SERS and Indicator Paper Sensing of Hydrogen Peroxide Using Au@Ag Nanorods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
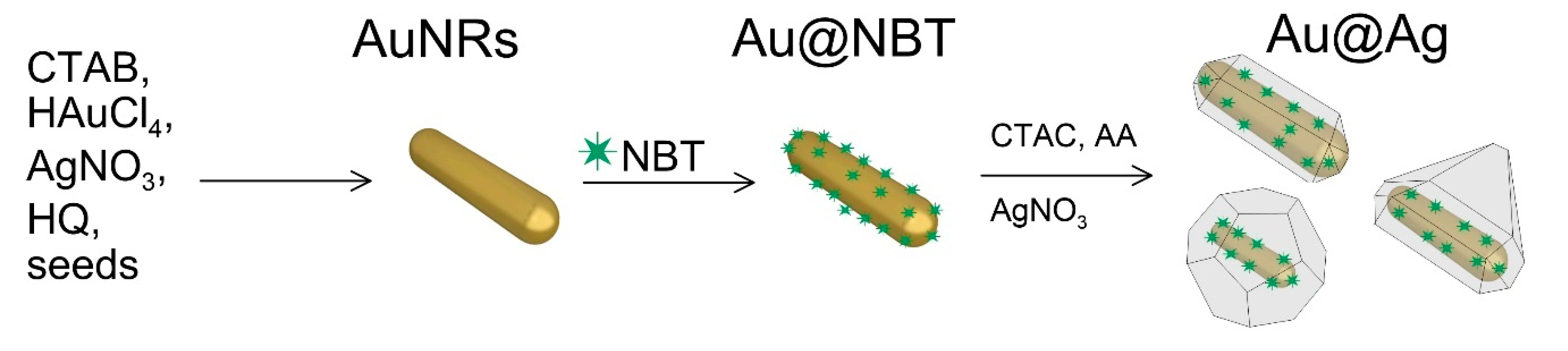
2.2. Synthesis of Au@Ag Nanorods with Embedded NBT
2.3. Nanoparticles Characterization
2.4. Detection of H2O2 in Colloid
2.5. Indicator Paper for the Detection of H2O2
3. Results
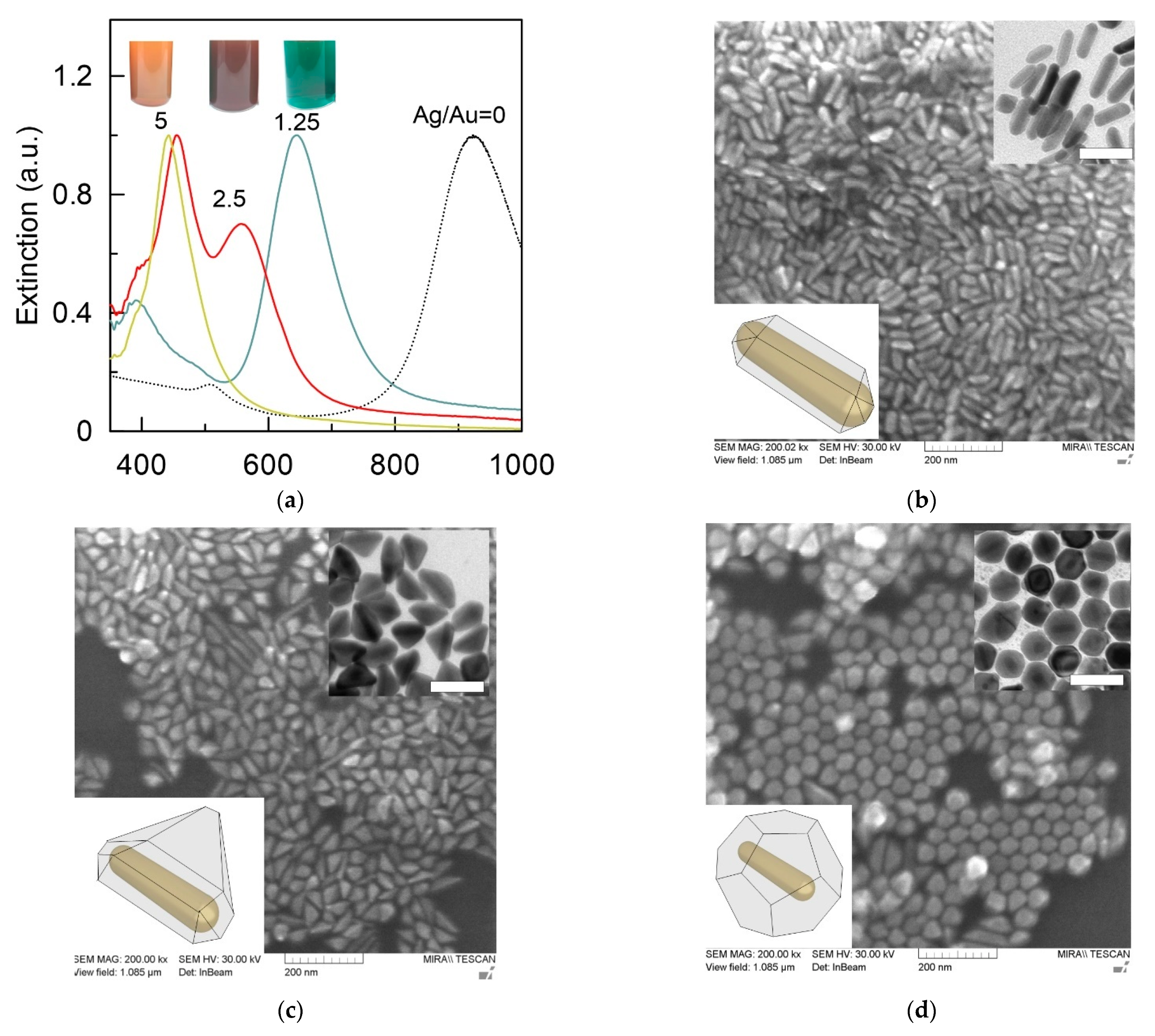
3.1. Size, Shape, and SERS Characterization of the Synthesized Nanoparticles
3.2. Colorimetric Detection of H2O2
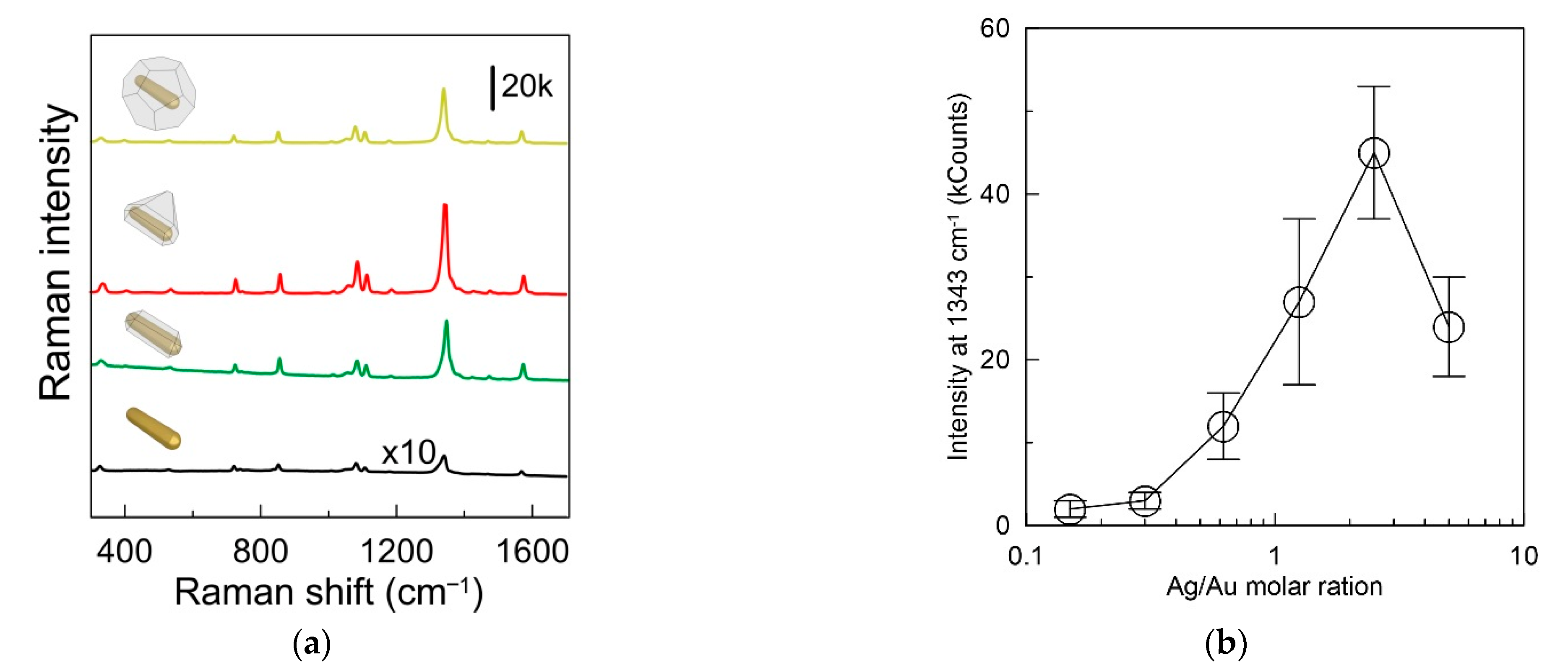
3.3. SERS Detection of H2O2
3.4. Anti-Interference and Stability of the Sensing
3.5. Indicator Paper for H2O2 Sensing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Guo, H.; Aleyasin, H.; Dickinson, B.C.; Haskew-Layton, R.E.; Ratan, R.R. Recent advances in hydrogen peroxide imaging for biological applications. Cell Biosci. 2014, 4, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijeratne, S.S.K.; Cuppett, S.L.; Schlegel, V. Hydrogen peroxide induced oxidative stress damage and antioxidant enzyme response in Caco-2 human colon cells. J. Agric. Food Chem. 2005, 53, 8768–8774. [Google Scholar] [CrossRef] [PubMed]
- Ransy, C.; Vaz, C.; Lombès, A.; Bouillaud, F. Use of H2O2 to cause oxidative stress, the catalase issue. Int. J. Mol. Sci. 2020, 21, 9149. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Gallegos, A.; Pletcher, D. The removal of low level organics via hydrogen peroxide formed in a reticulated vitreous carbon cathode cell, Part 1. The electrosynthesis of hydrogen peroxide in aqueous acidic solutions. Electrochim. Acta 1998, 44, 853–861. [Google Scholar] [CrossRef]
- Kosaka, K.; Yamada, H.; Shishida, K.; Echigo, S.; Minear, R.A.; Tsuno, H.; Matsui, S. Evaluation of the treatment performance of a multistage ozone/hydrogen peroxide process by decomposition by-products. Water Res. 2001, 35, 3587–3594. [Google Scholar] [CrossRef]
