Abstract
Hydrogen (H2) has gradually become a substitute for traditional energy, but its potential danger cannot be ignored. In this study, litchi-like g-C3N4/In2O3 composites were synthesized by a hydrothermal method and used to develop H2 sensors. The morphology characteristics and chemical composition of the samples were characterized to analyze the gas-sensing properties. Meanwhile, a series of sensors were tested to evaluate the gas-sensing performance. Among these sensors, the sensor based on the 3 wt% g-C3N4/In2O3 (the mass ratio of g-C3N4 to In2O3 is 3:100) showeds good response properties to H2, exhibiting fast response/recovery time and excellent selectivity to H2. The improvement in the gas-sensing performance may be related to the special morphology, the oxygen state and the g-C3N4/In2O3 heterojunction. To sum up, a sensor based on 3 wt% g-C3N4/In2O3 exhibits preeminent performance for H2 with high sensitivity, fast response, and excellent selectivity.
1. Introduction
Hydrogen (H2) is a potential clean energy that has rich application prospects in automobile, aerospace, and other fields [1]. However, due to its flammable and explosive characteristics, H2 can very easily cause disaster during utilization and storage [2]. Therefore, an effective survey of H2 has become an important problem to be solved. At present, detection methods of H2 include gas chromatography, mass spectrometry, gas sensors, and so on [3]. In the above methods, gas sensors have become the best choice, with the advantages of low cost, simple preparation, and small size [4]. Furthermore, the type of gas sensor include semiconductor oxide [5], catalytic combustion [6], solid electrolyte [7,8], etc. Among them, a semiconductor oxide sensor is widely used in people’s lives because of the outstanding performance [9]. So far, many semiconductor oxides have been researched as sensing-materials in H2 sensors, such as TiO2, ZnO, SnO2, NiO, and In2O3. Some reported H2 sensors based on different semiconductor oxides are summarized in Table 1.
In2O3 is a common semiconductor with a band gap of 3.6 eV, which is broadly used in gas sensors [10]. Up to now, preparing In2O3 with distinct morphologies has proved to be an available measure to heighten the performance of gas sensors. However, a single In2O3-based sensor has the defects of low response and poor selectivity in practical applications [11]. Many researchers have made great efforts to enhance the gas-sensing performance, such as doping noble metals [12], fabricating heterojunctions [13], and constructing 2D materials or polymer composites [14,15]. Graphitic carbon nitride (g-C3N4) is a typical polymer semiconductor with a band gap of 2.76 eV, which has been used in gas sensors owing to high chemical stability, large surface area, and good catalytic function [16,17]. It is reported that In2O3-based sensors can elevate gas-sensing performance through the g-C3N4 composite. Liu et al. prepared In2O3, and g-C3N4 was compounded by way of MOF, and the response value of the sensor achieved 294 to 100 ppm NOx at RT [18]. Sun et al. synthesized g-C3N4/In2O3 through a calcination annealing process, and the response value of the sensor reached 1405 at 119 °C to 100 ppm formaldehyde [19]. The enhanced performance of the In2O3 sensor can be attributed to the formation of g-C3N4/In2O3 heterojunction different oxygen species content [20].


Table 1.
Gas-sensing properties of H2 sensor made by some semiconductor oxides.
Table 1.
Gas-sensing properties of H2 sensor made by some semiconductor oxides.
| Sensing Materials | Conc. (ppm) | Res. | τres. (s) | τrec. (s) | Ref. |
|---|---|---|---|---|---|
| WO3-TiO2 | 10000 | 5.62 c | 48 | 5 | [21] |
| Ag/ZnO | 300 | 479% b | 175 | 655 | [22] |
| Pd/SnO2 | 1000 | 1.2 a | 214 | 51.5 | [23] |
| Pt@NiO | 5000 | 4.25 c | 91 | 8 | [24] |
| Pd-doped In2O3 | 100 | 3.6 a | 4 | 7 | [25] |
| 3 wt% g-C3N4/In2O3 | 100 | 180% b | 2 | 2.4 | This work |
Conc.: Concentration; Res.: Response; τres.: Response time; τrec.: Recovery time; Ref.: reference; a R = Ra/Rg; b R = (Ra − Rg)/Rg × 100%, c R = Rg/Ra.
In this paper, a litchi-like g-C3N4/In2O3 composite was successfully prepared by the hydrothermal method. Additionally, the morphology and composition of C3N4/In2O3 were characterized by XRD, SEM, TEM, and XPS. Sensors based on different amount of g-C3N4 composite were fabricated to investigate their gas-sensing specifics. Among them, the performance of the sensor based on 3 wt% g-C3N4/In2O3 was significantly improved for H2, giving it the merits of a fast response and excellent selectivity.
2. Experiment
2.1. Synthesis of Peachcore-like Pure In2O3
All chemicals are purchased from Aladdin Reagent and are analytical grade without being further purified for use. In a typical process, 147.5 mg of InCl3·4H2O was dissolved in 15 mL of deionized water, then 15 mL of glycerol was added and stirred until it was homogeneous. Afterwards, 520 mg of Na3C6H5O7·2H2O was added into the above solution and stirred vigorously for 20 min. Finally, 250 μL of NaOH (0.1 M) aqueous solution was added slowly to form a uniform solution. The obtained solution was transferred to an autoclave lined with 50 mL PTFE for a hydrothermal reaction at 190 °C for 16 h. After being cooled to room temperature, the precipitate was centrifuged with deionized water and absolute ethanol for several times. The powder was collected and dried at 80 °C overnight. The dried sample was placed in an Al2O3 boat and calcined in a muffle furnace at 400 °C for 2 h (2 °C/min) to obtain peachcore-like pure In2O3.
