Combination of Clinical and Gait Measures to Classify Fallers and Non-Fallers in Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. History of Falls and Classification
2.3. Clinical and Gait Assessments
2.4. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Clinical Measures
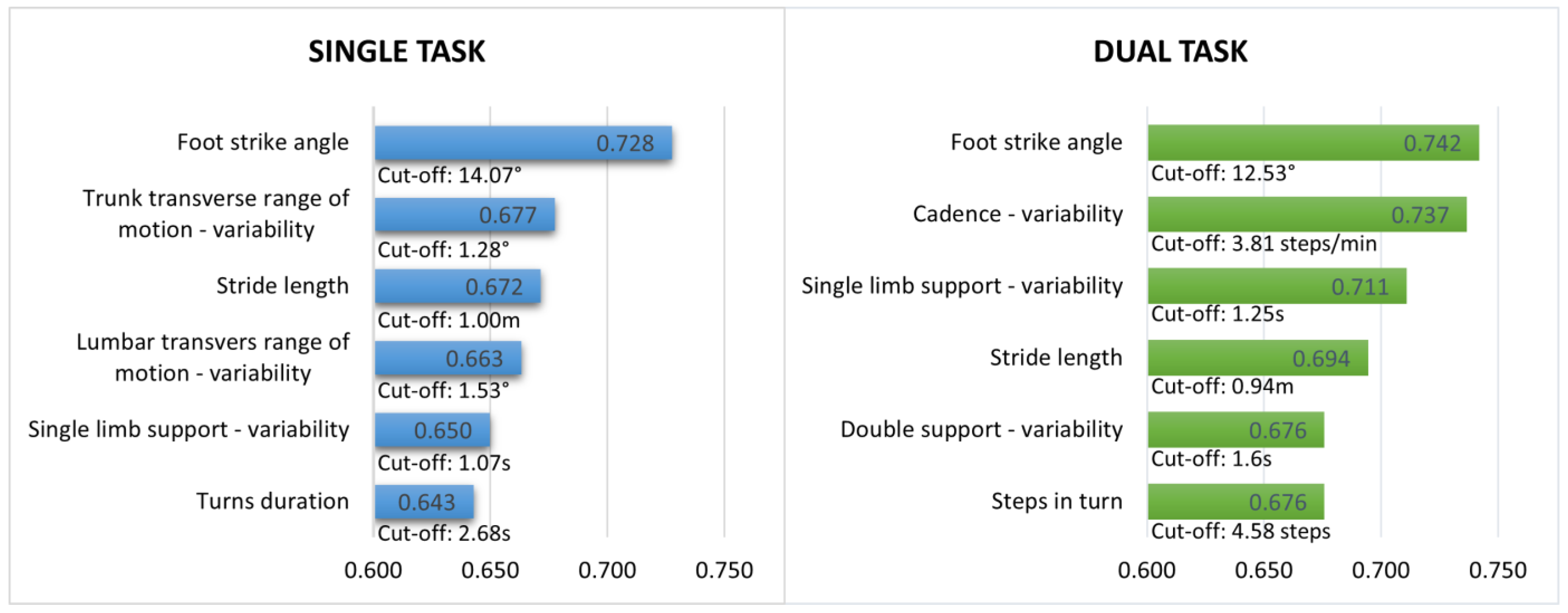
3.3. Gait Measures during Single and Dual-Task Walking
3.4. Combination of Clinical and Gait Measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, N.E.; Canning, C.G.; Almeida, L.R.S.; Bloem, B.R.; Keus, S.H.; Löfgren, N.; Nieuwboer, A.; Verheyden, G.S.; Yamato, T.P.; Sherrington, C. Interventions for preventing falls in Parkinson’s disease. Cochrane Database Syst. Rev. 2022, 6, 12–38. [Google Scholar] [CrossRef]
- Paul, S.S.; Dibble, L.E.; Peterson, D.S. Motor learning in people with Parkinson’s disease: Implications for fall prevention across the disease spectrum. Gait Posture 2018, 61, 311–319. [Google Scholar] [CrossRef]
- Wilczyński, J.; Ścipniak, M.; Ścipniak, K.; Margiel, K.; Wilczyński, I.; Zieliński, R.; Sobolewski, P. Assessment of Risk Factors for Falls among Patients with Parkinson’s Disease. BioMed Res. Int. 2021, 2021, 5531331. [Google Scholar] [CrossRef]
- Ashburn, A.; Pickering, R.; McIntosh, E.; Hulbert, S.; Rochester, L.; Roberts, H.C.; Nieuwboer, A.; Kunkel, D.; Goodwin, V.A.; Lamb, S.E.; et al. Exercise- and strategy-based physiotherapy-delivered intervention for preventing repeat falls in people with Parkinson’s: The PDSAFE RCT. Health Technol. Assess. 2019, 23, 1–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tung, T.; Zhang, C.; Shi, L. Systematic review for the prevention and management of falls and fear of falling in patients with Parkinson’s disease. Brain Behav. 2022, 12, e2690. [Google Scholar] [CrossRef]
- Vitorio, R.; Mancini, M.; Carlson-Kuhta, P.; Horak, F.B.; Shah, V.V. Should we use both clinical and mobility measures to identify fallers in Parkinson’s disease? Park. Relat. Disord. 2023, 106, 105235. [Google Scholar] [CrossRef]
- Owen, C.L.; Ibrahim, K.; Dennison, L.; Roberts, H.C. Falls Self-Management Interventions for People with Parkinson’s Disease: A Systematic Review. J. Park. Dis. 2019, 9, 283–299. [Google Scholar] [CrossRef]
- Lima, D.P.; De-Almeida, S.B.; Bonfadini, J.D.C.; Carneiro, A.H.S.; de Luna, J.R.G.; de Alencar, M.S.; Viana-Júnior, A.B.; Rodrigues, P.G.B.; Pereira, I.D.S.; Roriz-Filho, J.D.S.; et al. Falls in Parkinson’s disease: The impact of disease progression, treatment, and motor complications. Dement. Neuropsychol. 2022, 16, 153–161. [Google Scholar] [CrossRef]
- Miri, A.; Araújo, H.; Gil, A.; de Oliveira, M.; Volpe, R.; Angelo, E.; Smaili, S.M. Analysis of handgrip strength, pulling force using the upper limbs, and ground reaction forces in the task of boarding a bus between healthy elderly individuals and those with Parkinson’s disease. Physiother. Theory Pract. 2022, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Canning, C.G.; Hausdorff, J.M.; Lord, S.; Rochester, L. Falls in Parkinson’s disease: A complex and evolving picture. Mov. Disord. 2017, 32, 1524–1536. [Google Scholar] [CrossRef]
