Ultrasound Evaluation of the Primary α Phase Grain Size Based on Generative Adversarial Network
Abstract
:1. Introduction
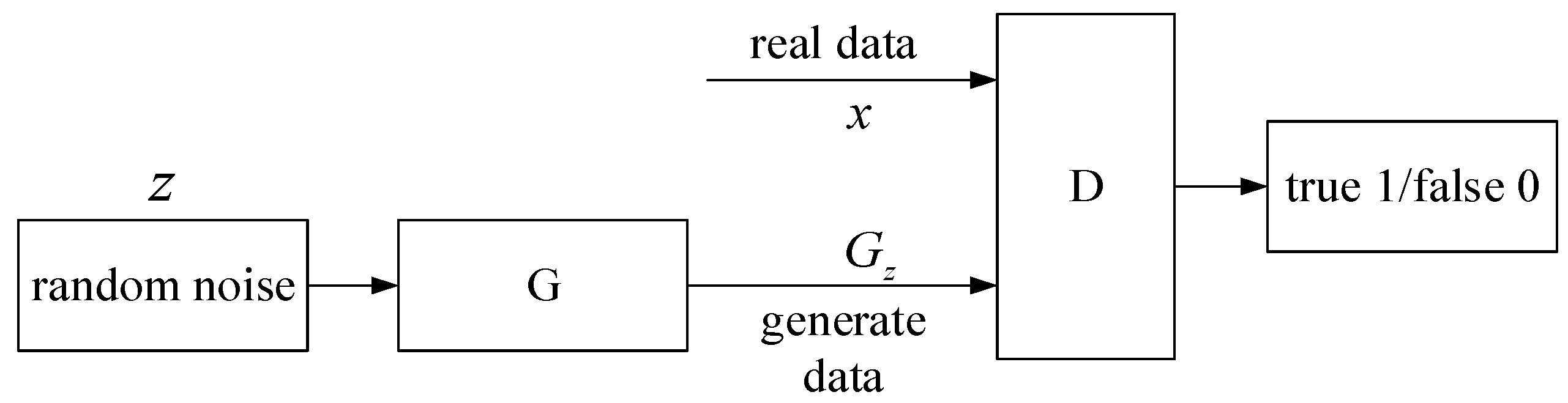
2. Virtual Sample Generation Technology
2.1. Definition of Virtual Samples
2.2. Introduction of Virtual Samples
3. Ultrasound Evaluation Model of the Grain Size of Primary α Phase
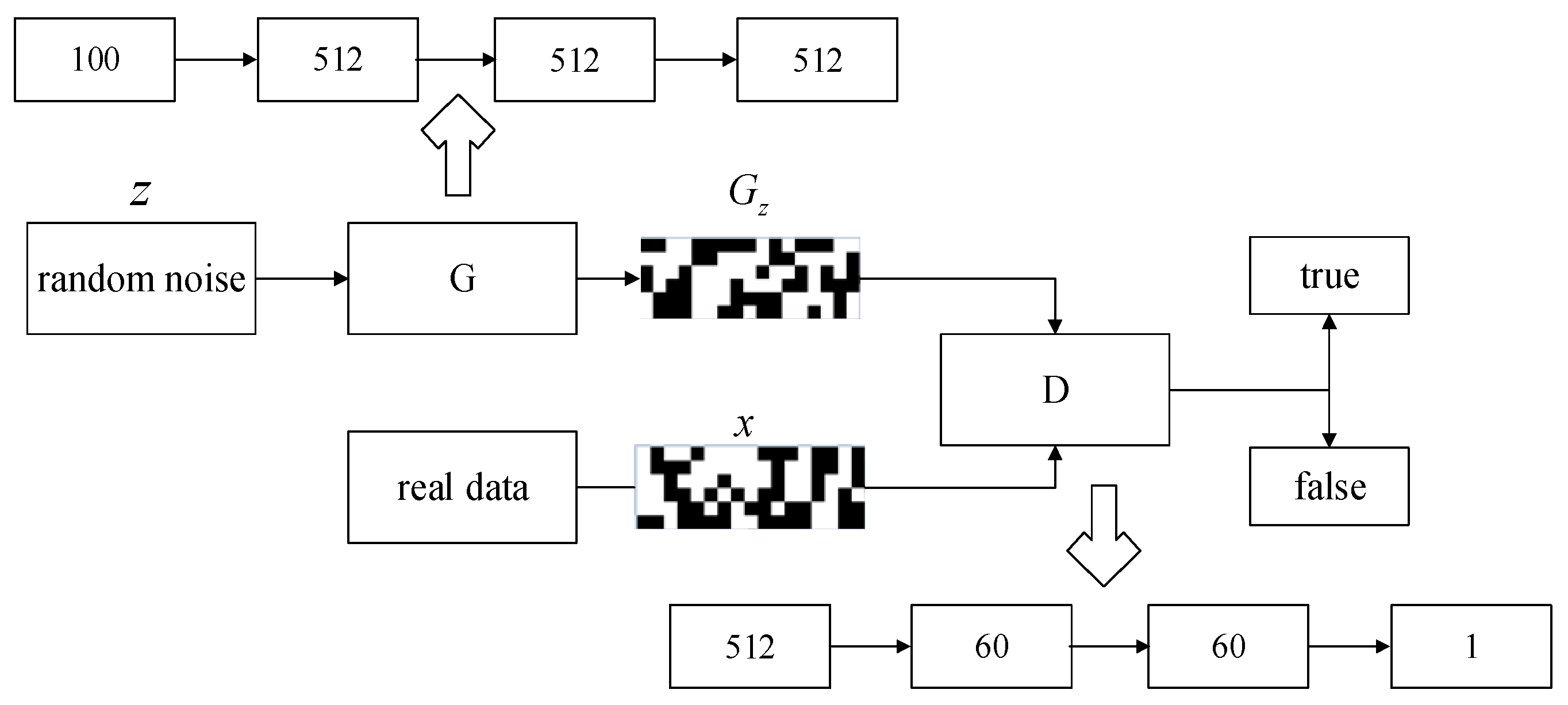
3.1. Virtual Sample Generation
3.2. Virtual Sample Validity Analysis and Screening Process