2.2. Synthesis of Litchi-like g-C3N4/In2O3
Firstly, 20 g of urea was added into a lidded crucible and heated in a muffle furnace at 550 °C for 2 h (2 °C/min) to prepare g-C3N4. Then, the prepared g-C3N4 was dispersed in 15 mL deionized water and sonicated for 2 h to ensure dispersion. Subsequently, 147.5 mg of InCl3·4H2O and 15 mL of glycerol was added the above solution in turn and stirred until homogeneous. Thereafter, 520 mg of Na3C6H5O7·2H2O was added and stirred vigorously for 20 min. Finally, 250 μL of NaOH (0.1 M) aqueous solution was added to form a uniform solution. The subsequent process was the same as the preparation of pure In2O3. According to the different contents of g-C3N4, 1 wt% g-C3N4/In2O3, 3 wt% g-C3N4/In2O3, and 5 wt% g-C3N4/In2O3 composites were prepared, respectively.
2.3. Material Characterization
The crystal structures of as-samples were determined by an X-ray diffractometer (XRD, Rigaku Miniflex 600 X, Cu Kα1 radiation, λ = 1.5406 Å) operated at 40 kV and 15 mA. The morphology characteristics of samples were characterized by a scanning electron microscope (SEM, PHENOM SCIENTIFIC ProX G5, The Netherlands) and a transmission electron microscope (TEM, FEI Tecnai G2 F30, USA). X-ray photoelectron spectroscopy (XPS, Thermo escalab 250Xi, USA) was used for the chemical composition analysis of samples.
2.4. Fabrication and Measurement of Gas Sensors
The device structure of the gas sensor is shown in Figure 1. As-prepared samples were mixed with deionized water to form a uniform slurry and coated on an alumina ceramic tube as the sensing layer. The coated sensing layer was baked under an infrared lamp for 15 min. Then, the ceramic tube was calcined at 300 °C for 1 h (2 °C/min). Finally, the heating wire was passed through the ceramic tube and welded to a six-legged base to make a gas sensor. In addition, the components of the test gas are the target gas and the component gas, wherein the standard value of the target gas is 1% mol/mol and that of the component gas is nitrogen.
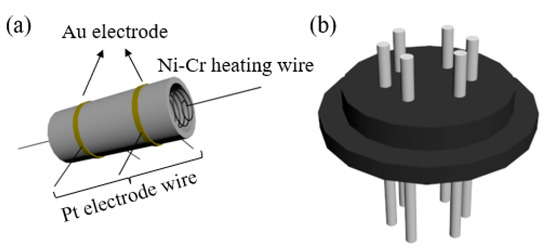
Figure 1.
Sensor device structure (a) ceramic tube; (b) tube base.
The evaluation of gas-sensing performances was carried out in a static test system (50% RH, 25 °C), as shown in Figure 2. The heating current of the sensor was provided by the DC-regulated power supply, and the resistance value was recorded by the multimeter. The response value is defined as R = (Ra − Rg)/Rg × 100%, wherein Ra and Rg are the resistance value of the sensor respectively exposed to clean air and target gas. Additionally, the response/recovery time is defined as the time for 90% of the resistance change.
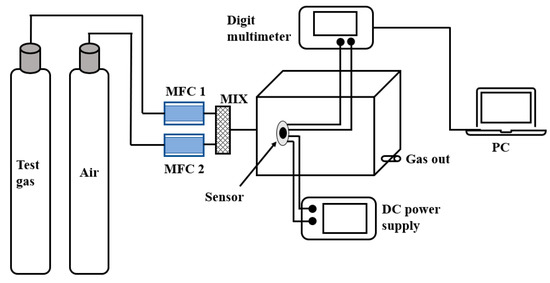
Figure 2.
The gas-sensing test system.
3. Results and Discussion
3.1. Characterization of Material Structure
The crystal phase of the as-prepared samples was obtained by XRD, as shown in Figure 3. The g-C3N4 has two peaks at 2θ angles of 13.36° and 27.38°, which are related to the tris-triazine units and aromatic systems, respectively [26]. The diffraction peaks of pure In2O3 at 2θ angles of 22.37°, 30.99°, 32.61°, 45.61°, 50.25°, 57.20° and 58.19° are index to the crystal planes (012), (104), (110), (024), (116), (214) and (300) of In2O3 (JCPDS 22-0366). However, the diffraction peaks of g-C3N4 are not obviously observed in the XRD pictures of g-C3N4/In2O3 composites, probably due to the low content of g-C3N4 [27].
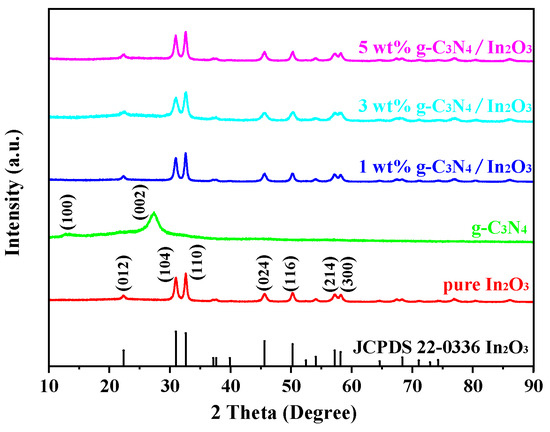
Figure 3.