- Allen, N.E.; Schwarzel, A.K.; Canning, C.G. Recurrent Falls in Parkinson’s Disease: A Systematic Review. Park. Dis. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Allen, N.E.; Canning, C.G.; Fung, V.S.C. Postural Instability in Patients with Parkinson’s Disease. CNS Drugs 2013, 27, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Da Conceição, N.R.; de Sousa, P.N.; Pereira, M.P.; Gobbi, L.; Vitório, R. Utility of center of pressure measures during obstacle crossing in prediction of fall risk in people with Parkinson’s disease. Hum. Mov. Sci. 2019, 66, 1–8. [Google Scholar] [CrossRef]
- Delval, A.; Betrouni, N.; Tard, C.; Devos, D.; Dujardin, K.; Defebvre, L.; Labidi, J.; Moreau, C. Do kinematic gait parameters help to discriminate between fallers and non-fallers with Parkinson’s disease? Clin. Neurophysiol. 2021, 132, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Dalrymple-Alford, J.C.; MacAskill, M.R.; Nakas, C.T.; Livingston, L.; Graham, C.; Crucian, G.P.; Melzer, T.R.; Kirwan, J.; Keenan, R.; Wells, S.; et al. The MoCA: Well-suited screen for cognitive impairment in Parkinson disease. Neurology 2010, 75, 1717–1725. [Google Scholar] [CrossRef]
- Royall, D.R.; A Cordes, J.; Polk, M. CLOX: An executive clock drawing task. J. Neurol. Neurosurg. Psychiatry 1998, 64, 588–594. [Google Scholar] [CrossRef]
- Sánchez-Cubillo, I.; Periáñez, J.A.; Adrover-Roig, D.; Rodríguez-Sánchez, J.M.; Ríos-Lago, M.; Tirapu, J.; Barceló, F. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J. Int. Neuropsychol. Soc. 2009, 15, 438–450. [Google Scholar] [CrossRef]
- Calamia, M.; Markon, K.; Denburg, N.L.; Tranel, D. Developing a Short Form of Benton’s Judgment of Line Orientation Test: An Item Response Theory Approach. Clin. Neuropsychol. 2011, 25, 670–684. [Google Scholar] [CrossRef]
- Yardley, L.; Beyer, N.; Hauer, K.; Kempen, G.; Piot-Ziegler, C.; Todd, C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005, 34, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, N.; Shah, V.V.; Carlson-Kuhta, P.; Nutt, J.G.; Horak, F.B.; Mancini, M. How to Select Balance Measures Sensitive to Parkinson’s Disease from Body-Worn Inertial Sensors—Separating the Trees from the Forest. Sensors 2019, 19, 3320. [Google Scholar] [CrossRef]
- Shah, V.V.; McNames, J.; Mancini, M.; Carlson-Kuhta, P.; Spain, R.I.; Nutt, J.G.; El-Gohary, M.; Curtze, C.; Horak, F.B. Quantity and quality of gait and turning in people with multiple sclerosis, Parkinson’s disease and matched controls during daily living. J. Neurol. 2020, 267, 1188–1196. [Google Scholar] [CrossRef]
