin, G.L. Drug-Testing Technologies and Applications. In Drugs of Abuse: Body Fluid Testing; Wong, R.C., Tse, H.Y., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 29–69. [Google Scholar]
- Forsgard, N.; Salehpour, M.; Possnert, G. Accelerator mass spectrometry in the attomolar concentration range for 14 C-labeled biologically active compounds in complex matrixes. J. Anal. At. Spectrom. 2010, 25, 74–78. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Aboul-Enein, H.Y.; Fleschin, S. Application of Fourier transform infrared spectrophotometry in pharmaceutical drugs analysis. Appl. Spectrosc. Rev. 2010, 45, 206–219. [Google Scholar] [CrossRef]
- de Oliveira Penido, C.A.F.; Pacheco, M.T.T.; Lednev, I.K.; Silveira, L., Jr. Raman spectroscopy in forensic analysis: Identification of cocaine and other illegal drugs of abuse. J. Raman Spectrosc. 2016, 47, 28–38. [Google Scholar] [CrossRef]
- Ali, E.M.; Edwards, H.G. The detection of flunitrazepam in beverages using portable Raman spectroscopy. Drug Test. Anal. 2017, 9, 256–259. [Google Scholar] [CrossRef]
- Trzybiński, D.; Niedziałkowski, P.; Ossowski, T.; Trynda, A.; Sikorski, A. Single-crystal X-ray diffraction analysis of designer drugs: Hydrochlorides of metaphedrone and pentedrone. Forensic Sci. Int. 2013, 232, e28–e32. [Google Scholar] [CrossRef] [PubMed]
- Yeager, K. Improvised explosives characteristics, detection, and analysis. In Forensic Investigation of Explosions; CRC Press: Boca Raton, FL, USA, 2011; pp. 531–576. [Google Scholar]
- Mocella, C.; Conkling, J.A. Chemistry of Pyrotechnics: Basic Principles and Theory; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Hopler, R.B. The History, Development, and Characteristics of Explosives and Propellants. In Forensic Investigation of Explosions; CRC Press: Boca Raton, FL, USA, 2011; Chapter 1; p. 18. [Google Scholar]
- Perigrin, T. Introductory Practical Pyrotechnics; Falcon Fireworks: Guyton, GA, USA, 1999; pp. 137–171. [Google Scholar]
- De Perre, C.; Prado, A.; McCord, B.R. Rapid and specific detection of urea nitrate and ammonium nitrate by electrospray ionization time-of-flight mass spectrometry using infusion with crown ethers. Rapid Commun. Mass Spectrom. 2012, 26, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Zeman, S.; Trzciński, W.A.; Matyáš, R. Some properties of explosive mixtures containing peroxides: Part I. Relative performance and detonation of mixtures with triacetone triperoxide. J. Hazard. Mater. 2008, 154, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Zeman, S.; Bartei, C. Some properties of explosive mixtures containing peroxides: Part II. Relationships between detonation parameters and thermal reactivity of the mixtures with triacetone triperoxide. J. Hazard. Mater. 2008, 154, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Ladbeck, R.; Kolla, P.; Karst, U. Trace analysis of peroxide-based explosives. Anal. Chem. 2003, 75, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Van De Craats, A.M.; Kok, E.M.; De Bruyn, P. Trace analysis of peroxide explosives by high performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry (HPLC-APCI-MS/MS) for forensic applications. J. Forensic Sci. 2004, 49, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Trends in analysis of explosives by microchip electrophoresis and conventional CE. Electrophoresis 2008, 29, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Piccin, E.; Dossi, N.; Cagan, A.; Carrilho, E.; Wang, J. Rapid and sensitive measurements of nitrate ester explosives using microchip electrophoresis with electrochemical detection. Analyst 2009, 134, 528–532. [Google Scholar] [CrossRef]
- Cordesman, A.H. The Developing Iraqi Insurgency: Status at End-2004, Working Draft. 2004. Available online: https://www.comw.org/warreport/fulltext/0412cordesman.pdf (accessed on 8 May 2023).
- Singh, S. Sensors—An effective approach for the detection of explosives. J. Hazard. Mater. 2007, 144, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Buttigieg, G.A.; Knight, A.K.; Denson, S.; Pommier, C.; Denton, M.B. Characterization of the explosive triacetone triperoxide and detection by ion mobility spectrometry. Forensic Sci. Int. 2003, 135, 53–59. [Google Scholar] [CrossRef]
- Moore, D.S. Instrumentation for trace detection of high explosives. Rev. Sci. Instruments 2004, 75, 2499–2512. [Google Scholar] [CrossRef]
- Andrew, T.L.; Swager, T.M. Detection of explosives via photolytic cleavage of nitroesters and nitramines. J. Org. Chem. 2011, 76, 2976–2993. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.H.; Simpson, G. Capabilities and limitations of ion mobility spectrometry for field screening applications. Field Anal. Chem. Technol. 1997, 1, 119–134. [Google Scholar] [CrossRef]
- Forensic Store. Pocket-ETK FS Explosives Testing Kit. 2023. Available online: https://www.forensicstore.com/product/pocket-etk-fs-explosives-testing-kit/ (accessed on 8 May 2023).