| Algorithm 1: Effective virtual sample generation algorithm ( algorithm) | |
| Input: real training sample set | |
| Output: optimal virtual sample set | |
| 1. | begin |
| 2. | /* initialize the number of iterations , initialize reconstructed sample set , initialize , initialize the virtual sample set after initial screening */ |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. | repeat |
| 8. | |
| 9. | repeat |
| 10. | |
| 11. | if then |
| 12. | |
| 13. | end if |
| 14. | until |
| 15. | |
| 16. | until |
| 17. | |
| 18. | end |
3.3. Constructing the Ultrasound Evaluation Model of the Grain Size of Primary α Phase
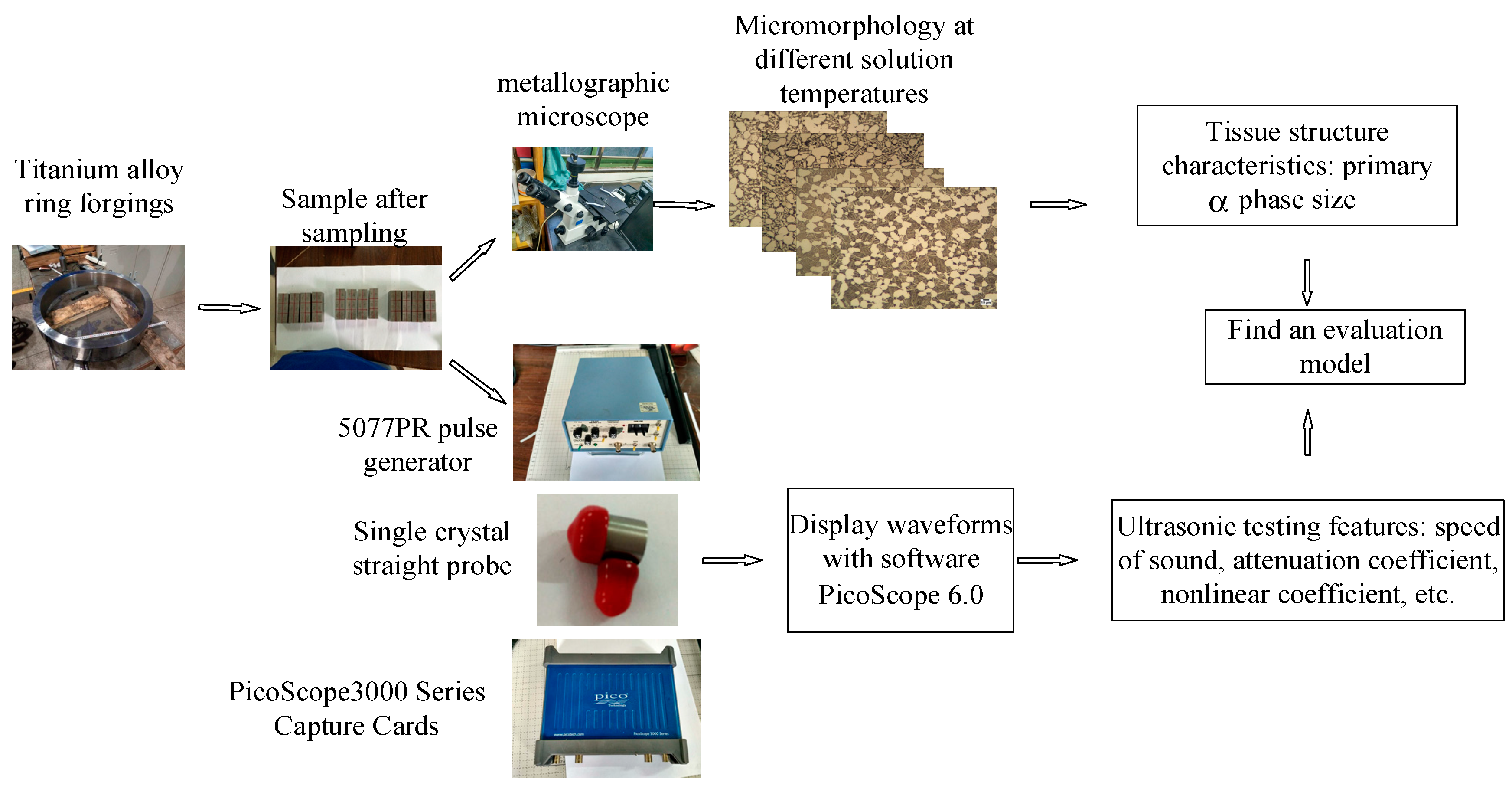
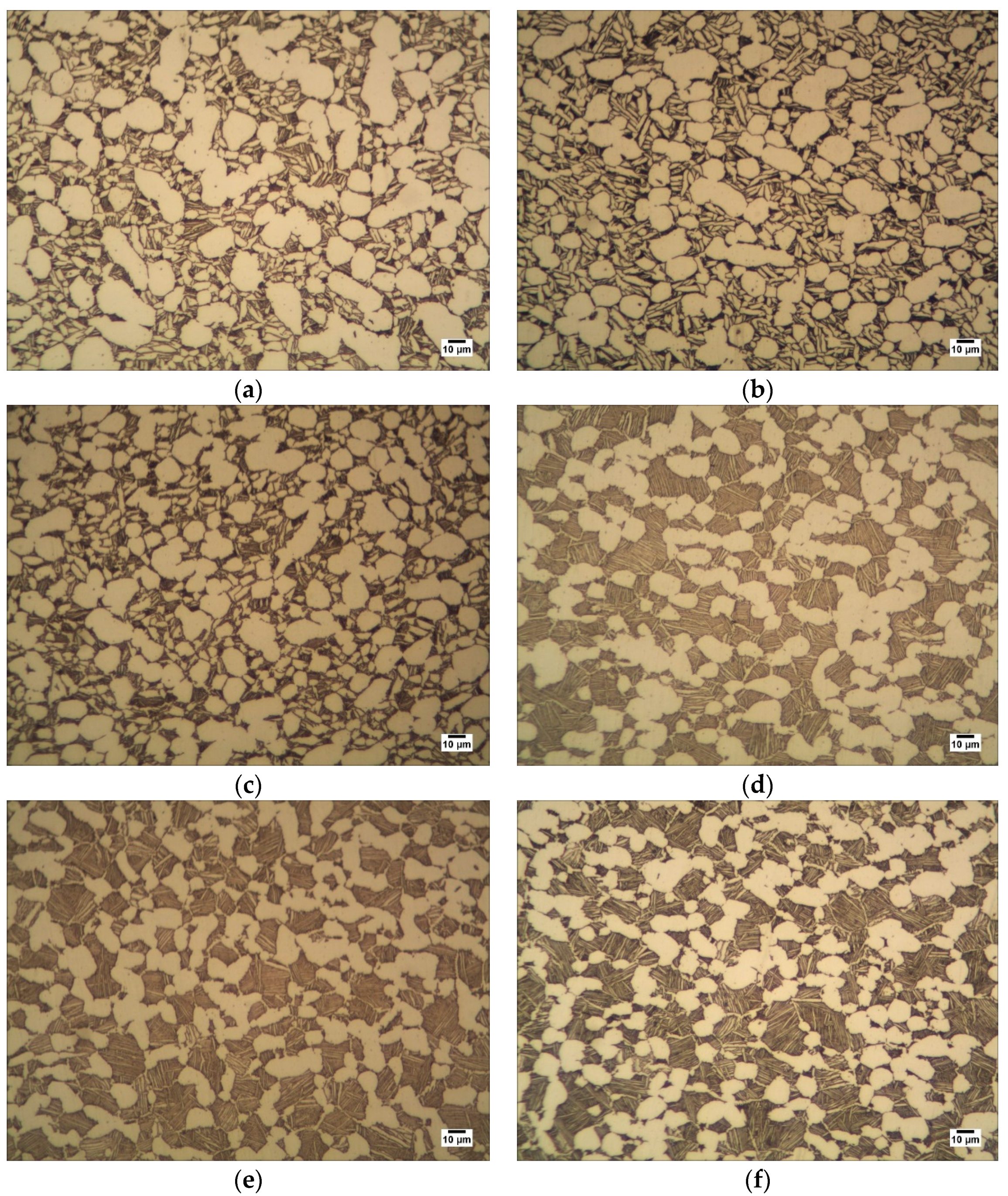
3.3.1. Ultrasonic Testing Experiment and Metallographic Observation Experiment
3.3.2. Constructing the Ultrasound Evaluation Model of the Grain Size of Primary α Phase
- (1)
- Virtual sample generation process.
- (2)
- Virtual sample validity analysis and screening process.
- (3)
- Modeling process.