- Mancini, M.; King, L.; Salarian, A.; Holmstrom, L.; McNames, J.; Horak, F.B. Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors. J. Bioeng. Biomed. Sci. 2013, 1–5. [Google Scholar] [CrossRef]
- Morris, R.; Stuart, S.; McBarron, G.; Fino, P.C.; Mancini, M.; Curtze, C. Validity of Mobility Lab (version 2) for gait assessment in young adults, older adults and Parkinson’s disease. Physiol. Meas. 2019, 40, 095003. [Google Scholar] [CrossRef] [PubMed]
- Washabaugh, E.P.; Kalyanaraman, T.; Adamczyk, P.G.; Claflin, E.S.; Krishnan, C. Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 2017, 55, 87–93. [Google Scholar] [CrossRef]
- Mancini, M.; Horak, F.B. Potential of APDM mobility lab for the monitoring of the progression of Parkinson’s disease. Expert Rev. Med. Devices 2017, 13, 455–462. [Google Scholar] [CrossRef]
- El-Gohary, M.; Pearson, S.; McNames, J.; Mancini, M.; Horak, F.; Mellone, S.; Chiari, L. Continuous Monitoring of Turning in Patients with Movement Disability. Sensors 2014, 14, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Perkins, N.J.; Schisterman, E.F. The Inconsistency of “Optimal” Cut-Points Using Two ROC Based Criteria. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef]
- Morrison, S.; Moxey, J.; Reilly, N.; Russell, D.M.; Thomas, K.M.; Grunsfeld, A.A. The relation between falls risk and movement variability in Parkinson’s disease. Exp. Brain Res. 2021, 239, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Venhovens, J.; Meulstee, J.; Bloem, B.R.; Verhagen, W.I.M. Neurovestibular Dysfunction and Falls in Parkinson’s Disease and Atypical Parkinsonism: A Prospective 1 Year Follow-Up Study. Front. Neurol. 2020, 11, 580285. [Google Scholar] [CrossRef]
- Kerr, G.K.; Worringham, C.J.; Cole, M.H.; Lacherez, P.F.; Wood, J.M.; Silburn, P.A. Predictors of future falls in Parkinson disease. Neurology 2010, 75, 116–124. [Google Scholar] [CrossRef]
- Almeida, L.R.; Valenca, G.T.; Negreiros, N.N.; Pinto, E.B.; Oliveira-Filho, J. Comparison of Self-report and Performance-Based Balance Measures for Predicting Recurrent Falls in People with Parkinson Disease: Cohort Study. Phys. Ther. 2016, 96, 1074–1084. [Google Scholar] [CrossRef]
- Wang, C.; Patriquin, M.; Vaziri, A.; Najafi, B. Mobility Performance in Community-Dwelling Older Adults: Potential Digital Biomarkers of Concern about Falling. Gerontology 2021, 67, 365–373. [Google Scholar] [CrossRef]
- Atrsaei, A.; Hansen, C.; Elshehabi, M.; Solbrig, S.; Berg, D.; Liepelt-Scarfone, I.; Maetzler, W.; Aminian, K. Effect of Fear of Falling on Mobility Measured During Lab and Daily Activity Assessments in Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 793. [Google Scholar] [CrossRef] [PubMed]
- Vitorio, R.; Hasegawa, N.; Carlson-Kuhta, P.; Nutt, J.G.; Horak, F.B.; Mancini, M.; Shah, V.V. Dual-Task Costs of Quantitative Gait Parameters While Walking and Turning in People with Parkinson’s Disease: Beyond Gait Speed. J. Park. Dis. 2021, 11, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Heinzel, S.; Maechtel, M.; Hasmann, S.E.; Hobert, M.A.; Heger, T.; Berg, D.; Maetzler, W. Motor dual-tasking deficits predict falls in Parkinson’s disease: A prospective study. Park. Relat. Disord. 2016, 26, 73–77. [Google Scholar] [CrossRef]