- Meditest. EXPRAY Explosives Detection Identification Field Test Kit. 2023. Available online: https://meditests.com/product/expray-explosives-detection-identification-field-test-kit-100-tests/ (accessed on 10 May 2023).
- Defence, R. XCAT Capillary Analysis Test. 2023. Available online: https://cbrnetechindex.com/SupportDocuments/b285d3fd-7be6-4c28-a1f7-0fecc6d1d516RedX-XCat.pdf (accessed on 10 May 2023).
- Peters, K. Development of Presumptive and Confirmatory Analytical Methods for the Simultaneous Detection of Multiple Improvised Explosives. Ph.D. Thesis, Florida International University, St. Miami, FL, USA, 2014. [Google Scholar]
- Cagan, A.; Schmidt, H.; Rodriguez, J.; Eiceman, G. Fast gas chromatography-differential mobility spectrometry of explosives from TATP to Tetryl without gas atmosphere modifiers. Int. J. Ion Mobil. Spectrom. 2010, 13, 157–165. [Google Scholar] [CrossRef]
- Schulte-Ladbeck, R.; Edelmann, A.; Quintas, G.; Lendl, B.; Karst, U. Determination of peroxide-based explosives using liquid chromatography with on-line infrared detection. Anal. Chem. 2006, 78, 8150–8155. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, T.; Sun, H.; Kannan, K. Perchlorate in tap water, groundwater, surface waters, and bottled water from China and its association with other inorganic anions and with disinfection byproducts. Arch. Environ. Contam. Toxicol. 2010, 58, 543–550. [Google Scholar] [CrossRef]
- Flanagan, R.J.; Perrett, D.; Whelpton, R. Electrochemical Detection in HPLC: Analysis of Drugs and Poisons; Royal Society of Chemistry: London, UK, 2005; Volume 10. [Google Scholar]
- Doyle, J.M.; Miller, M.L.; McCord, B.R.; McCollam, D.A.; Mushrush, G.W. A multicomponent mobile phase for ion chromatography applied to the separation of anions from the residue of low explosives. Anal. Chem. 2000, 72, 2302–2307. [Google Scholar] [CrossRef]
- Bender, E.C.; Beveridge, A.D. Investigation of Pipe Bombs. In Forensic Investigation of Explosions; CRC Press: Boca Raton, FL, USA, 2011; Chapter 11; pp. 431–481. [Google Scholar]
- Hutchinson, J.P.; Johns, C.; Breadmore, M.C.; Hilder, E.F.; Guijt, R.M.; Lennard, C.; Dicinoski, G.; Haddad, P.R. Identification of inorganic ions in post-blast explosive residues using portable CE instrumentation and capacitively coupled contactless conductivity detection. Electrophoresis 2008, 29, 4593–4602. [Google Scholar] [CrossRef] [PubMed]
- Burks, R.M.; Hage, D.S. Current trends in the detection of peroxide-based explosives. Anal. Bioanal. Chem. 2009, 395, 301–313. [Google Scholar] [CrossRef]
- Berk, R.E. Automated SEM/EDS Analysis of Airbag Residue.* I: Particle Identification. J. Forensic Sci. 2009, 54, 60–68. [Google Scholar] [CrossRef]
- Berk, R.E. Automated SEM/EDS analysis of airbag residue. II: Airbag residue as a source of percussion primer residue particles. J. Dorensic Sci. 2009, 54, 69–76. [Google Scholar] [CrossRef]
- Prabhakar, P.; Sen, R.K.; Dwivedi, N.; Khan, R.; Solanki, P.R.; Mishra, S.; Srivastava, A.K.; Dhand, C. 3D-Printed Microfluidic Device with Integrated Biosensors for Biomedical Applications. In Advanced Microfluidics Based Point-of-Care Diagnostics; CRC Press: Boca Raton, FL, USA, 2022; Chapter 6; pp. 148–166. [Google Scholar]
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.R.; Weigl, B.H. Microfluidic diagnostic technologies for global public health. Nature 2006, 442, 412–418. [Google Scholar] [CrossRef]
- Katoch, V.; Prakash, B. The Basic Concept for Microfluidics-Based Devices. In Advanced Microfluidics Based Point-of-Care Diagnostics; CRC Press: Boca Raton, FL, USA, 2022; Chapter 1; pp. 1–38. [Google Scholar]
- Vashist, S.K.; Mudanyali, O.; Schneider, E.M.; Zengerle, R.; Ozcan, A. Cellphone-based devices for bioanalytical sciences. Anal. Bioanal. Chem. 2014, 406, 3263–3277. [Google Scholar] [CrossRef] [PubMed]
