Monitoring Various Bioactivities at the Molecular, Cellular, Tissue, and Organism Levels via Biological Lasers
Abstract
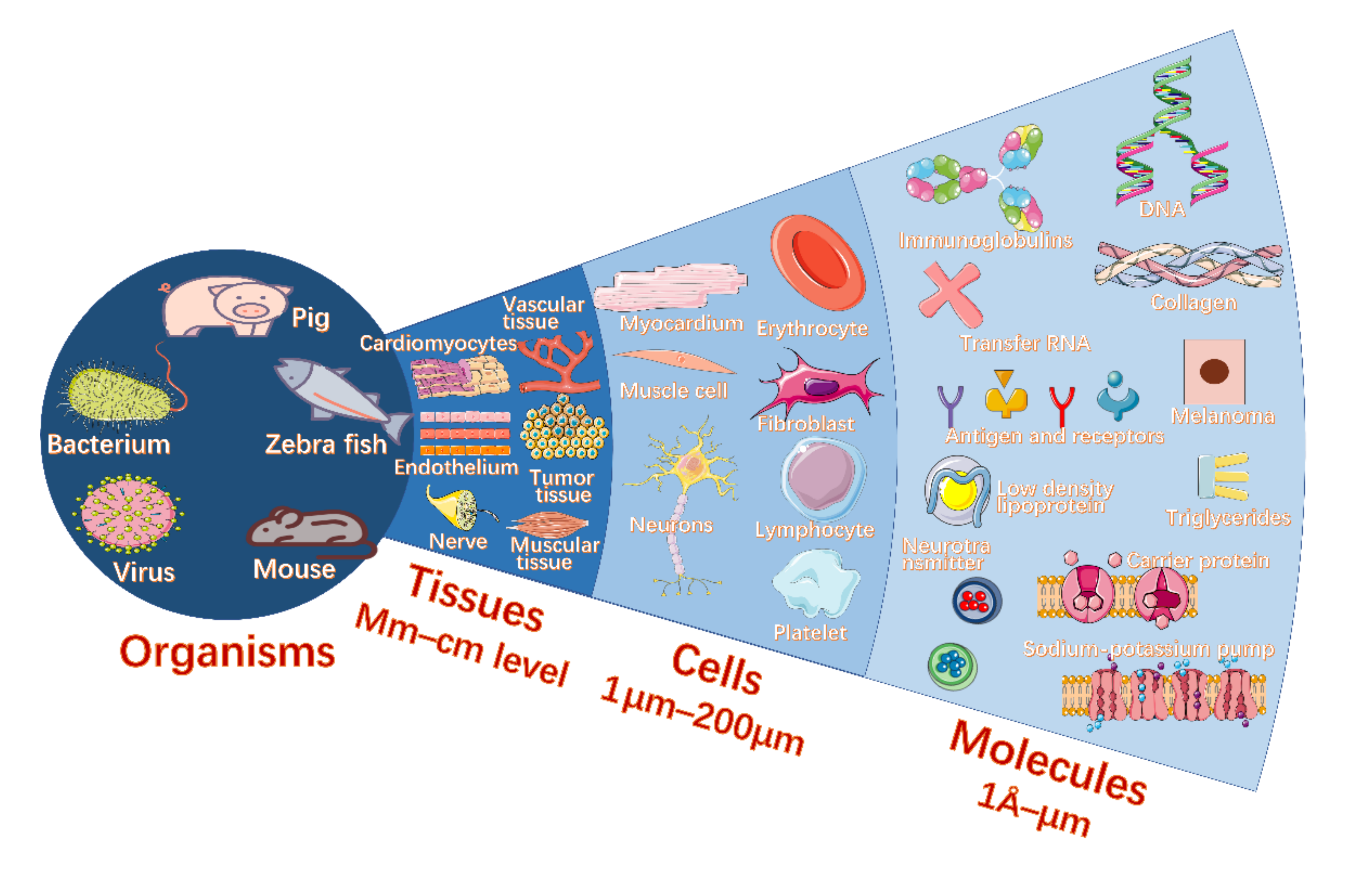
:1. Introduction
2. Biosensing with Biolasers
2.1. Environment and Molecular Detection with Biolasers
2.1.1. Biological Environment Detection
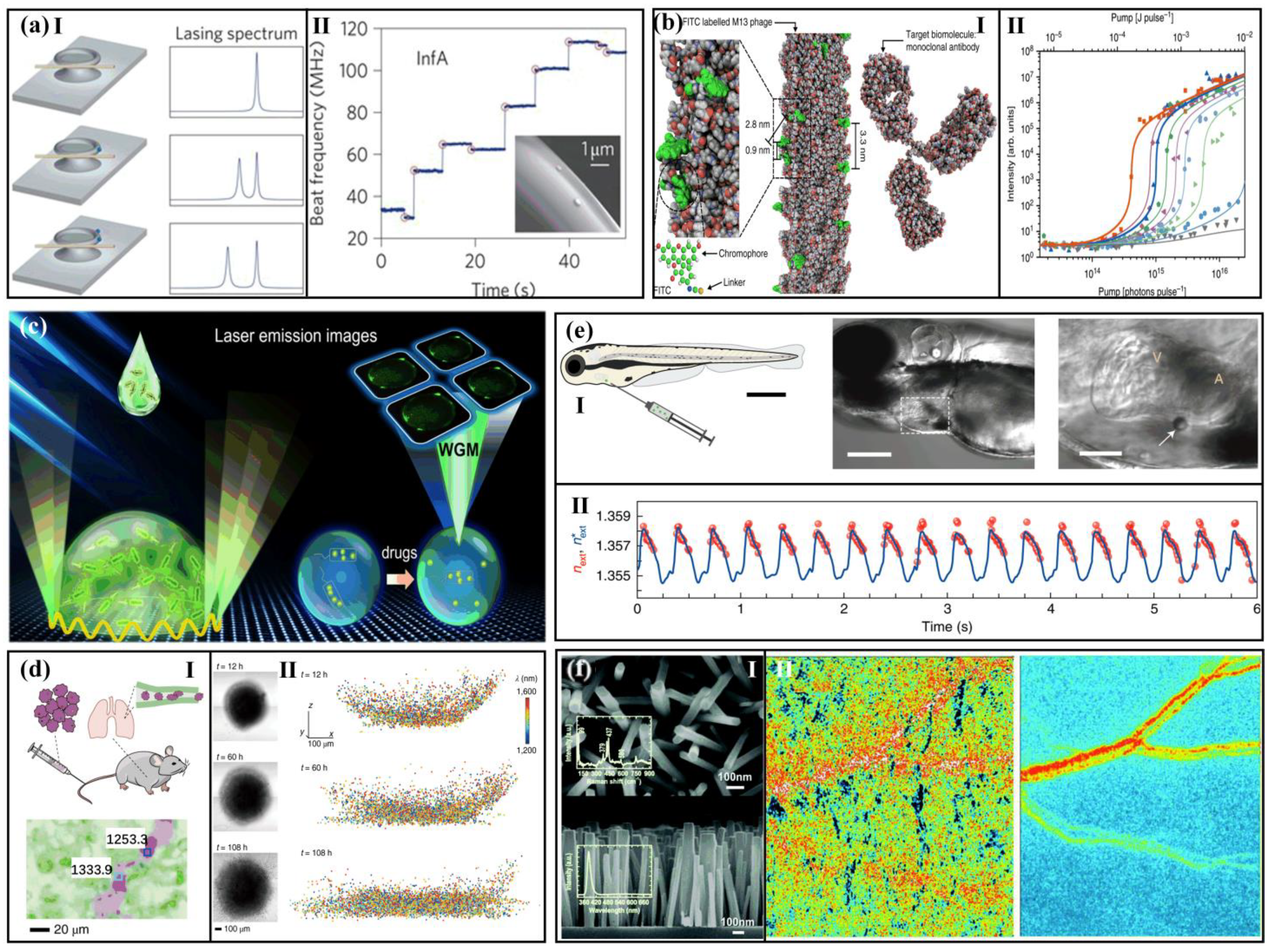
2.1.2. Protein and Protein Appendage Detection
2.1.3. DNA Detection
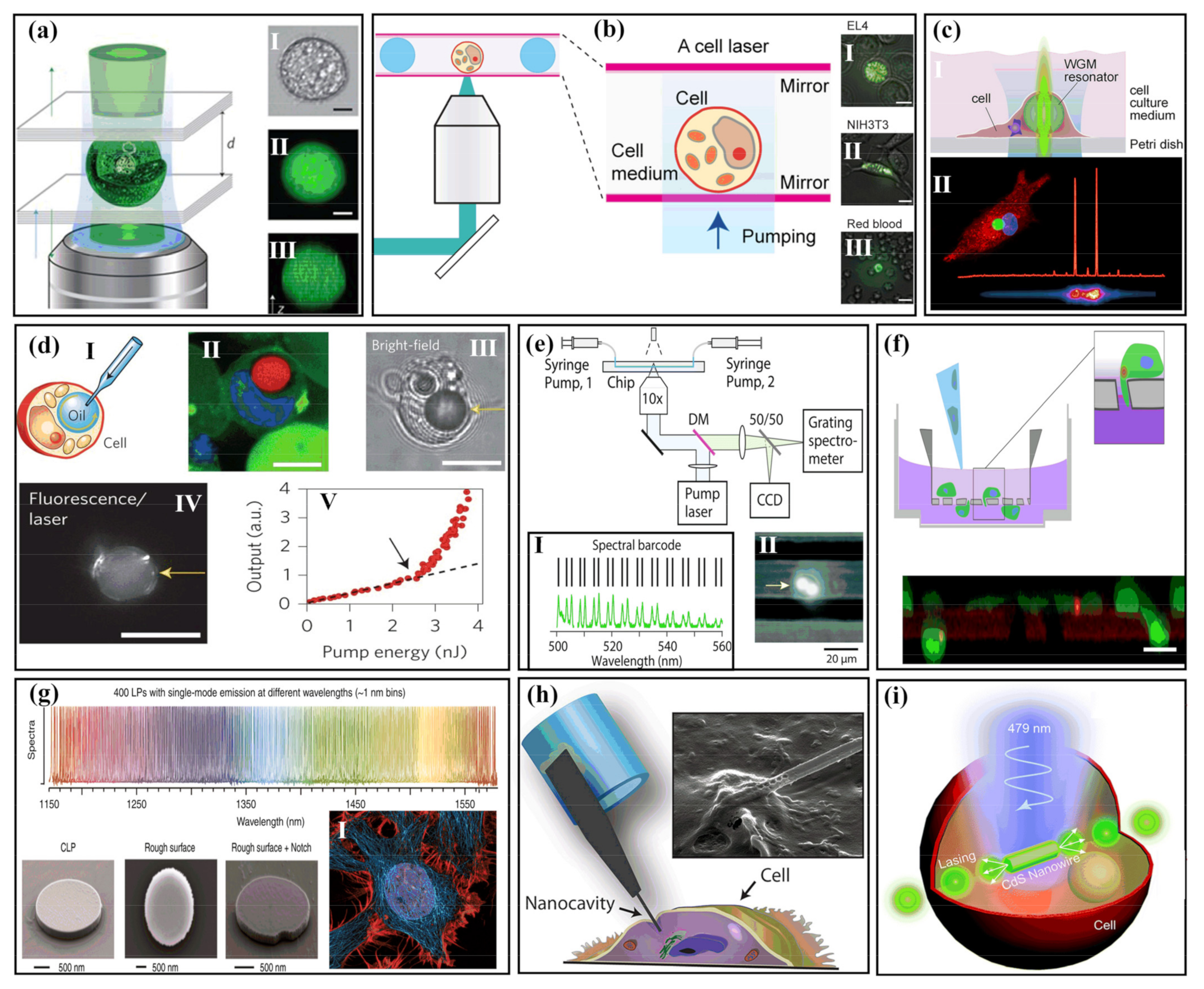
2.2. Cellular Detection with Biolasers
2.2.1. Extracellular Biolasers
2.2.2. Intracellular Biolasers
Intracellular Microlaser Based on Microspheres and Microdisks
Intracellular Biolasers Based on Nanowires
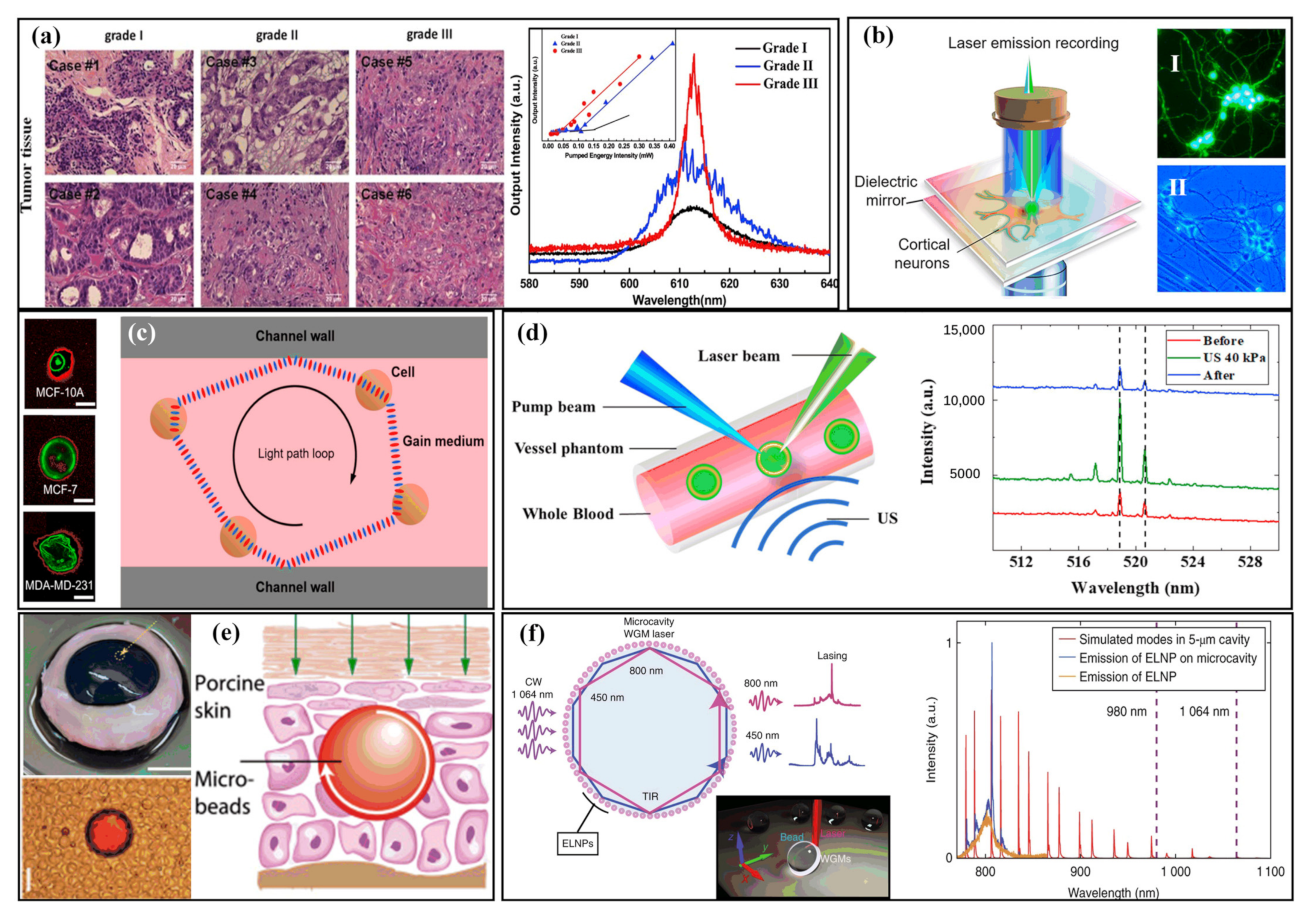
2.3. Tissue-Based Sensing with Biolasers
2.4. Monitoring the Life Activities of Living Organisms with Biolasers
3. Outlook, Challenges, and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laissue, P.P.; Alghamdi, R.A.; Tomanca, P.; Reynaud, E.G.; Shroff, H. Assessing phototoxicity in live fluorescence imaging. Nat. Methods 2017, 14, 