- Xu, S.; Peng, B.; Han, X. A third-generation H2O2 biosensor based on horseradish peroxidase-labeled Au nanoparticles self-assembled to hollow porous polymeric nanospheres. Biosens. Bioelectron. 2007, 22, 1807–1810. [Google Scholar] [CrossRef]
- Sunil, K.; Narayana, B. Spectrophotometric determination of hydrogen peroxide in water and cream samples. Bull. Environ. Contam. Toxicol. 2008, 81, 422–426. [Google Scholar] [CrossRef]
- Yu, X.; Gong, Y.; Xiong, W.; Li, M.; Zhao, J.; Che, Y. Turn-on fluorescent detection of hydrogen peroxide and triacetone triperoxide via enhancing interfacial interactions of a blended system. Anal. Chem. 2019, 91, 6967–6970. [Google Scholar] [CrossRef] [Green Version]
- Rezende, F.; Brandes, R.P.; Schröder, K. Detection of hydrogen peroxide with fluorescent dyes. Antioxid. Redox. Signal 2018, 29, 585–602. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Singh, S. Nanomaterials exhibiting enzyme-like properties (Nanozymes): Current advances and future perspectives. Front. Chem. 2019, 7, 46. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef]
- Kaur, B.; Santosh, K.; Kaushik, B.K. Recent advancements in optical biosensors for cancer detection. Biosens. Bioelectron. 2022, 197, 113805. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Pereira, T.S.; Facure, M.H.M.; Santos, D.M.; Mercante, L.A.; Mattoso, L.H.C.; Correa, D.S. Current progress in plant pathogen detection enabled by nanomaterials-based (bio)sensors. Sens. Actuators Rep. 2022, 4, 100068. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lou, X.-Y.; Liang, F. Surface-functionalized gold and silver nanoparticles for colorimetric and fluorescent sensing of metal ions and biomolecules. Coord. Chem. Rev. 2022, 45915, 214461. [Google Scholar] [CrossRef]
- Zannotti, M.; Vicomandi, V.; Rossi, A.; Minicucci, M.; Ferraro, S.; Petetta, L.; Giovannetti, R. Tuning of hydrogen peroxide etching during the synthesis of silver nanoparticles. An application of triangular nanoplates as plasmon sensors for Hg2+ in aqueous solution. J. Mol. Liq. 2020, 309, 113238. [Google Scholar] [CrossRef]