- Beduk, T.; Beduk, D.; Hasan, M.R.; Guler Celik, E.; Kosel, J.; Narang, J.; Salama, K.N.; Timur, S. Smartphone-based multiplexed biosensing tools for health monitoring. Biosensors 2022, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tian, Z.; Bachman, H.; Zhang, P.; Huang, T.J. A cell-phone-based acoustofluidic platform for quantitative point-of-care testing. ACS Nano 2020, 14, 3159–3169. [Google Scholar] [CrossRef]
- Isiksacan, Z.; Guler, M.T.; Aydogdu, B.; Bilican, I.; Elbuken, C. Rapid fabrication of microfluidic PDMS devices from reusable PDMS molds using laser ablation. J. Micromech. Microeng. 2016, 26, 035008. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, Z.; Hou, Z.; Song, J. Processing and Profile Control of Microhole Array for PDMS Mask with Femtosecond Laser. Micromachines 2022, 13, 340. [Google Scholar] [CrossRef]
- Behroodi, E.; Latifi, H.; Bagheri, Z.; Ermis, E.; Roshani, S.; Salehi Moghaddam, M. A combined 3D printing/CNC micro-milling method to fabricate a large-scale microfluidic device with the small size 3D architectures: An application for tumor spheroid production. Sci. Rep. 2020, 10, 22171. [Google Scholar] [CrossRef] [PubMed]
- Opalski, A.S.; Makuch, K.; Derzsi, L.; Garstecki, P. Split or slip–passive generation of monodisperse double emulsions with cores of varying viscosity in microfluidic tandem step emulsification system. RSC Adv. 2020, 10, 23058–23065. [Google Scholar] [CrossRef]
- Goral, V.N.; Hsieh, Y.C.; Petzold, O.N.; Faris, R.A.; Yuen, P.K. Hot embossing of plastic microfluidic devices using poly (dimethylsiloxane) molds. J. Micromech. Microeng. 2010, 21, 017002. [Google Scholar] [CrossRef]
- Narasimhan, J.; Papautsky, I. Rapid fabrication of hot embossing tools using PDMS. Proc. SPIE 2003, 4982, 110–119. [Google Scholar]
- Khan, M.S.; Lachmayer, R.; Roth, B. Maskless lithography for versatile and low cost fabrication of polymer based micro optical structures. OSA Contin. 2020, 3, 2808–2816. [Google Scholar] [CrossRef]
- Ma, X.; Li, R.; Jin, Z.; Fan, Y.; Zhou, X.; Zhang, Y. Injection molding and characterization of PMMA-based microfluidic devices. Microsyst. Technol. 2020, 26, 1317–1324. [Google Scholar] [CrossRef]
- Jeon, J.S.; Chung, S.; Kamm, R.D.; Charest, J.L. Hot embossing for fabrication of a microfluidic 3D cell culture platform. Biomed. Microdevices 2011, 13, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Kreutz, J.E.; Schneider, T.; Yen, G.S.; Shah, E.S.; Wu, L.; Chiu, D.T. A reinforced PDMS mold for hot embossing of cyclic olefin polymer in the fabrication of microfluidic chips. Lab Chip 2022, 22, 4729–4734. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. 2007, 119, 1340–1342. [Google Scholar] [CrossRef]
- Sen, A.K.; Nath, A.; Sudeepthi, A.; Jain, S.K.; Banerjee, U. Microfluidics-Based Point-of-Care Diagnostic Devices. In Advanced Microfluidics Based Point-of-Care Diagnostics; CRC Press: Boca Raton, FL, USA, 2022; Chapter 4; pp. 99–120. [Google Scholar]
- Wang, X.; Cheng, C.; Wang, S.; Liu, S. Electroosmotic pumps and their applications in microfluidic systems. Microfluid. Nanofluidics 2009, 6, 145–162. [Google Scholar] [CrossRef]
- Guttenberg, Z.; Müller, H.; Habermüller, H.; Geisbauer, A.; Pipper, J.; Felbel, J.; Kielpinski, M.; Scriba, J.; Wixforth, A. Planar chip device for PCR and hybridization with surface acoustic wave pump. Lab Chip 2005, 5, 308–317. [Google Scholar] [CrossRef]
- Garcia, A.A.; Egatz-Gomez, A.; Lindsay, S.A.; Dominguez-Garcia, P.; Melle, S.; Marquez, M.; Rubio, M.A.; Picraux, S.; Yang, D.; Aella, P.; et al. Magnetic movement of biological fluid droplets. J. Magn. Magn. Mater. 2007, 311, 238–243. [Google Scholar] [CrossRef]
- Chiou, P.; Park, S.Y.; Wu, M.C. Continuous optoelectrowetting for picoliter droplet manipulation. Appl. Phys. Lett. 2008, 93, 221110. [Google Scholar] [CrossRef]