4. Experiment
4.1. Analysis of Virtual Sample Effectiveness
4.1.1. Data Description
4.1.2. Parameter Selection
4.1.3. Analysis of Effectiveness
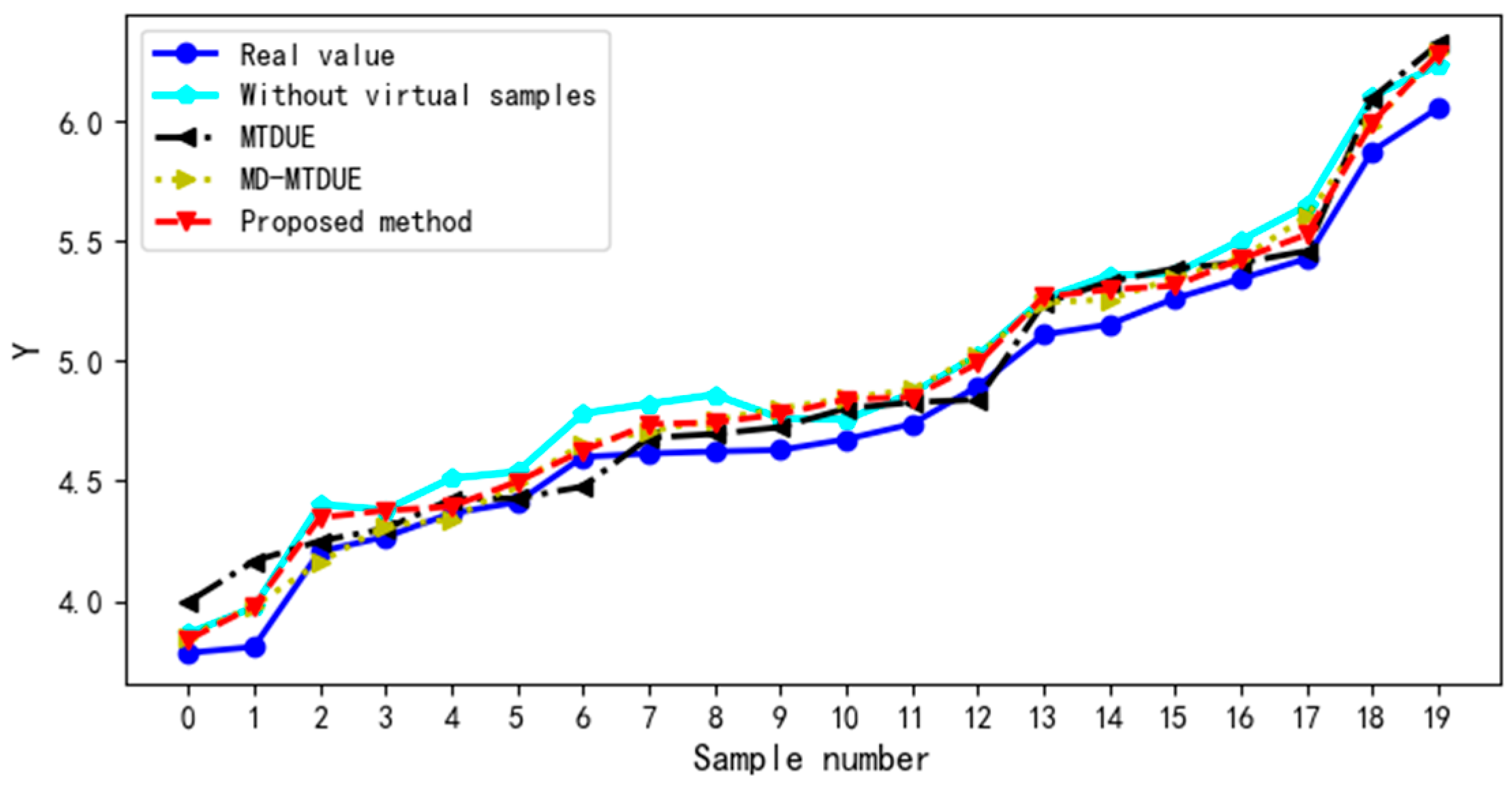
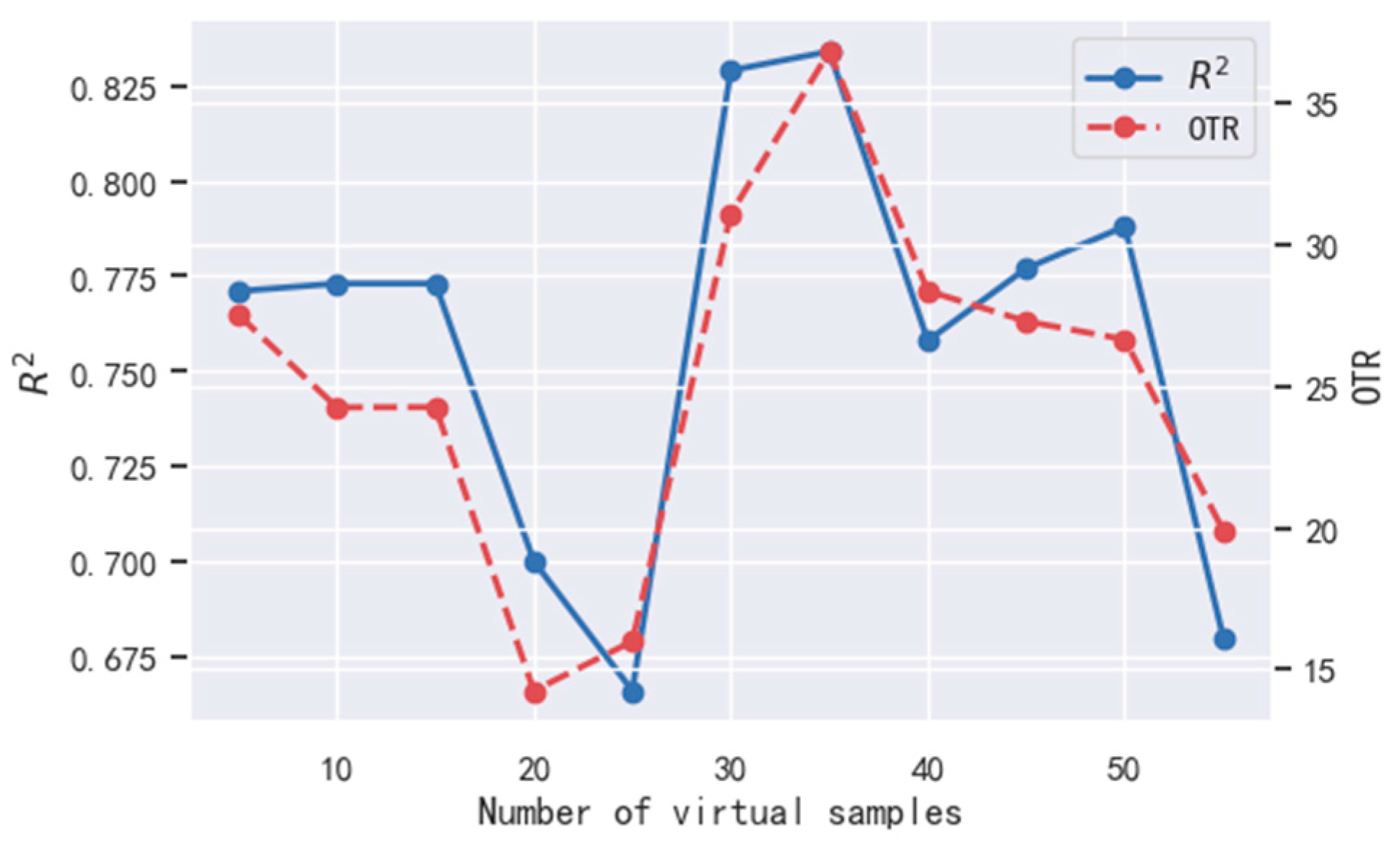
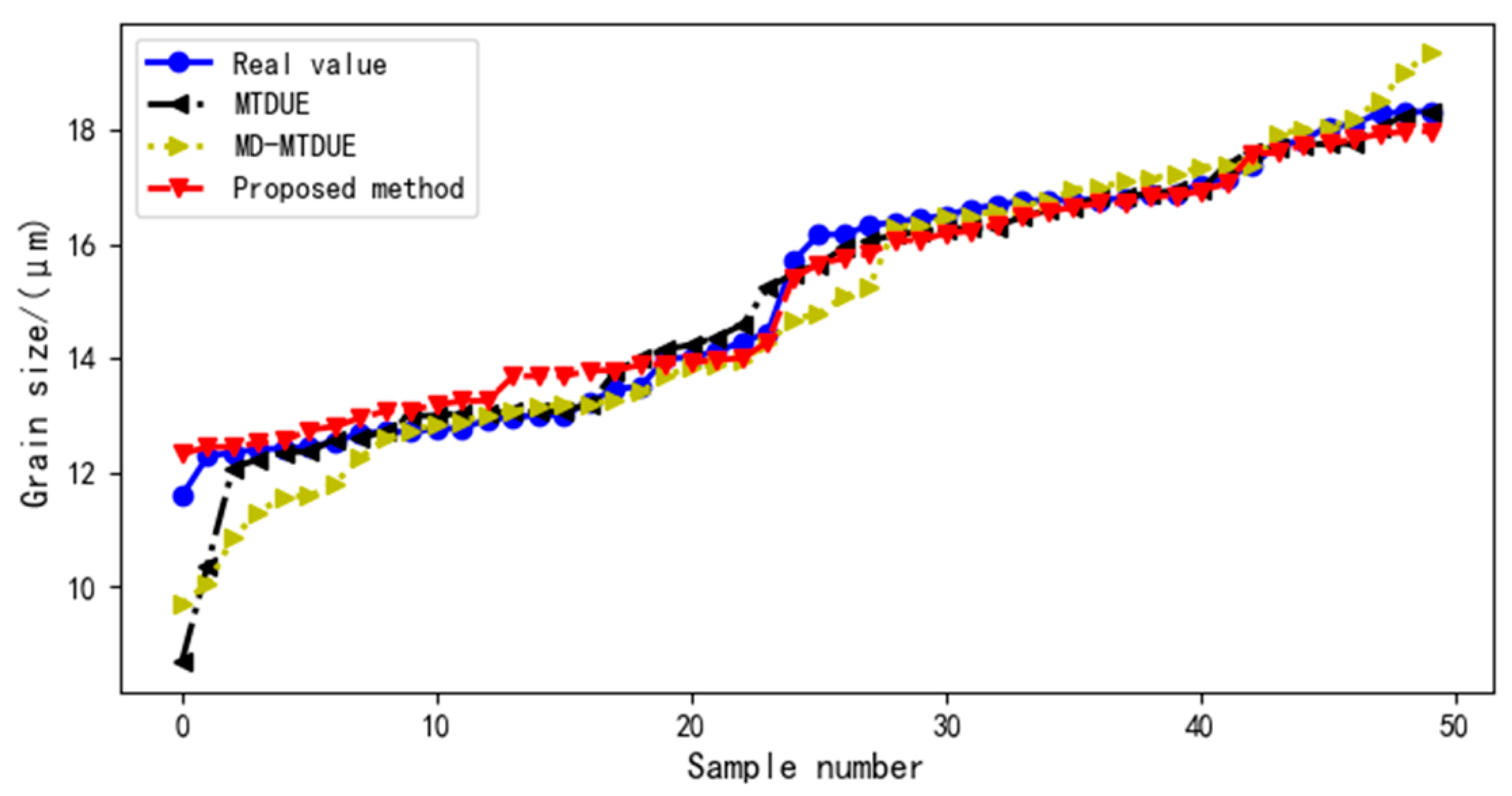
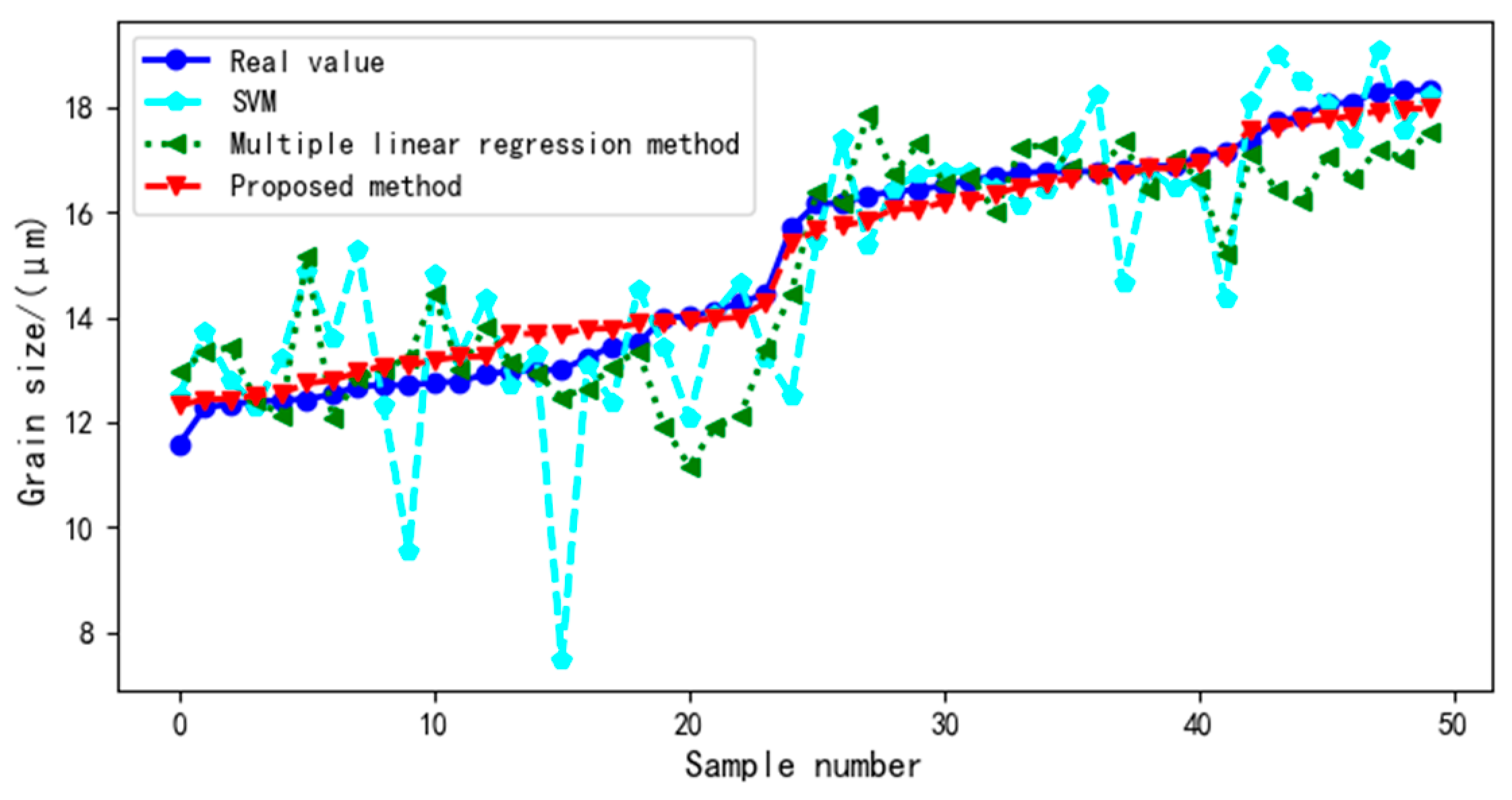
4.2. Impact of Virtual Samples on Ultrasound Evaluation Model
4.2.1. Impact of Virtual Sample Number on Ultrasound Evaluation Model
4.2.2. Impact of Different Virtual Sample Generation Methods on Ultrasound Evaluation Model
4.2.3. Comparison with Traditional Ultrasound Evaluation Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, B.; Li, P.T.; Zhang, C.Y.; Wang, X.B. Effect of ultrasonic vibration direction on milling characteristics of TC4 titanium alloy. Acta Aeronaut. Astronaut. Sin. 2020, 41, 623301. [Google Scholar]
- Wenzheng, Z.; Xiwang, Q.; Guoqing, W.; Hai, N.; Huang, Z. Non-destructive Evaluation Method of Large-scale Casting Piece Based on Metallographic Structure Statistical Analysis. J. Aeronaut. Mater. 2018, 38, 89–95. [Google Scholar]
- Nikolas, H.; Thomas, G.H.; Timothy, Q. Fatigue properties of a titanium alloy (Ti-6Al-4V) fabricated via electron beam melting (EBM): Effects of internal defects and residual stress. Int. J. Fatigue 2017, 94, 202–210. [Google Scholar]
- Wang, Y.Q.; Liu, W.; Wang, L.Y. Microstructure and Properties of TC25 Titanium Alloy. In Proceedings of the 12th Annual Conference on Materials Science and Alloy Processing of China Nonferrous Metals Society, Qingdao, China, 25–28 September 2007; pp. 191–196. [Google Scholar]
- Chlebus, E.; Gruber, K.; Kuźnicka, B.; Kurzac, J.; Kurzynowski, T. Effect of heat treatment on the microstructure and mechanical properties of TC25 titanium alloy bars. Mater. Rev. 2014, 28, 417–437. [Google Scholar]