- Lu, H.; Yu, C.; Quan, S.; Xu, S. A novel dual response ratiometric fluorescent probe for the determination of H2O2 and glucose via etching of silver nanoparticles. Analyst 2019, 144, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S.; Jeon, H.-J.; Ahn, C.W. Role of capping agents in controlling silver nanoparticles size, antibacterial activity and potential application as optical hydrogen peroxide sensor. RSC Adv. 2016, 6, 36171–36179. [Google Scholar] [CrossRef]
- Hou, W.; Liu, X.; Lu, Q.; Liu, M.; Zhang, Y.; Yao, S. Etching and anti–etching strategy for sensitive colorimetric sensing of H2O2 and biothiols based on silver/carbon nanomaterial. Colloids Surf. B Biointerfaces 2018, 162, 118–125. [Google Scholar] [CrossRef]
- Roy, K.; Sarkar, C.K.; Ghosh, C.K. Fast colourimetric detection of H2O2 by biogenic silver nanoparticles synthesised using Benincasa hispida fruit extract. Nanotechnol. Rev. 2016, 5, 251–258. [Google Scholar] [CrossRef]
- Pham, X.-H.; Seong, B.; Bock, S.; Hahm, E.; Huynh, K.-H.; Kim, Y.-H.; Kim, W.; Kim, J.; Kim, D.E.; Jun, B.-H. Nonenzymatic hydrogen peroxide detection using surface-enhanced Raman scattering of gold–silver core–shell-assembled silica nanostructures. Nanomaterials 2021, 11, 2748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hou, W.; Lu, Q.; Liu, M.; Chen, C.; Zhang, Y.; Yao, S. Colorimetric detection of hydrogen peroxide and lactate based on the etching of the carbon based Au-Ag bimetallic nanocomposite synthesized by carbon dots as the reductant and stabilizer. Anal. Chim. Acta 2016, 947, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Li, M.; Wang, J.; Feng, F.; Zhang, Y.; Qiu, Z.; Chen, Y.; Meteku, B.E.; Wen, C.; Yan, Z.; et al. Ag@Au core/shell triangular nanoplates with dual enzyme-like properties for the colorimetric sensing of glucose. Chin. Chem. Lett. 2019, 31, 5. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L. Colorimetric detection of hydrogen peroxide using silver nanoparticles with three different morphologies. Anal. Meth. 2016, 8, 6691–6695. [Google Scholar] [CrossRef]