- Gervais, L.; De Rooij, N.; Delamarche, E. Microfluidic chips for point-of-care immunodiagnostics. Adv. Mater. 2011, 23, H151–H176. [Google Scholar] [CrossRef] [PubMed]
- Gubala, V.; Harris, L.F.; Ricco, A.J.; Tan, M.X.; Williams, D.E. Point of care diagnostics: Status and future. Anal. Chem. 2012, 84, 487–515. [Google Scholar] [CrossRef]
- Lynn, N.S.; Dandy, D.S. Passive microfluidic pumping using coupled capillary/evaporation effects. Lab Chip 2009, 9, 3422–3429. [Google Scholar] [CrossRef] [PubMed]
- Dalili, A.; Samiei, E.; Hoorfar, M. A review of sorting, separation and isolation of cells and microbeads for biomedical applications: Microfluidic approaches. Analyst 2019, 144, 87–113. [Google Scholar] [CrossRef]
- Lenshof, A.; Ahmad-Tajudin, A.; Jarås, K.; Sward-Nilsson, A.M.; Åberg, L.; Marko-Varga, G.; Malm, J.; Lilja, H.; Laurell, T. Acoustic whole blood plasmapheresis chip for prostate specific antigen microarray diagnostics. Anal. Chem. 2009, 81, 6030–6037. [Google Scholar] [CrossRef]
- Dow, P.; Kotz, K.; Gruszka, S.; Holder, J.; Fiering, J. Acoustic separation in plastic microfluidics for rapid detection of bacteria in blood using engineered bacteriophage. Lab Chip 2018, 18, 923–932. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Wang, X.B.; Becker, F.F.; Gascoyne, P.R. Differential analysis of human leukocytes by dielectrophoretic field-flow-fractionation. Biophys. J. 2000, 78, 2680–2689. [Google Scholar] [CrossRef]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Electrochemical detection for paper-based microfluidics. Anal. Chem. 2009, 81, 5821–5826. [Google Scholar] [CrossRef]
- Sanjay, S.T.; Fu, G.; Dou, M.; Xu, F.; Liu, R.; Qi, H.; Li, X. Biomarker detection for disease diagnosis using cost-effective microfluidic platforms. Analyst 2015, 140, 7062–7081. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Yin, Y.; Pan, Y.; Wang, Y.; Song, Y. Nanomaterial-assisted microfluidics for multiplex assays. Microchim. Acta 2022, 189, 139. [Google Scholar] [CrossRef]
- Du, W.; Li, L.; Nichols, K.P.; Ismagilov, R.F. SlipChip. Lab Chip 2009, 9, 2286–2292. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Crooks, R.M. Paper-based SlipPAD for high-throughput chemical sensing. Anal. Chem. 2013, 85, 4263–4267. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Wang, Y.; Magaud, P.; Liu, Y.; Zeng, F.; Yang, J.; Baldas, L.; Song, Y. Nanocatalysis meets microfluidics: A powerful platform for sensitive bioanalysis. TrAC Trends Anal. Chem. 2023, 158, 116887. [Google Scholar] [CrossRef]
- Seetasang, S.; Kaneta, T. Analytical Devices with Instrument-Free Detection Based on Paper Microfluidics. In Advanced Microfluidics Based Point-of-Care Diagnostics; CRC Press: Boca Raton, FL, USA, 2022; Chapter 10; pp. 249–269. [Google Scholar]
- Radhapyari, K.; Aribam, N.G.; Datta, S.; Dutta, S.; Barman, R.; Khan, R. Chromatographic Separation and Visual Detection on Wicking Microfluidics Devices. In Advanced Microfluidics Based Point-of-Care Diagnostics; CRC Press: Boca Raton, FL, USA, 2022; Chapter 14; pp. 339–364. [Google Scholar]
- Li, H.; Steckl, A.J. Paper microfluidics for point-of-care blood-based analysis and diagnostics. Anal. Chem. 2018, 91, 352–371. [Google Scholar] [CrossRef] [PubMed]
- Lepowsky, E.; Ghaderinezhad, F.; Knowlton, S.; Tasoglu, S. Paper-based assays for urine analysis. Biomicrofluidics 2017, 11, 051501. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Piety, N.Z.; Vignes, S.M.; Benton, M.S.; Kanter, J.; Shevkoplyas, S.S. Simple paper-based test for measuring blood hemoglobin concentration in resource-limited settings. Clin. Chem. 2013, 59, 1506–1513. [Google Scholar] [CrossRef]
- Nie, Z.; Deiss, F.; Liu, X.; Akbulut, O.; Whitesides, G.M. Integration of paper-based microfluidic devices with commercial electrochemical readers. Lab Chip 2010, 10, 3163–3169. [Google Scholar] [CrossRef]