XRD patterns of as-prepared samples.
The SEM images of pure In2O3 and 3 wt% g-C3N4/In2O3 are shown in Figure 4. Pure In2O3 exhibits a peachcore-like structure with a diameter of about 250 nm in Figure 4a,b. The morphology of 3 wt% g-C3N4/In2O3 possesses a distinctive litchi-like structure, and the diameter of 3 wt% g-C3N4/In2O3 is only 130 nm, as shown in Figure 4c,d. Apparently, the morphology of In2O3 was changed from peachcore-like to litchi-like, and the diameter of 3 wt% g-C3N4/In2O3 is smaller about 100 nm than that of pure In2O3. Moreover, the size of 3 wt% g-C3N4/In2O3 is more uniform than that of pure In2O3. The crystallite sizes of as-prepared samples were calculated by the Scherrer formula:
where K is Scherrer constant of 0.9, is the X-ray wavelength of 0.15406 nm, is the half-width of the diffraction peak, and is the Bragg diffraction angle. The average size of pure In2O3, 1 wt% g-C3N4/In2O3, 3 wt% g-C3N4/In2O3, and 3 wt% g-C3N4/In2O3 is about 15 nm, 14.4 nm, 13.9 nm and 12.6 nm, respectively. Based on the above results, it is inferred that the g-C3N4 may inhibit the growth of In2O3 crystal, thus leading to morphological changes [28].
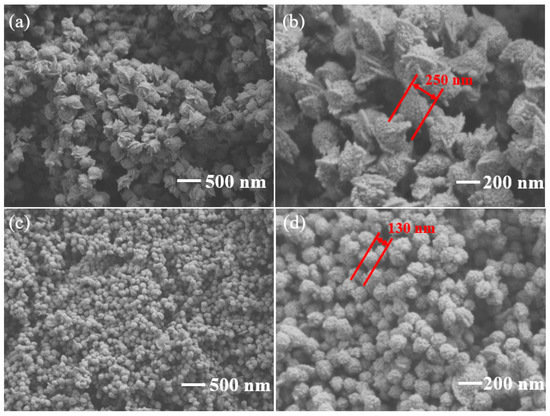
Figure 4.
SEM images of (a,b) pure In2O3; (c,d) 3 wt% g-C3N4/In2O3.
The microstructure of 3 wt% g-C3N4/In2O3 was characterized by TEM and HRTEM, as shown in Figure 5. Through TEM in Figure 5a, we can clearly see that the 3 wt% g-C3N4/In2O3 presents a litchi-like structure with uniform size. Furthermore, HRTEM in Figure 5b is manifested by the different lattice spacings of In2O3, which plainly indicates In2O3 with a highly crystalline form. The three lattice spacings are 0.12 nm, 0.18 nm and 0.17 nm, which are assigned to the (012), (110) and (104) planes of In2O3, respectively. In addition, the element mapping is shown in Figure 5c–h. From element mapping, it can be explicitly noticed that the In, O, C, and N elements are evenly distributed; this can be evidence that the 3 wt% g-C3N4/In2O3 composite was successfully prepared.
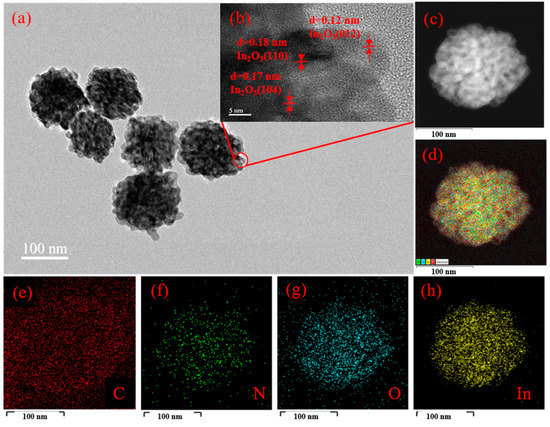
Figure 5.
3 wt% g-C3N4/In2O3 (a) TEM image; (b) HRTEM; (c–h) elemental mapping.
To analyze the chemical composition of pure In2O3 and 3 wt% g-C3N4/In2O3, the XPS is shown in Figure 6. As shown in Figure 6a, the In 3d of pure In2O3 and 3 wt% g-C3N4/In2O3 have two strong peaks at 451.6 eV, 444.1 eV and 451.7 eV, 444.2 eV, which correspond to In 3d5/2 and In 3d3/2, respectively [29]. The O 1s peak spectrums in Figure 6b are decomposed into three fitting peaks around 532.1 eV, 531.2 eV, 529.5 eV and 532.2 eV, 531.2 eV, 529.6 eV, wherein the fitting peaks are assigned to the hydroxyl (OH) or chemisorbed oxygen (OC), oxygen vacancy (OV), and lattice oxygen (OL) [30,31]. Furthermore, the OC, OV, and OL content of pure In2O3 and 3 wt% g-C3N4/In2O3 are about 8.56%, 23.59%, 67.85% and 9.14%, 33.42%, 57.44%, respectively. It is worth noting that the content of OC and OV increases with the introduction of g-C3N4. It may be one of the reasons why the gas-sensing performance of 3wt% g-C3N4/In2O3-based sensors has improved [32,33]. The C 1s spectrum displays three peaks at 288.3 eV, 286.3 eV, and 284.8 eV, as shown in Figure 6c. These three peaks of C 1s respectively belong to the sp2- bonded carbon (N–C=N) and the sp3- fitted carbon bond from surface defects of g-C3N4 and carbon atoms (C-C) [34]. However, the N 1s peak appears to be a weak peak at 398.8eV, as shown in Figure 6d, which may be caused by the low content of g-C3N4 [35].