- Zhang, R.; Zhong, Q.; Liu, Y.; Ji, J.; Liu, B. Monodispersed silver-gold nanorods controllable etching for ultrasensitive SERS detection of hydrogen peroxide-involved metabolites. Talanta 2022, 243, 123382. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Fu, C.; Wu, Y.; Cao, H.; Shia, W.; Jung, Y.M. A simple enzyme-free SERS sensor for the rapid and sensitive detection of hydrogen peroxide in food. Analyst 2020, 145, 607–612. [Google Scholar] [CrossRef]
- Gao , C.; Lu , Z.; Liu , Y.; Zhang, Q.; Chi, M.; Cheng, Q.; Yin, Y. Highly stable silver nanoplates for surface plasmon resonance biosensing. Angew. Chem. Int. Ed. 2012, 51, 5629–5633. [Google Scholar] [CrossRef] [PubMed]
- de Barros, M.R.; Winiarski, J.P.; Elias, W.C.; de Campos, C.E.M.; Jost, C.L. Au-on-Pd bimetallic nanoparticles applied to the voltammetric determination and monitoring of 4-nitroaniline in environmental samples. J. Environ. Chem. Eng. 2021, 9, 105821. [Google Scholar] [CrossRef]
- Bao, C.; Shi, M.; Ma, W.; Li, J.; Huang, X.; Cheng, H. Simultaneous determination of aesculin and aesculetin and their interactions with DNA using carbon fiber microelectrode modified by Pt–Au bimetallic nanoparticles. Anal. Chim. Acta 2022, 1202, 339664. [Google Scholar] [CrossRef]
- Pusch, J.M.E.; Brondani, D.; Luza, L.; Dupont, J.; Vieira, I.C. Pt–Pd bimetallic nanoparticles dispersed in an ionic liquid and peroxidase immobilized on nanoclay applied in the development of a biosensor. Analyst 2013, 138, 4898–4906. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, K.; Tan, X.; Zhang, Y.; Bai, L.; Xie, W. Etchable SERS nanosensor for accurate pH and hydrogen peroxide sensing in living cells. Chem. Commun. 2019, 55, 12996–12999. [Google Scholar]