- Mark, P.B.; Daniel, E. The influence of texture and phase distortion on ultrasonic attenuation in Ti-6Al-4V. J. Nondestruct. Eval. 2001, 20, 1–16. [Google Scholar]
- Yang, L.; Li, J.; Lobkis, O.I.; Rokhlin, S.I. Ultrasonic propagation and scattering in duplex microstructures with application to titanium alloys. J. Nondestruct. Eval. 2012, 31, 270–283. [Google Scholar] [CrossRef]
- Li, J.; Lobkis, O.I.; Yang, L.; Rokhlin, S.I. Integrated method of ultrasonic attenuation and backscattering for characterization of microstructures in polycrystals. AIP Conf. Proc. 2012, 1430, 1397–1404. [Google Scholar]
- Mahadevan, S.; Nath, P.; Hu, Z. The influence of In718 grain size on ultrasonic multi-objective optimization backscatter signal and its non-destructive evaluation method. Acta Metall. Sin. 2016, 52, 378–384. [Google Scholar]
- Ogi, H.; Hirao, M.; Honda, T. Evaluation of Grain Size Using the Ultrasonic Attenuation Rate. J. Mech. Eng. 2015, 51, 1–7. [Google Scholar]
- He, Y.L.; Wang, P.J.; Zhang, M.Q.; Zhu, Q.X.; Xu, Y. A novel and effective nonlinear interpolation virtual sample generation method for enhancing energy prediction and analysis on small data problem: A case study of Ethylene industry. Energy 2018, 147, 418–427. [Google Scholar] [CrossRef]
- Chang, C.J.; Li, D.C.; Huang, Y.H.; Chen, C.C. A novel gray forecasting model based on the box plot for small manufacturing data sets. Appl. Math. Comput. 2015, 265, 400–408. [Google Scholar] [CrossRef]
- Nicole, A.; Julian, F. When small data beats big data. Stat. Probab. Lett. 2018, 136, 142–145. [Google Scholar]
- Piercesare, S. On the role of statistics in the era of big data: A call for a debate. Stat. Probab. Lett. 2018, 136, 10–14. [Google Scholar]
- Chang, C.J.; Li, D.C.; Chen, C.C. A forecasting model for small non-equigapdata sets considering data weights and occurrence possibilities. Comput. Ind. Eng. 2014, 67, 139–145. [Google Scholar] [CrossRef]