- Yu, J.; Ge, L.; Huang, J.; Wang, S.; Ge, S. Microfluidic paper-based chemiluminescence biosensor for simultaneous determination of glucose and uric acid. Lab Chip 2011, 11, 1286–1291. [Google Scholar] [CrossRef]
- Delaney, J.L.; Hogan, C.F.; Tian, J.; Shen, W. Electrogenerated chemiluminescence detection in paper-based microfluidic sensors. Anal. Chem. 2011, 83, 1300–1306. [Google Scholar] [CrossRef]
- Rankin-Turner, S.; Turner, M.A.; Kelly, P.F.; King, R.S.P.; Reynolds, J.C. Transforming presumptive forensic testing: In situ identification and age estimation of human bodily fluids. Chem. Sci. 2019, 10, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Mozayani, A.; Noziglia, C. The Forensic Laboratory Handbook Procedures and Practice; Springer Science & Business Media: Berlin, Germany, 2011. [Google Scholar]
- Harbison, S.; Fleming, R. Forensic body fluid identification: State of the art. Res. Rep. Forensic Med. Sci. 2016, 6, 11–23. [Google Scholar] [CrossRef]
- Vidaki, A.; Kayser, M. From forensic epigenetics to forensic epigenomics: Broadening DNA investigative intelligence. Genome Biol. 2017, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cromartie, R.L.; Wardlow, A.; Duncan, G.; McCord, B.R. Development of a microfluidic device (μPADs) for forensic serological analysis. Anal. Methods 2019, 11, 587–595. [Google Scholar] [CrossRef]
- Ansari, N.; Trambadiya, N.; Lodha, A.; Menon, S. A portable microfluidic paper-based analytical device for blood detection and typing assay. Aust. J. Forensic Sci. 2021, 53, 407–418. [Google Scholar] [CrossRef]
- Quinn, A.A.; Elkins, K.M. The differentiation of menstrual from venous blood and other body fluids on various substrates using ATR FT-IR spectroscopy. J. Forensic Sci. 2017, 62, 197–204. [Google Scholar] [CrossRef]
- Layne, T.R.; Nouwairi, R.L.; Fleming, R.; Blair, H.; Landers, J.P. Rapid Microchip Electrophoretic Separation of Novel Transcriptomic Body Fluid Markers for Forensic Fluid Profiling. Micromachines 2022, 13, 1657. [Google Scholar] [CrossRef]
- Roeder, A.D.; Haas, C. Body Fluid Identification Using mRNA Profiling. In Forensic DNA Typing Protocols; Goodwin, W., Ed.; Springer: New York, NY, USA, 2016; pp. 13–31. [Google Scholar]
- O’Leary, K.R.; Glynn, C.L. Investigating the isolation and amplification of microRNAs for forensic body fluid identification. MicroRNA 2018, 7, 187–194. [Google Scholar] [CrossRef]
- Mayes, C.; Seashols-Williams, S.; Hughes-Stamm, S. A capillary electrophoresis method for identifying forensically relevant body fluids using miRNAs. Leg. Med. 2018, 30, 1–4. [Google Scholar] [CrossRef]
- Liu, B.; Song, F.; Yang, Q.; Zhou, Y.; Shao, C.; Shen, Y.; Zhao, Z.; Tang, Q.; Hou, Y.; Xie, J. Characterization of tissue-specific biomarkers with the expression of circRNAs in forensically relevant body fluids. Int. J. Leg. Med. 2019, 133, 1321–1331. [Google Scholar] [CrossRef]
- Song, F.; Luo, H.; Xie, M.; Zhu, H.; Hou, Y. Microarray expression profile of circular RNAs in human body fluids. Forensic Sci. Int. Genet. Suppl. Ser. 2017, 6, e55–e56. [Google Scholar] [CrossRef]
- Albani, P.P.; Fleming, R. Novel messenger RNAs for body fluid identification. Sci. Justice 2018, 58, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.L.; Ouyang, Y.; Duarte, G.R.; Carrilho, E.; Krauss, S.T.; Landers, J.P. Inexpensive, rapid prototyping of microfluidic devices using overhead transparencies and a laser print, cut and laminate fabrication method. Nat. Protoc. 2015, 10, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.M. Advanced Topics in Forensic DNA Typing: Methodology; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- U.S.D. of Justice Federal Bureau of Investigation Science; Branch, T. Guide to All Things Rapid DNA. 2022. Available online: http://www.lsp.org/pdf/FBI_Guide_to_All_Things_Rapid_DNA_01_27_2022.pdf (accessed on 12 May 2023).