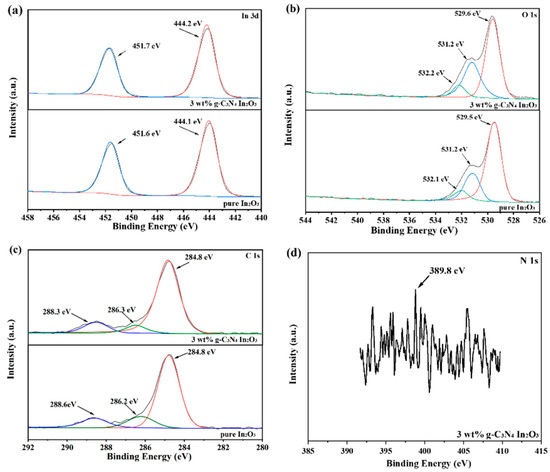
Figure 6.
XPS spectra of as-prepared samples (a) In 3d; (b) O 1s; (c) C 1s; (d) N 1s.
3.2. Gas-Sensing Properties
To determine the optimal operating temperature of the sensors, the different sensors based on g-C3N4/In2O3 composites with different proportions were evaluated from 225 °C to 300 °C, as shown in Figure 7. When the operating temperature reaches 275 °C, the response value is 180% to 100 ppm H2 of 3 wt% g-C3N4/In2O3 sensor in Figure 7a, which is 3.5 times that of pure In2O3 sensor. The operating temperature can be explained according to the desorption equilibrium of the gas molecules and the chemical reaction kinetics. When the working temperature is too low, the gas molecules do not have enough heat energy and kinetic energy to react on the In2O3 surface, so the adsorption capacity of the gas is reduced. However, when the operating temperature is too high, the gas molecules adsorbed on the In2O3 surface will have a high activity and escape before the electron carrier transfer, resulting in a reduced response [3,36]. In addition, the response values of different sensors were tested with different concentrations of H2 (10~1000 ppm) at 275 °C. As exhibited in Figure 7b, the response value of the 3 wt% g-C3N4/In2O3 sensor is greatly improved compared with other sensors within the whole range of H2 concentrations. With the increase in the H2 concentration, the rising trend of the response value curve is gradually stable, which may indicate that the sensor is gradually saturated.
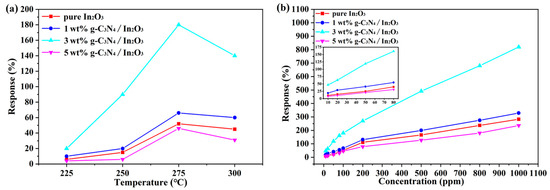
Figure 7.
(a) Response values of sensors vs. operating temperatures to 100 ppm H2; (b) response values of sensors vs. different concentrations H2 at 275 °C.
The response/recovery time of the different sensors at 275 °C is shown in Figure 8a–d. It is not hard to notice that the increase of g-C3N4 content, which may have a positive action in the sensor. Although g-C3N4 is beneficial to the response and recovery characteristics of the sensor, excessive g-C3N4 may play a reverse role. Furthermore, the 3 wt% g-C3N4/In2O3 sensor exhibits fast response/recovery time (2 s/2.4 s) to 100 ppm H2. From Figure 8e, even if it is in a high concentration H2 atmosphere, the 3 wt% g-C3N4/In2O3 sensor also demonstrated extremely fast response/recovery time. The above results reveal that the 3 wt% g-C3N4/In2O3 sensor has great potential in practical application. The Ra of different sensors at different temperatures are shown in Figure 8f; the Ra decrease with the increase in temperature. In addition, the Ra of sensors based on g-C3N4/In2O3 is lower than that of the pure In2O3 and decreased with the increasing amount of g-C3N4 in the composites. The reason for the increase in conductivity is firstly due to the increase in oxygen vacancy in g-C3N4/In2O3. The second reason is attributed to the C–N bond breaking, which releases a lot of electrons [37]. Based on the above reasons, it can be proven that the sensor based on g-C3N4/In2O3 has high conductivity.
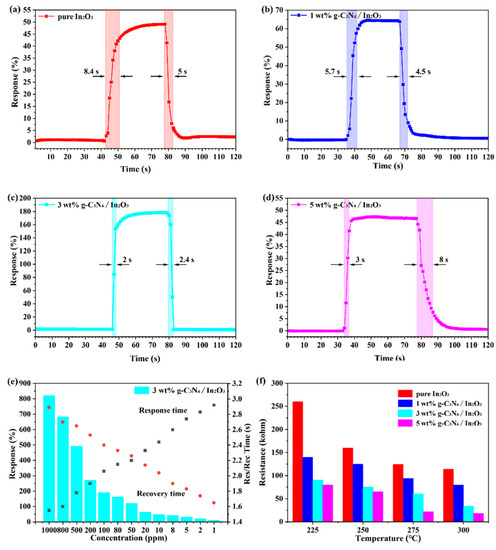
Figure 8.
(a–d) Response/recovery time of different sensors; (e) response/recovery time of 3 wt% g-C3N4/In2O3 to different concentrations of H2 at 275 °C; (f) Ra at different temperatures of different sensors.