- Vance, R.C.; Healy, D.G.; Galvin, R.; French, H.P. Dual Tasking with the Timed “Up & Go” Test Improves Detection of Risk of Falls in People with Parkinson Disease. Phys. Ther. 2015, 95, 95–102. [Google Scholar] [CrossRef]
- Maranesi, E.; Casoni, E.; Baldoni, R.; Barboni, I.; Rinaldi, N.; Tramontana, B.; Amabili, G.; Benadduci, M.; Barbarossa, F.; Luzi, R.; et al. The Effect of Non-Immersive Virtual Reality Exergames versus Traditional Physiotherapy in Parkinson’s Disease Older Patients: Preliminary Results from a Randomized-Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 14818. [Google Scholar] [CrossRef]

| Variables | All Participants (n = 127) | Fallers (n = 31) | Non-Fallers (N = 96) | p-Value |
|---|---|---|---|---|
| Age (years) | 69.65 ± 7.67 | 70.73 ± 7.08 | 68.93 ± 8.00 | 0.06 |
| Height (m) | 1.68 ± 0.01 | 1.69 ± 0.09 | 1.68 ± 0.01 | 0.70 |
| Weight (kg) | 80.72 ± 17.56 | 77. 16 ± 14.54 | 83.08 ± 19.03 | 0.09 |
| Education (years) | 14.60 ± 3.40 | 14.64 ± 3.43 | 14.57 ± 3.41 | 0.79 |
| Disease duration (years) | 6.04 ± 5.23 | 7.18 ± 6.31 | 5.28 ± 4.26 | 0.13 |
| MoCA (score) | 26.98 ± 2.64 | 26.51 ± 2.87 | 27.29 ± 2.46 | 0.08 |
| MDS-UPDRS III (score) | 35.20 ± 16.17 | 39.42 ± 15.04 | 32.43 ± 16.40 | 0.02 * |
| HY (stage) | 2.10 ± 0.65 | 2.27 ± 0.58 | 1.99 ± 0.68 | 0.03 * |
| 1 | 20 (15.9%) | 2 (6.5%) | 18 (18.9%) | - |
| 2 | 72 (57.1%) | 16 (51.6%) | 56 (58.9%) | - |
| 3 | 34 (27.0%) | 13 (41.9%) | 21 (22.1%) | - |
| Measures | AUC | Cutoff | Sensitivity | 1−Specificity | ||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | ||||
| FES-I | NFOGQ | UPDRS-III | HY | TMT B | 0.809 | 2.5 | 0.710 | 0.208 |
| FES-I | NFOGQ | HY | TMT B | 0.809 | 1.5 | 0.806 | 0.344 | |
| NFOGQ | UPDRS-III | HY | TMT B | 0.808 | 1.5 | 0.839 | 0.292 | |
| NFOGQ | HY | TMT B | 0.802 | 1.5 | 0.677 | 0.188 | ||
| FES-I | UPDRS-III | HY | TMT B | 0.791 | 2.5 | 0.613 | 0.156 | |
| Measures | AUC | Cutoff | Sensitivity | 1−Specificity | ||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | ||||
| Single task walking | ||||||||
| Foot Strike Angle | Trunk Transverse ROM SD | Stride Length | Lumbar Transverse ROM SD | Single Limb Support SD | 0.788 | 3.5 | 0.613 | 0.135 |
| Trunk Transverse ROM SD | Stride Length | Lumbar Transverse ROM SD | Single Limb Support SD | 0.787 | 2.5 | 0.613 | 0.177 | |
| Foot Strike Angle | Trunk Transverse ROM SD | Stride Length | Lumbar Transverse ROM SD | 0.784 | 2.5 | 0.742 | 0.219 | |
| Foot Strike Angle | Stride Length | Lumbar Transverse ROM SD | Single Limb Support SD | 0.779 | 2.5 | 0.645 | 0.208 | |
| Trunk Transverse ROM SD | Stride Length | Lumbar Transverse ROM SD | 0.776 | 1.5 | 0.774 | 0.365 | ||