- Horsman, K.M.; Bienvenue, J.M.; Blasier, K.R.; Landers, J.P. Forensic DNA analysis on microfluidic devices: A review. J. Forensic Sci. 2007, 52, 784–799. [Google Scholar] [CrossRef]
- Bruijns, B.; Knotter, J.; Tiggelaar, R. A Systematic Review on Commercially Available Integrated Systems for Forensic DNA Analysis. Sensors 2023, 23, 1075. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Johnson, M.; Hill, P.; Gale, B.K. Microfluidic sample preparation: Cell lysis and nucleic acid purification. Integr. Biol. 2009, 1, 574–586. [Google Scholar] [CrossRef]
- Di Carlo, D.; Jeong, K.H.; Lee, L.P. Reagentless mechanical cell lysis by nanoscale barbs in microchannels for sample preparation. Lab Chip 2003, 3, 287–291. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, J.H.; Lim, H.K.; Cho, E.C.; Huh, N.; Ko, C.; Park, J.C.; Choi, J.W.; Lee, S.S. Electrochemical cell lysis device for DNA extraction. Lab Chip 2010, 10, 626–633. [Google Scholar] [CrossRef]
- Gac, S.L.; van den Berg, A. Cell Capture and Lysis on a Chip. In Unravelling Single Cell Genomics; The Royal Society of Chemistry: London, UK, 2010; Chapter 12; pp. 150–184. [Google Scholar]
- Kutter, J.P.; Jacobson, S.C.; Ramsey, J.M. Solid phase extraction on microfluidic devices. J. Microcolumn Sep. 2000, 12, 93–97. [Google Scholar] [CrossRef]
- Price, C.W.; Leslie, D.C.; Landers, J.P. Nucleic acid extraction techniques and application to the microchip. Lab Chip 2009, 9, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Reinholt, S.J.; Baeumner, A.J. Microfluidic isolation of nucleic acids. Angew. Chem. Int. Ed. 2014, 53, 13988–14001. [Google Scholar] [CrossRef]
- Tian, H.; Hühmer, A.F.; Landers, J.P. Evaluation of silica resins for direct and efficient extraction of DNA from complex biological matrices in a miniaturized format. Anal. Biochem. 2000, 283, 175–191. [Google Scholar] [CrossRef]
- Duarte, G.R.; Price, C.W.; Augustine, B.H.; Carrilho, E.; Landers, J.P. Dynamic solid phase DNA extraction and PCR amplification in polyester-toner based microchip. Anal. Chem. 2011, 83, 5182–5189. [Google Scholar] [CrossRef]
- Chong, K.W.Y.; Thong, Z.; Syn, C.K.C. Recent trends and developments in forensic DNA extraction. Wiley Interdiscip. Rev. Forensic Sci. 2021, 3, e1395. [Google Scholar] [CrossRef]
- Clark, C.; Turiello, R.; Cotton, R.; Landers, J.P. Analytical approaches to differential extraction for sexual assault evidence. Anal. Chim. Acta 2021, 1141, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.V.; Evander, M.; Horsman-Hall, K.M.; Nilsson, J.; Laurell, T.; Landers, J.P. Acoustic differential extraction for forensic analysis of sexual assault evidence. Anal. Chem. 2009, 81, 6089–6095. [Google Scholar] [CrossRef] [PubMed]
- Belgrader, P.I.; Yuan, B. Sonication to Selectively Lyse Different Cell Types. U.S. Patent 7,785,869, 31 August 2010. [Google Scholar]
- Inci, F.; Ozen, M.O.; Saylan, Y.; Miansari, M.; Cimen, D.; Dhara, R.; Chinnasamy, T.; Yuksekkaya, M.; Filippini, C.; Kumar, D.K.; et al. A novel on-Chip method for differential extraction of sperm in forensic cases. Adv. Sci. 2018, 5, 1800121. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.U.; Mello, A.J.d.; Manz, A. Chemical amplification: Continuous-flow PCR on a chip. Science 1998, 280, 1046–1048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ozdemir, P. Microfluidic DNA amplification—A review. Anal. Chim. Acta 2009, 638, 115–125. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.R. A review on continuous-flow microfluidic PCR in droplets: Advances, challenges and future. Anal. Chim. Acta 2016, 914, 7–16. [Google Scholar] [CrossRef]
- Theberge, A.B.; Courtois, F.; Schaerli, Y.; Fischlechner, M.; Abell, C.; Hollfelder, F.; Huck, W.T. Microdroplets in microfluidics: An evolving platform for discoveries in chemistry and biology. Angew. Chem. Int. Ed. 2010, 49, 5846–5868. [Google Scholar] [CrossRef]
- Gu, H.; Duits, M.H.; Mugele, F. Droplets formation and merging in two-phase flow microfluidics. Int. J. Mol. Sci. 2011, 12, 2572–2597. [Google Scholar] [CrossRef]
- Ahrberg, C.D.; Manz, A.; Chung, B.G. Polymerase chain reaction in microfluidic devices. Lab Chip 2016, 16, 3866–3884. [Google Scholar] [CrossRef]
- Estes, M.D.; Yang, J.; Duane, B.; Smith, S.; Brooks, C.; Nordquist, A.; Zenhausern, F. Optimization of multiplexed PCR on an integrated microfluidic forensic platform for rapid DNA analysis. Analyst 2012, 137, 5510–5519. [Google Scholar] [CrossRef]