- Jin, X.; Khlebtsov, B.N.; Khanadeev, V.A.; Khlebtsov, N.G.; Ye, J. Rational design of ultrabright SERS probes with embedded reporters for bioimaging and photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 30387–30397. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, B.; Khanadeev, V.; Khlebtsov, N. Surface-enhanced Raman scattering inside Au@Ag core/shell nanorods. Nano Res. 2016, 9, 2303–2318. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Bratashov, D.N.; Khlebtsov, N.G. Tip functionalized Au@Ag nanorods as ultra-bright SERS probes for bioimaging in of-resonance mode. J. Phys. Chem. C 2018, 122, 17983–17993. [Google Scholar] [CrossRef]
- Xiang, Y.; Wu, X.; Liu, D.; Li, Z.; Chu, W.; Feng, L.; Zhang, K.; Zhou, W.; Xie, S. Gold nanorod-seeded growth of silver nanostructures: from homogeneous coating to anisotropic coating. Langmuir 2008, 24, 3465–3470. [Google Scholar] [CrossRef]
- Chen, Q.; Lin, T.; Huang, J.; Chen, Y.; Guo, L.; Fu, F. Colorimetric detection of residual hydrogen peroxide in soaked food based on Au@Ag nanorods. Anal. Methods 2018, 10, 504–507. [Google Scholar] [CrossRef]
- Vigderman, L.; Zubarev, E.R. High-yield synthesis of gold nanorods with longitudinal SPR peak greater than 1200 nm using hydroquinone as a reducing agent. Chem. Mater. 2013, 25, 1450–1457. [Google Scholar] [CrossRef]
- Abdelsalam, M.E. Surface enhanced Raman scattering of aromatic thiols adsorbed on nanostructured gold surfaces. Cent. Eur. J. Chem. 2009, 7, 446–453. [Google Scholar] [CrossRef]
- Orendorff, C.J.; Gearheart, L.; Jana, N.R.; Murphy, C.J. Aspect ratio dependence on surface enhanced Raman scattering using silver and gold nanorod substrates. Phys. Chem. Chem. Phys. 2006, 8, 165–170. [Google Scholar] [CrossRef]
- Jiang, X.; Tan, Z.; Lin, L.; He, J.; He, C.; Thackray, B.D.; Zhang, Y.; Ye, J. Surface-enhanced Raman nanoprobes with embedded standards for quantitative cholesterol detection. Small Methods 2018, 2, 1800182. [Google Scholar] [CrossRef]