657–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vangindertael, J.; Camacho, R.; Sempels, W.; Mizuno, H.; Dedecker, P.; Janssen, K. An introduction to optical super-resolution microscopy for the adventurous biologist. Methods Appl. Fluoresc. 2018, 6, 022003. [Google Scholar] [CrossRef] [PubMed]
- Schermelleh, L.; Ferrand, A.; Huser, T.; Eggeling, C.; Sauer, M.; Biehlmaier, O.; Drummen, G. Super-resolution microscopy demystified. Nat. Cell Biol. 2019, 21, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Betzig, E.; Patterson, G.; Sougrat, R.; Lindwasser, O.; Olenych, S.; Bonifacino, J.; Davidson, M.; Lippincott-Schwartz, J.; Hess, H. Imaging intracellular fluorescent proteins at nanometer resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef] [Green Version]
- Manley, S.; Gillette, J.M.; Lippincottschwartz, J. Single-Particle Tracking Photoactivated Localization Microscopy for Mapping Single-Molecule Dynamics. Methods Enzymol. 2010, 475, 109–120. [Google Scholar]
- Müller, D.T.; Schumann, C.; Kraegeloh, A. STED microscopy and its applications: New insights into cellular processes on the nanoscale. Chemphyschem 2012, 13, 1986–2000. [Google Scholar] [CrossRef]