- Poggio, T.; Vetter, T. Recognition and Structure from One 2D Model View: Observations on Prototypes, Object Classes, and Symmetries; A. I. Memo No. 1347; Artificial Intelligence Laboratory, Massachusetts Institute of Technology: Cambridge, MA, USA, 1992. [Google Scholar]
- Huang, C.F.; Claudio, M. A diffusion-neural-network for learning from small samples. Int. J. Approx. Reason. 2003, 35, 137–161. [Google Scholar] [CrossRef] [Green Version]
- Li, D.C.; Wu, C.S.; Tsai, T.I.; Lina, Y.S. Using mega-trend-diffusion and artificial samples in small data set learning for early flexible manufacturing system scheduling knowledge. Comput. Oper. Res. 2007, 34, 966–982. [Google Scholar] [CrossRef]
- Chen, Z.S.; Zhu, B.; He, Y.L. A PSO based virtual samples generation method for small sample sets: Applications to regression datasets. Eng. Appl. Artif. Intell. 2017, 59, 236–243. [Google Scholar] [CrossRef]
- Yu, X.; Yang, J.; Xie, Z.Q. Research on Virtual Sample Generation Technology. Comput. Sci. 2011, 38, 16–19. [Google Scholar]
- Nordestgarrd, B.; Anette, V. Triglycerides and cardiovascular disease. Lancet 2014, 384, 626–635. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Sun, J.; Zhang, J.; Zissimos, M. Gray bootstrap method for estimating frequency-varying random vibration signals with small samples. Chin. J. Aeronaut. 2014, 27, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Li, D.C.; Wen, I.H. A genetic algorithm-based virtual sample generation technique to improve small data set learning. Neurocomputing 2014, 143, 222–230. [Google Scholar] [CrossRef]
- Niyogi, P.; Girosi, F.; Poggio, T. Incorporating prior information in machine learning by creating virtual examples. Proc. IEEE 1998, 86, 2196–2209. [Google Scholar] [CrossRef] [Green Version]