Stability and reproducibility are important indicators of a sensor’s performance, and the evaluation result of the 3 wt% g-C3N4/In2O3 sensor at 275 °C is shown in Figure 9. As shown in Figure 9a, the dynamic curves of the 3 wt% g-C3N4/In2O3 sensor is exhibited. Within the variation range of H2 concentrations, the response and recovery characteristics of the 3 wt% g-C3N4/In2O3 sensor are stable. Meanwhile, the detection limit was tested to 1 ppm H2, and the response value is 10%. Reproducibility was evaluated by continuous exposure to 50 ppm H2 for five cycles, as shown in Figure 9b. In the five-cycle experiment, the response/recovery time and response value were basically stable without significant change. By assessing the gas-sensing properties of the 3 wt% g-C3N4/In2O3 sensor, it was sufficienly proven that the sensor has fine stability and reproducibility.
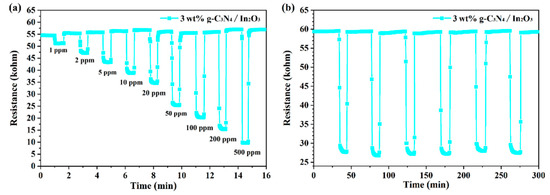
Figure 9.
(a) Dynamic response curve of sensor to different concentrations of H2 at 275 °C; (b) the five-cycle response/recovery curve of the sensor to 50 ppm H2 at 275 °C.
Selectivity is a momentous parameter for the practical application of the sensor. Various gases were tested at 275 °C for the appraisement of different sensors, as presented in Figure 10. Among them, 1 wt% g-C3N4/In2O3 and 3 wt% g-C3N4/In2O3 sensors exhibit similar selectivity characteristics, which possess a higher response value to 50 ppm H2 than other gases (50 ppm SO2, 50 ppm NH3, 50 ppm CO2, 50 ppm CO, 50 ppm CH4, 100 ppm NO2). Besides, the response value of the 3 wt% g-C3N4/In2O3 sensor is three times that of the 1 wt% g-C3N4/In2O3 sensor to 50 ppm H2. Meanwhile, the response value of the 3 wt% g-C3N4/In2O3 sensor to 50 ppm H2 is 7.5 times that of 50 ppm SO2 (the secondary response gas). As we all know, CO, CH4, and H2 are common fuel gases, and the response values of the 3 wt% g-C3N4/In2O3 sensor to H2 are 20 times and 40 times that of CO and CH4 of 50 ppm, respectively. The improvement of H2 selectivity is due to the better dispersion of litchi-like 3wt% g-C3N4/In2O3 compared with pure In2O3, which makes H2 with the smallest molecular size easy to diffuse [38]. In addition, at 275 °C for the sensor based on the 3 wt% g-C3N4/In2O3 surface, the adsorption energy of H2 gas is much higher than that of other gases [22]. From the above results, the 3 wt% g-C3N4/In2O3 sensor exhibits excellent selectivity in H2 detection.
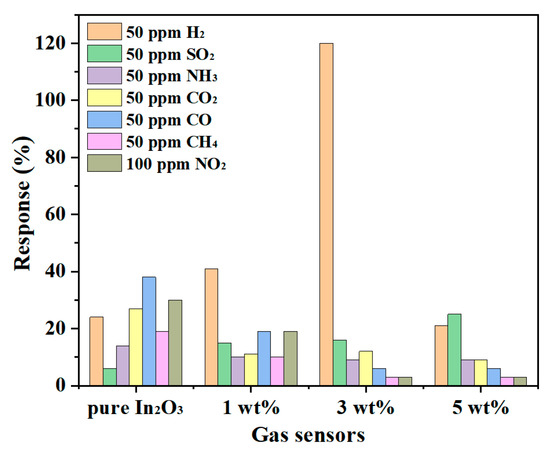
Figure 10.
Gas selectivity of different sensors at 275 °C.
The long-term stability is shown in Figure 11, which was evaluated by continuous exposure to 50 ppm H2 at 275 °C within 2 weeks. It is distinctly perceived from Figure 11a that the response value to 50 ppm H2 is not significantly changed. Moreover, the transient curves for different days are shown in Figure 11b–d, and it is noteworthy that the air resistance (Ra) and response features of the sensor are basically stable. In view of the above assessment, it can be assured that the 3 wt% g-C3N4/In2O3 sensor has good long-term stability for H2 detection.
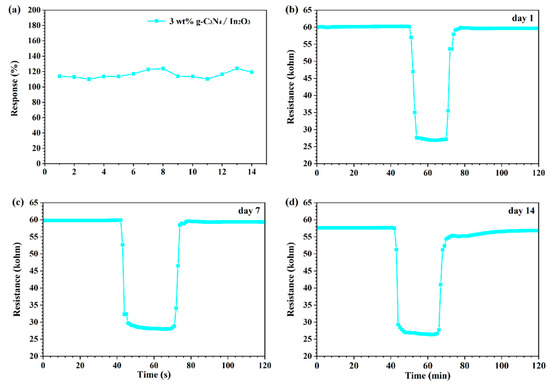
Figure 11.
(a) Long-term stability over 2 weeks for the sensor to 50 ppm H2 at 275 °C; (b–d) transient curve of different days.
3.3. Gas Sensing Mechanism
In order to understand the enhanced properties of the H2 sensor based on g-C3N4/In2O3, the gas-sensing mechanism of the In2O3 sensor was analyzed. In air at different temperatures, the oxygen molecules on the surface of In2O3 materials will become O2- (<147 °C), O− (147 °C–397 °C), O2- (>397 °C), as follows in Equations (2)–(5) [29].