- DuVall, J.A.; Le Roux, D.; Thompson, B.L.; Birch, C.; Nelson, D.A.; Li, J.; Mills, D.L.; Tsuei, A.C.; Ensenberger, M.G.; Sprecher, C.; et al. Rapid multiplex DNA amplification on an inexpensive microdevice for human identification via short tandem repeat analysis. Anal. Chim. Acta 2017, 980, 41–49. [Google Scholar] [CrossRef]
- Cornelis, S.; Tytgat, O.; Fauvart, M.; Gansemans, Y.; Vander Plaetsen, A.S.; Wiederkehr, R.S.; Deforce, D.; Van Nieuwerburgh, F.; Stakenborg, T. Silicon μPCR chip for forensic STR profiling with hybeacon probe melting curves. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Landers, J.P. Handbook of Capillary and Microchip Electrophoresis and Associated Microtechniques; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Nouwairi, R.L.; O’Connell, K.C.; Gunnoe, L.M.; Landers, J.P. Microchip electrophoresis for fluorescence-based measurement of polynucleic acids: Recent developments. Anal. Chem. 2020, 93, 367–387. [Google Scholar] [CrossRef]
- Sang, F.; Ren, J. Capillary electrophoresis of double-stranded DNA fragments using a new fluorescence intercalating dye EvaGreen. J. Sep. Sci. 2006, 29, 1275–1280. [Google Scholar] [CrossRef]
- Hopwood, A.J.; Hurth, C.; Yang, J.; Cai, Z.; Moran, N.; Lee-Edghill, J.G.; Nordquist, A.; Lenigk, R.; Estes, M.D.; Haley, J.P.; et al. Integrated microfluidic system for rapid forensic DNA analysis: Sample collection to DNA profile. Anal. Chem. 2010, 82, 6991–6999. [Google Scholar] [CrossRef]
- Bruijns, B.; Tiggelaar, R.; Gardeniers, H. A microfluidic approach for biosensing DNA within forensics. Appl. Sci. 2020, 10, 7067. [Google Scholar] [CrossRef]
- Pehrsson, A.; Blencowe, T.; Vimpari, K.; Langel, K.; Engblom, C.; Lillsunde, P. An evaluation of on-site oral fluid drug screening devices DrugWipe® 5+ and rapid STAT® using oral fluid for confirmation analysis. J. Anal. Toxicol. 2011, 35, 211–218. [Google Scholar] [CrossRef]
- Greenway, G.M.; Nelstrop, L.J.; Port, S.N. Tris (2, 2-bipyridyl) ruthenium (II) chemiluminescence in a microflow injection system for codeine determination. Anal. Chim. Acta 2000, 405, 43–50. [Google Scholar] [CrossRef]
- Ribeiro, M.F.M.; Bento, F.; Ipolito, A.J.; de Oliveira, M.F. Development of a pencil drawn paper-based analytical device to detect Lysergic Acid Diethylamide (LSD). J. Forensic Sci. 2020, 65, 2121–2128. [Google Scholar] [CrossRef]
- Musile, G.; Wang, L.; Bottoms, J.; Tagliaro, F.; McCord, B. The development of paper microfluidic devices for presumptive drug detection. Anal. Methods 2015, 7, 8025–8033. [Google Scholar] [CrossRef]
- Krauss, S.T.; Remcho, T.P.; Lipes, S.M.; Aranda, R., IV; Maynard, H.P., III; Shukla, N.; Li, J.; Tontarski, R.E., Jr.; Landers, J.P. Objective method for presumptive field-testing of illicit drug possession using centrifugal microdevices and smartphone analysis. Anal. Chem. 2016, 88, 8689–8697. [Google Scholar] [CrossRef]
- Bruijns, B.; Veciana, A.; Tiggelaar, R.; Gardeniers, H. Cyclic olefin copolymer microfluidic devices for forensic applications. Biosensors 2019, 9, 85. [Google Scholar] [CrossRef]
- Lockwood, T.L.E.; Leong, T.X.; Bliese, S.L.; Helmke, A.; Richard, A.; Merga, G.; Rorabeck, J.; Lieberman, M. idPAD: Paper analytical device for presumptive identification of illicit drugs. J. Forensic Sci. 2020, 65, 1289–1297. [Google Scholar] [CrossRef]
- Hermann, T.; Patel, D.J. Adaptive recognition by nucleic acid aptamers. Science 2000, 287, 820–825. [Google Scholar] [CrossRef]
- Stojanovic, M.N.; de Prada, P.; Landry, D.W. Fluorescent sensors based on aptamer self-assembly. J. Am. Chem. Soc. 2000, 122, 11547–11548. [Google Scholar] [CrossRef]
- Stojanovic, M.N.; De Prada, P.; Landry, D.W. Aptamer-based folding fluorescent sensor for cocaine. J. Am. Chem. Soc. 2001, 123, 4928–4931. [Google Scholar] [CrossRef]
- Baker, B.R.; Lai, R.Y.; Wood, M.S.; Doctor, E.H.; Heeger, A.J.; Plaxco, K.W. An electronic, aptamer-based small-molecule sensor for the rapid, label-free detection of cocaine in adulterated samples and biological fluids. J. Am. Chem. Soc. 2006, 128, 3138–3139. [Google Scholar] [CrossRef] [PubMed]
- Swensen, J.S.; Xiao, Y.; Ferguson, B.S.; Lubin, A.A.; Lai, R.Y.; Heeger, A.J.; Plaxco, K.W.; Soh, H.T. Continuous, real-time monitoring of cocaine in undiluted blood serum via a microfluidic, electrochemical aptamer-based sensor. J. Am. Chem. Soc. 2009, 131, 4262–4266. [Google Scholar] [CrossRef]