- Aydemir, M.S.; Bilgin, G. Semi-supervised sparse representation classifier (S3RC) with deep features on small sample sized hyperspectral images. Neurocomputing 2020, 399, 213–226. [Google Scholar] [CrossRef]
- Zhang, X.H.; Xu, Y.; He, Y.L.; Zhu, Q.X. Novel manifold learning based virtual sample generation for optimizing soft sensor with small data. ISA Trans. 2020, 109, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, W.; Alessandro, C. Adel AJ A novel virtual sample generation method to overcome the small sample size problem in computer aided medical diagnosing. Algorithms 2019, 12, 160. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.X.; Chen, Z.S.; Zhang, X.H.; Rajabifard, A.; Xu, Y.; Chen, Y.Q. Dealing with small sample size problems in process industry using virtual sample generation: A Kriging-based approach. Soft Comput. 2020, 24, 6889–6902. [Google Scholar] [CrossRef]
- Tao, H.; Wang, P.; Chen, Y.; Stojanovic, V.; Yang, H. An unsupervised fault diagnosis method for rolling bearing using STFT and generative neural networks. J. Frankl. Inst. 2020, 357, 7286–7307. [Google Scholar] [CrossRef]
- Li, D.C.; Lin, L.S. Generating information for small data sets with a multimodal distribution. Decis. Support Syst. 2014, 66, 71–81. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.; Xie, Z.Q. A novel virtual sample generation method based on Gaussian distribution. Knowl.-Based Syst. 2011, 24, 740–748. [Google Scholar] [CrossRef]
- Nitesh, V.C.; Kevin, W.B.; Lawrence, O.H. SMOTE: Synthetic minority over-sampling technique. Artif. Intell. Res 2002, 16, 321–357. [Google Scholar]
- Goodfellow Ian Pouget-Abadie, J.; Mirza, M. Generative adversarial nets. Adv. Neural Inf. Process. Syst. 2014, 1, 2672–2680. [Google Scholar]


| Number | ||||||
|---|---|---|---|---|---|---|
| 1 | 6148.172 | 0.197 | 1.351 | 1.226 | 3.5 | 17.670 |
| 2 | 6160.178 | 0.199 | 1.268 | 1.060 | 3.48 | 17.858 |