O2 → O2(ads)
O2(ads) + e− → O2−(ads)
O2−(ads) + e− → 2O−(ads)
O−(ads) + e− → O2−(ads)
2 H + O−(ads) → H2O + e−
In this paper, the optimum operating temperature was determined to be 275°C, so most of the oxygen molecules are converted into O−. When the In2O3 sensor exposure to H2, the H molecules are oxidized by O− to form H2O and free electrons as in Equation (6) [22]. The reaction process is shown in Figure 12a. The elevated gas-sensing performance of sensor based on 3 wt% g-C3N4/In2O3 may be attributed to the morphology characteristic, the oxygen state and the g-C3N4/In2O3 heterojunctions. The first reason is the distinctive litchi-like morphology of 3 wt% g-C3N4/In2O3. Through observing SEM and TEM photos, the 3 wt% g-C3N4/In2O3 has smaller size and better dispersion than pure In2O3. The performance of the sensor based on 3 wt% g-C3N4/In2O3 may be improved due to the unique morphology configuration.
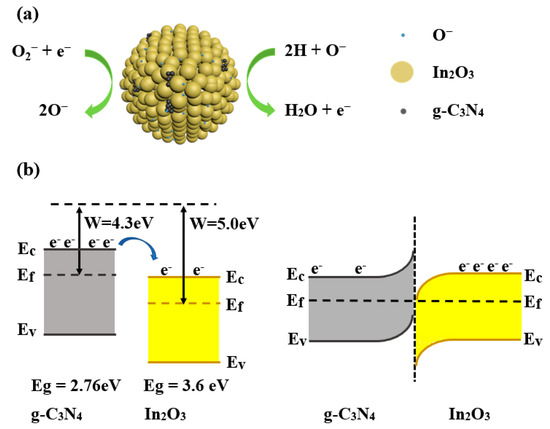
Figure 12.
(a) Sketch map of gas-sensing mechanism; (b) energy band diagram of In2O3 and g-C3N4.
The second reason can be attributed to the adjustment of the oxygen state. The XPS results show that the OV and OC contents in 3 wt% g-C3N4/In2O3 are higher than pure in In2O3. For the sensor based on 3 wt% g-C3N4/In2O3, a large amount of OV provides more active sites for the adsorption of active oxygen, and the increase in OC content indicates that more chemically adsorbed oxygen participates in the redox reaction [19]. Therefore the performance of the 3 wt% g-C3N4/In2O3 sensor may be improved. The last reason is the formation of the g-C3N4/In2O3 heterojunction [19]. The work functions and band gaps of g-C3N4 and In2O3 are W = 4.3 [39], Eg = 2.76 eV [16] and W = 5.0 [13], Eg = 3.6 eV [10], respectively, as exhibited in Figure 12b. The electrons flow from g-C3N4 to In2O3 to the new equilibrium of the Fermi level. Therefore, the sensor based on 3 wt% g-C3N4/In2O3 shows excellent response characteristics [40].
4. Conclusions
In this work, the g-C3N4/In2O3 composite was prepared by a hydrothermal method. The morphology features and chemical compositions of g-C3N4/In2O3 were characterized by XRD, SEM, TEM and XPS. The sensor based on 3 wt% g-C3N4/In2O3 perform excellent gas-sensing behavior. The response value of 3 wt% g-C3N4/In2O3 was 180% to 100 ppm H2 at 275 °C, which is 3.5 times higher than that of the pure In2O3 sensor. Furthermore, the 3 wt% g-C3N4/In2O3 sensor exhibits fast response/recovery time (2 s/2.4 s) to 100 ppm H2 and excellent selectivity (R50 ppm H2/R50 ppm CH4 = 40, R50 ppm H2/R50 ppm CO = 20). The improvement of sensor performance based on 3 wt% g-C3N4/In2O3 can be attributed to the special morphology characteristic, the state of oxygen, and the g-C3N4/In2O3 heterojunctions. In conclusion, this work provides a useful composite for preparing highly efficient H2 sensors and proves that this composite has certain application value.