- Meng, F.; Zhu, X.; Miao, P. Study of autocatalytic oxidation reaction of silver nanoparticles and the application for nonenzymatic H2O2 assay. Chem. Phys. Lett. 2015, 635, 213–216. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Obare, S.O.; Murphy, C.J. Anisotropic chemical reactivity of gold spheroids and nanorods. Langmuir 2002, 18, 922–927. [Google Scholar] [CrossRef]
- Mantri, Y.; Davidi, B.; Lemaster, J.E.; Hariric, A.; Jokerst, J.V. Iodide-doped precious metal nanoparticles: Measuring oxidative stress in vivo via photoacoustic imaging. Nanoscale 2020, 12, 10511–10520. [Google Scholar] [CrossRef] [PubMed]
- Bendich, A.; Machlin, L.; Scandurra, O.; Burton, G.; Wayner, D. The antioxidant role of vitamin C. Adv. Free Radic. Biol. Med. 1986, 2, 419–444. [Google Scholar] [CrossRef]



| Sample | Added, mM | Found, mM | Recovery (%) | Standard Deviation (n = 3), % |
|---|---|---|---|---|
| 1 | 0.5 | 0.45 | 90% | 4% |
| 2 | 2 | 2.15 | 107% | 5% |
| 3 | 2.5 | 2.7 | 108% | 3% |
| 4 | 3 | 3.5 | 115% | 12% |
| 5 | 5 | 5.5 | 110% | 6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khlebtsov, B.N.; Burov, A.M.; Zakharevich, A.M.; Khlebtsov, N.G. SERS and Indicator Paper Sensing of Hydrogen Peroxide Using Au@Ag Nanorods. Sensors 2022, 22, 3202. https://doi.org/10.3390/s22093202
Khlebtsov BN, Burov AM, Zakharevich AM, Khlebtsov NG. SERS and Indicator Paper Sensing of Hydrogen Peroxide Using Au@Ag Nanorods. Sensors. 2022; 22(9):3202. https://doi.org/10.3390/s22093202
Chicago/Turabian StyleKhlebtsov, Boris N., Andrey M. Burov, Andrey M. Zakharevich, and Nikolai G. Khlebtsov. 2022. "SERS and Indicator Paper Sensing of Hydrogen Peroxide Using Au@Ag Nanorods" Sensors 22, no. 9: 3202. https://doi.org/10.3390/s22093202
APA StyleKhlebtsov, B. N., Burov, A. M., Zakharevich, A. M., & Khlebtsov, N. G. (2022). SERS and Indicator Paper Sensing of Hydrogen Peroxide Using Au@Ag Nanorods. Sensors, 22(9), 3202. https://doi.org/10.3390/s22093202