| 3 | 6192.273 | 0.248 | 1.309 | 1.392 | 4.28 | 18.831 |
| 6137.756 | 0.206 | 1.5072 | 1.2791 | 3.75 | 17.090 | |
| 6186.24 | 0.211 | 1.517 | 1.351 | 3.32 | 18.687 |
| Training data (30) | 0.957 | 0.857 | 0.872 | 0.837 | 0.318 | 4.684 |
| 0.765 | 0.243 | 0.805 | 0.392 | 0.139 | 4.761 | |
| 0.033 | 0.058 | 0.265 | 0.210 | 0.506 | 4.812 | |
| Test data (20) | 0.020 | 0.824 | 0.573 | 0.413 | 0.701 | 5.153 |
| 0.575 | 0.310 | 0.743 | 0.060 | 0.099 | 5.343 | |
| 0.230 | 0.798 | 0.623 | 0.776 | 0.560 | 4.366 |
| Evaluation Method | MAPE (%) |
|---|---|
| SVM | 3.317 |
| MTDUE | 3.05 |
| MD-MTDUE | 2.939 |
| Proposed method | 2.531 |
| Evaluation Methods | MAPE (%) |
|---|---|
| SVM | 7.091 |
| MTDUE | 6.518 |
| MD-MTDUE | 6.357 |
| Proposed method | 4.479 |
| Evaluation Methods | MAPE (%) |
|---|---|
| First-order sound velocity method | 9.814 |
| Second-order sound velocity method | 9.718 |
| Multiple linear regression method | 5.607 |
| SVM | 7.091 |
| Proposed method | 4.479 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, S.; Chen, X.; Wu, G.; Li, M.; Chen, H. Ultrasound Evaluation of the Primary α Phase Grain Size Based on Generative Adversarial Network. Sensors 2022, 22, 3274. https://doi.org/10.3390/s22093274
Peng S, Chen X, Wu G, Li M, Chen H. Ultrasound Evaluation of the Primary α Phase Grain Size Based on Generative Adversarial Network. Sensors. 2022; 22(9):3274. https://doi.org/10.3390/s22093274
Chicago/Turabian StylePeng, Siqin, Xi Chen, Guanhua Wu, Ming Li, and Hao Chen. 2022. "Ultrasound Evaluation of the Primary α Phase Grain Size Based on Generative Adversarial Network" Sensors 22, no. 9: 3274. https://doi.org/10.3390/s22093274
APA StylePeng, S., Chen, X., Wu, G., Li, M., & Chen, H. (2022). Ultrasound Evaluation of the Primary α Phase Grain Size Based on Generative Adversarial Network. Sensors, 22(9), 3274. https://doi.org/10.3390/s22093274