Author Contributions
Writing—Original draft, J.Z.; writing—Review, X.L.; resources Q.P.; writing—Editing, T.L.; conceptualization and supervision, Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by the National Nature Science Foundation of China (61803172), Hainan Provincial Natural Science Foundation of China (621RC509) and the Start-Up Research Foundation of Hainan University (KYQD(ZR)1910).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data of this study can be obtained from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liang, J.-Y.; Chou, Y.-J.; Yin, C.-W.; Lin, W.-C.; Lin, H.-J.; Chen, P.-W.; Tseng, Y.-C. Realization of an H2/CO dual-gas sensor using CoPd magnetic structures. Appl. Phys. Lett. 2018, 113, 182401. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Viespe, C. Development of Pd/TiO2 Porous Layers by Pulsed Laser Deposition for Surface Acoustic Wave H2 Gas Sensor. Nanomaterials 2020, 10, 760. [Google Scholar] [CrossRef]
- Meng, X.; Bi, M.; Xiao, Q.; Gao, W. Ultrasensitive gas sensor based on Pd/SnS2/SnO2 nanocomposites for rapid detection of H2. Sens. Actuators B Chem. 2022, 359, 131612. [Google Scholar] [CrossRef]
- Kumaresan, M.; Venkatachalam, M.; Saroja, M.; Gowthaman, P. TiO2 nanofibers decorated with monodispersed WO3 heterostruture sensors for high gas sensing performance towards H2 gas. Inorg. Chem. Commun. 2021, 129, 108663. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, L.; Cao, Z.; Li, C.; Li, X.; Liu, F.; Sun, P.; Lu, G. Microwave-assisted hydrothermal synthesis of Pt/SnO2 gas sensor for CO detection. Chin. Chem. Lett. 2020, 31, 2029–2032. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, M.M.; Zhang, D.; Gao, Z. Improving the Performance of Catalytic Combustion Type Methane Gas Sensors Using Nanostructure Elements Doped with Rare Earth Cocatalysts. Sensors 2010, 11, 19–31. [Google Scholar] [CrossRef]
- Cao, Z.; Gao, Q.; Zhou, M.; Li, X.; Wang, Q. LaNiTiO3-SE-based stabilized zirconium oxide mixed potentiometric SO2 gas sensor. Ceram. Int. 2021, 48, 9269–9276. [Google Scholar] [CrossRef]
- Islam, S.; Bhardwaj, A.; Mathur, L.; Kim, I.-H.; Park, J.-Y.; Song, S.-J. Effects of electrolyte variation on ammonia sensing temperature for BiVO4 sensing electrode in mixed potential gas sensor. Sens. Actuators B Chem. 2022, 371, 132504. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, F.; Lin, J.; Lu, G. Gas-sensing properties of In-Sn oxides composites synthesized by hydrothermal method. Sens. Actuators B Chem. 2016, 234, 130–136. [Google Scholar] [CrossRef]
- Guo, S.-Q.; Zhang, X.; Hao, Z.-W.; Gao, G.-D.; Li, G.; Liu, L. In2O3 cubes: Synthesis, characterization and photocatalytic properties. RSC Adv. 2014, 4, 31353–31361. [Google Scholar] [CrossRef]
- Hu, J.; Sun, Y.; Xue, Y.; Zhang, M.; Li, P.; Lian, K.; Zhuiykov, S.; Zhang, W.; Chen, Y. Highly sensitive and ultra-fast gas sensor based on CeO2-loaded In2O3 hollow spheres for ppb-level hydrogen detection. Sens. Actuators B Chem. 2018, 257, 124–135. [Google Scholar] [CrossRef]
- Liu, W.; Xie, Y.; Chen, T.; Lu, Q.; Ur Rehman, S.; Zhu, L. Rationally designed mesoporous In2O3 nanofibers functionalized Pt catalysts for high-performance acetone gas sensors. Sens. Actuators B Chem. 2019, 298, 126871. [Google Scholar] [CrossRef]
- Zhang, K.; Qin, S.; Tang, P.; Feng, Y.; Li, D. Ultra-sensitive ethanol gas sensors based on nanosheet-assembled hierarchical ZnO-In2O3 heterostructures. J. Hazard. Mater. 2020, 391, 122191. [Google Scholar] [CrossRef] [PubMed]
- Hussain, C.M.; Thomas, S. Handbook of Polymer and Ceramic Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Achary, L.S.K.; Kumar, A.; Barik, B.; Nayak, P.S.; Tripathy, N.; Kar, J.P.; Dash, P. Reduced graphene oxide-CuFe2O4 nanocomposite: A highly sensitive room temperature NH3 gas sensor. Sens. Actuators B Chem. 2018, 272, 100–109. [Google Scholar] [CrossRef]
- Hou, M.; Gao, J.; Yang, L.; Guo, S.; Hu, T.; Li, Y. Room temperature gas sensing under UV light irradiation for Ti3C2Tx MXene derived lamellar TiO2-C/g-C3N4 composites. Appl. Surf. Sci. 2021, 535, 147666. [Google Scholar] [CrossRef]
- Maji, B.; Achary, L.S.K.; Barik, B.; Jyotsna Sahoo, S.; Mohanty, A.; Dash, P. MnCo2O4 decorated (2D/2D) rGO/g-C3N4-based Non-Enzymatic sensor for highly selective and sensitive detection of Chlorpyrifos in water and food samples. J. Electroanal. Chem. 2022, 909, 116115. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Pan, Q.; Pan, K.; Zhang, G. Metal-organic framework (MOF) derived In2O3 and g-C3N4 composite for superior NOx gas-sensing performance at room temperature. Sens. Actuators B Chem. 2021, 352, 131001. [Google Scholar] [CrossRef]
- Sun, D.; Wang, W.; Zhang, N.; Liu, C.; Li, X.; Zhou, J.; Ruan, S. G-C3N4/In2O3 composite for effective formaldehyde detection. Sens. Actuators B Chem. 2022, 358, 131414. [Google Scholar] [CrossRef]