- Wang, L.; Musile, G.; McCord, B.R. An aptamer-based paper microfluidic device for the colorimetric determination of cocaine. Electrophoresis 2018, 39, 470–475. [Google Scholar] [CrossRef]
- Kawano, R.; Osaki, T.; Sasaki, H.; Takinoue, M.; Yoshizawa, S.; Takeuchi, S. Rapid detection of a cocaine-binding aptamer using biological nanopores on a chip. J. Am. Chem. Soc. 2011, 133, 8474–8477. [Google Scholar] [CrossRef]
- Mobini Far, H.R.; Torabi, F.; Danielsson, B.; Khayyami, M. ELISA on a microchip with a photodiode for detection of amphetamine in plasma and urine. J. Anal. Toxicol. 2005, 29, 790–793. [Google Scholar]
- Miyaguchi, H.; Takahashi, H.; Ohashi, T.; Mawatari, K.; Iwata, Y.T.; Inoue, H.; Kitamori, T. Rapid analysis of methamphetamine in hair by micropulverized extraction and microchip-based competitive ELISA. Forensic Sci. Int. 2009, 184, 1–5. [Google Scholar] [CrossRef]
- Tian, T.; Wei, X.; Jia, S.; Zhang, R.; Li, J.; Zhu, Z.; Zhang, H.; Ma, Y.; Lin, Z.; Yang, C.J. Integration of target responsive hydrogel with cascaded enzymatic reactions and microfluidic paper-based analytic devices (μPADs) for point-of-care testing (POCT). Biosens. Bioelectron. 2016, 77, 537–542. [Google Scholar] [CrossRef]
- Yang, G.; Liu, H. Application of monolithic stationary phases in solid-phase extraction and pharmaceutical analysis. Curr. Pharm. Anal. 2010, 6, 213–224. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Zeng, P.; Cao, Q. A butyl methacrylate monolithic column prepared in situ on a microfluidic chip and its applications. Sensors 2009, 9, 3437–3446. [Google Scholar] [CrossRef]
- Du, Y.; Wang, E. Separation and detection of narcotic drugs on a microchip using micellar electrokinetic chromatography and electrochemiluminescence. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2008, 20, 643–647. [Google Scholar] [CrossRef]
- Qiang, W.; Zhai, C.; Lei, J.; Song, C.; Zhang, D.; Sheng, J.; Ju, H. Disposable microfluidic device with ultraviolet detection for highly resolved screening of illicit drugs. Analyst 2009, 134, 1834–1839. [Google Scholar] [CrossRef]
- Bai, H.Y.; Lin, S.L.; Chan, S.A.; Fuh, M.R. Characterization and evaluation of two-dimensional microfluidic chip-HPLC coupled to tandem mass spectrometry for quantitative analysis of 7-aminoflunitrazepam in human urine. Analyst 2010, 135, 2737–2742. [Google Scholar] [CrossRef]
- United States Department of State Publication Bureau of Counterterrorism; Extremism, C.V. Country Reports on Terrorism 2015. 2016. Available online: https://2009-2017.state.gov/documents/organization/258249.pdf (accessed on 12 May 2023).
- Verpoorte, E. Microfluidic chips for clinical and forensic analysis. Electrophoresis 2002, 23, 677–712. [Google Scholar] [CrossRef]
- Wang, J.; Polsky, R.; Tian, B.; Chatrathi, M.P. Voltammetry on microfluidic chip platforms. Anal. Chem. 2000, 72, 5285–5289. [Google Scholar] [CrossRef] [PubMed]
- Hilmi, A.; Luong, J.H. Electrochemical detectors prepared by electroless deposition for microfabricated electrophoresis chips. Anal. Chem. 2000, 72, 4677–4682. [Google Scholar] [CrossRef]
- Wang, J.; Tian, B.; Sahlin, E. Micromachined electrophoresis chips with thick-film electrochemical detectors. Anal. Chem. 1999, 71, 5436–5440. [Google Scholar] [CrossRef]
- Peters, K.L.; Corbin, I.; Kaufman, L.M.; Zreibe, K.; Blanes, L.; McCord, B.R. Simultaneous colorimetric detection of improvised explosive compounds using microfluidic paper-based analytical devices (μPADs). Anal. Methods 2015, 7, 63–70. [Google Scholar] [CrossRef]
- Pesenti, A.; Taudte, R.V.; McCord, B.; Doble, P.; Roux, C.; Blanes, L. Coupling paper-based microfluidics and lab on a chip technologies for confirmatory analysis of trinitro aromatic explosives. Anal. Chem. 2014, 86, 4707–4714. [Google Scholar] [CrossRef] [PubMed]
- Salles, M.O.; Meloni, G.N.; De Araujo, W.; Paixão, T.R.L.C.d. Explosive colorimetric discrimination using a smartphone, paper device and chemometrical approach. Anal. Methods 2014, 6, 2047–2052. [Google Scholar] [CrossRef]
- Chabaud, K.R.; Thomas, J.L.; Torres, M.N.; Oliveira, S.; McCord, B.R. Simultaneous colorimetric detection of metallic salts contained in low explosives residue using a microfluidic paper-based analytical device (μPAD). Forensic Chem. 2018, 9, 35–41. [Google Scholar] [CrossRef]
- Boyd-Moss, M.; Baratchi, S.; Di Venere, M.; Khoshmanesh, K. Self-contained microfluidic systems: A review. Lab Chip 2016, 16, 3177–3192. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).