| Dual task walking | ||||||||
| Foot Strike Angle | Cadence SD | Single Limb Support SD | Double Support SD | 0.790 | 2.5 | 0.613 | 0.152 | |
| Foot Strike Angle | Cadence SD | Single Limb Support SD | Stride Length | Double Support SD | 0.787 | 2.5 | 0.742 | 0.293 |
| Foot Strike Angle | Cadence SD | Single Limb Support SD | 0.783 | 1.5 | 0.774 | 0.293 | ||
| Foot Strike Angle | Cadence SD | Single Limb Support SD | Stride Length | 0.780 | 1.5 | 0.806 | 0.359 | |
| Cadence SD | Single Limb Support SD | Stride Length | Double Support SD | 0.779 | 2.5 | 0.677 | 0.185 | |
| Measures | AUC | Cutoff | Sensitivity | 1−Specificity | ||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | ||||
| Single task walking | ||||||||
| FES-I | NFOGQ | Foot Strike Angle | Trunk Transverse ROM SD | 0.850 | 2.5 | 0.710 | 0.146 | |
| FES-I | NFOGQ | Trunk Transverse ROM SD | 0.835 | 1.5 | 0.839 | 0.250 | ||
| NFOGQ | Foot Strike Angle | Trunk Transverse ROM SD | 0.831 | 1.5 | 0.774 | 0.250 | ||
| FES-I | NFOGQ | Foot Strike Angle | UPDRS-III | Trunk Transverse ROM SD | 0.828 | 2.5 | 0.806 | 0.240 |
| FES-I | NFOGQ | Foot Strike Angle | 0.817 | 1.5 | 0.806 | 0.240 | ||
| Dual task walking | ||||||||
| FES-I | Foot Strike Angle | NFOGQ | Cadence SD | Single Limb Support SD | 0.842 | 2.5 | 0.839 | 0.261 |
| FES-I | Foot Strike Angle | NFOGQ | Single Limb Support SD | 0.838 | 2.5 | 0.742 | 0.163 | |
| FES-I | Foot Strike Angle | NFOGQ | Cadence SD | 0.837 | 2.5 | 0.742 | 0.141 | |
| FES-I | NFOGQ | Cadence SD | Single Limb Support SD | 0.836 | 2.5 | 0.742 | 0.174 | |
| FES-I | NFOGQ | Single Limb Support SD | 0.832 | 1.5 | 0.871 | 0.250 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, H.A.G.O.; Smaili, S.M.; Morris, R.; Graham, L.; Das, J.; McDonald, C.; Walker, R.; Stuart, S.; Vitório, R. Combination of Clinical and Gait Measures to Classify Fallers and Non-Fallers in Parkinson’s Disease. Sensors 2023, 23, 4651. https://doi.org/10.3390/s23104651
Araújo HAGO, Smaili SM, Morris R, Graham L, Das J, McDonald C, Walker R, Stuart S, Vitório R. Combination of Clinical and Gait Measures to Classify Fallers and Non-Fallers in Parkinson’s Disease. Sensors. 2023; 23(10):4651. https://doi.org/10.3390/s23104651
Chicago/Turabian StyleAraújo, Hayslenne A. G. O., Suhaila M. Smaili, Rosie Morris, Lisa Graham, Julia Das, Claire McDonald, Richard Walker, Samuel Stuart, and Rodrigo Vitório. 2023. "Combination of Clinical and Gait Measures to Classify Fallers and Non-Fallers in Parkinson’s Disease" Sensors 23, no. 10: 4651. https://doi.org/10.3390/s23104651
APA StyleAraújo, H. A. G. O., Smaili, S. M., Morris, R., Graham, L., Das, J., McDonald, C., Walker, R., Stuart, S., & Vitório, R. (2023). Combination of Clinical and Gait Measures to Classify Fallers and Non-Fallers in Parkinson’s Disease. Sensors, 23(10), 4651. https://doi.org/10.3390/s23104651