- Ullah, M.; Lv, H.; Liu, Z.; Bai, X.; Chen, J.; Zhang, Y.; Wang, J.; Sun, B.; Li, L.; Shi, K. Rational fabrication of a g-C3N4/NiO hierarchical nanocomposite with a large surface area for the effective detection of NO2 gas at room temperature. Appl. Surf. Sci. 2021, 550, 149368. [Google Scholar] [CrossRef]
- Li, H.; Wu, C.-H.; Liu, Y.-C.; Yuan, S.-H.; Chiang, Z.-X.; Zhang, S.; Wu, R.-J. Mesoporous WO3-TiO2 heterojunction for a hydrogen gas sensor. Sens. Actuators B Chem. 2021, 341, 130035. [Google Scholar] [CrossRef]
- Agarwal, S.; Kumar, S.; Agrawal, H.; Moinuddin, M.G.; Kumar, M.; Sharma, S.K.; Awasthi, K. An efficient hydrogen gas sensor based on hierarchical Ag/ZnO hollow microstructures. Sens. Actuators B Chem. 2021, 346, 130510. [Google Scholar] [CrossRef]
- Kadhim, I.H.; Abu Hassan, H.; Abdullah, Q.N. Hydrogen Gas Sensor Based on Nanocrystalline SnO2 Thin Film Grown on Bare Si Substrates. Nano-Micro Lett. 2016, 8, 20–28. [Google Scholar] [CrossRef]
- Wu, C.-H.; Zhu, Z.; Chang, H.-M.; Jiang, Z.-X.; Hsieh, C.-Y.; Wu, R.-J. Pt@NiO core–shell nanostructure for a hydrogen gas sensor. J. Alloys Compd. 2020, 814, 151815. [Google Scholar] [CrossRef]
- Chen, L.; He, X.; Liang, Y.; Sun, Y.; Zhao, Z.; Hu, J. Synthesis and gas sensing properties of palladium-doped indium oxide microstructures for enhanced hydrogen detection. J. Mater. Sci. Mater. Electron. 2016, 27, 11331–11338. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Akhtar, A.; Jiao, C.; Chu, X.; Liang, S.; Dong, Y.; He, L. Acetone sensing properties of the g–C3N4–CuO nanocomposites prepared by hydrothermal method. Mater. Chem. Phys. 2021, 265, 124375. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Chu, X.; Liang, S.; Kong, L. Preparation of g–C3N4–SnO2 composites for application as acetic acid sensor. J. Alloys Compd. 2020, 832, 153355. [Google Scholar] [CrossRef]
- Li, S.; Xie, L.; He, M.; Hu, X.; Luo, G.; Chen, C.; Zhu, Z. Metal-Organic frameworks-derived bamboo-like CuO/In2O3 Heterostructure for high-performance H2S gas sensor with Low operating temperature. Sens. Actuators B Chem. 2020, 310, 127828. [Google Scholar] [CrossRef]
- Ma, J.; Fan, H.; Zheng, X.; Wang, H.; Zhao, N.; Zhang, M.; Yadav, A.K.; Wang, W.; Dong, W.; Wang, S. Facile metal-organic frameworks-templated fabrication of hollow indium oxide microstructures for chlorine detection at low temperature. J. Hazard. Mater. 2020, 387, 122017. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Reddeppa, M.; Chougule, S.; Bak, N.-H.; Nam, D.-J.; Jung, N.; Cho, H.D.; Kim, S.-G.; Kim, M.-D. High performance langasite based SAW NO2 gas sensor using 2D g-C3N4@TiO2 hybrid nanocomposite. J. Hazard. Mater. 2022, 427, 128174. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, C.; Jiang, F.; Liu, J.; Liu, G.; Ma, X.; Liu, P.; Huang, R.; Xu, J.; Wang, L. Flexible fiber-shaped hydrogen gas sensor via coupling palladium with conductive polymer gel fiber. J. Hazard. Mater. 2021, 411, 125008. [Google Scholar] [CrossRef]
- Han, D.; Zhai, L.; Gu, F.; Wang, Z. Highly sensitive NO2 gas sensor of ppb-level detection based on In2O3 nanobricks at low temperature. Sens. Actuators B Chem. 2018, 262, 655–663. [Google Scholar] [CrossRef]
- Cao, S.W.; Liu, X.F.; Yuan, Y.P.; Zhang, Z.Y.; Liao, Y.S.; Fang, J.; Loo, S.C.J.; Sum, T.C.; Xue, C. Solar-to-fuels conversion over In2O3/g-C3N4 hybrid photocatalysts. Appl. Catal. B—Environ. 2014, 147, 940–946. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Sun, G.; Luo, N.; Zhang, B.; Zhang, Z. Synthesis of a Flower-Like g-C3N4/ZnO Hierarchical Structure with Improved CH4 Sensing Properties. Nanomaterials 2019, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Akbar, S.A.; Morris, P.A. Synergistic effects in gas sensing semiconducting oxide nano-heterostructures: A review. Sens. Actuators B Chem. 2019, 286, 624–640. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Wang, T.; Li, X.; Fu, Y.; Zhao, G.; Xu, X. g-C3N4 templated synthesis of 3DOM SnO2/CN enriched with oxygen vacancies for superior NO2 gas sensing. Appl. Surf. Sci. 2022, 604, 154618. [Google Scholar] [CrossRef]
- Xie, F.; Li, W.; Zhang, Q.; Zhang, S. Highly Sensitive and Selective CO/NO/H2/NO2 Gas Sensors Using Noble Metal (Pt, Pd) Decorated MOx (M = Sn, W) Combined With SiO2 Membrane. IEEE Sens. J. 2019, 19, 10674–10679. [Google Scholar] [CrossRef]
- Guo, W.W.; Huang, L.L.; Zhang, J.; He, Y.Z.; Zeng, W. Ni-doped SnO2/g-C3N4 nanocomposite with enhanced gas sensing performance for the eff;ective detection of acetone in diabetes diagnosis. Sens. Actuator B Chem. 2021, 334, 11. [Google Scholar] [CrossRef]
- Patrick, D.S.; Govind, A.; Bharathi, P.; Mohan, M.K.; Harish, S.; Archana, J.; Navaneethan, M. Hierarchical ZnO/g-C3N4 nanocomposites for enhanced NO2 gas sensing applications. Appl. Surf. Sci. 2023, 609, 155337. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).