- Vicidomini, G.; Bianchini, P.; Diaspro, A. STED super-resolved microscopy. Nat. Methods 2018, 15, 173–182. [Google Scholar] [CrossRef]
- Tam, J.; Merino, D. Stochastic optical reconstruction microscopy (STORM) in comparison with stimulated emission depletion (STED) and other imaging methods. J. Neurochem. 2015, 135, 643–658. [Google Scholar] [CrossRef]
- Wu, Y.; Shroff, H. Author Correction: Faster, sharper, and deeper: Structured illumination microscopy for biological imaging. Nat. Methods 2018, 15, 1011–1019. [Google Scholar] [CrossRef]
- Guo, Y.; Li, D.; Zhang, S.; Yang, Y.; Liu, J.J.; Wang, X.; Liu, C.; Milkie, D.E.; Moore, R.P.; Tulu, U.S. Visualizing intracellular organelle and cytoskeletal interactions at nanoscale resolution on millisecond timescales. Cell 2018, 175, 1430–1442. [Google Scholar] [CrossRef] [Green Version]
- Schöneberg, J.; Dambournet, D.; Liu, T.L.; Forster, R.; Hockemeyer, D.; Betzig, E.; Drubin, D.G. 4D cell biology: Big data image analytics and lattice light-sheet imaging reveal dynamics of clathrin-mediated endocytosis in stem cell-derived intestinal organoids. Biophys. J. 2019, 116, 167a. [Google Scholar] [CrossRef] [Green Version]
- Wldchen, F.; Schlegel, J.; Gtz, R.; Luciano, M.; Sauer, M. Whole-cell imaging of plasma membrane receptors by 3D lattice light-sheet dSTORM. Nat. Commun. 2020, 11, 887. [Google Scholar] [CrossRef]
- Mir, M.; Reimer, A.; Haines, J.E.; Li, X.Y.; Darzacq, X. Dense Bicoid hubs accentuate binding along the morphogen gradient. Genes Dev. 2017, 31, 1784–1794. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Zhong, G.; Zhuang, X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 2013, 339, 452–456. [Google Scholar] [CrossRef] [Green Version]
- Bger, C.; Hafner, A.S.; Schlichthrle, T.; Strauss, M.T.; Malkusch, S.; Endesfelder, U.; Jungmann, R.; Schuman, E.M.; Heilemann, M. Super-resolution imaging and estimation of protein copy numbers at single synapses with DNA-point accumulation for imaging in nanoscale topography. Neurophotonics 2019, 6, 035008. [Google Scholar] [CrossRef]
- Hak, L.S.; Chaoyi, J.; En, C.; Pinghua, G.; Yuji, I.; Wen, T.K.; De, T.A.A.; Duncan, N.; Murat, B.; Okunola, J. Super-resolution imaging of synaptic and Extra-synaptic AMPA receptors with different-sized fluorescent probes. eLife 2017, 6, 27744. [Google Scholar]
- Schedin-Weiss, S.; Caesar, I.; Winblad, B.; Blom, H.; Tjernberg, L.O. Super-resolution microscopy reveals γ-secretase at both sides of the neuronal synapse. Acta Neuropathol. Commun. 2016, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.C.; Legant, W.R.; Wang, K.; Shao, L.; Milkie, D.E.; Davidson, M.W.; Janetopoulos, C.; Wu, X.S.; Hammer, J.A.; Liu, Z. Lattice light-sheet microscopy: Imaging molecules to embryos at high spatiotemporal resolution. Science 2014, 346, 1257998. [Google Scholar] [CrossRef] [Green Version]
- Aguet, F.; Upadhyayula, S.; Gaudin, R.; Chou, Y.Y.; Kirchhausen, T. Membrane dynamics of dividing cells imaged by lattice light-sheet microscopy. Mol. Biol. Cell 2016, 27, 3418–3435. [Google Scholar] [CrossRef]
- Macropinosome formation by tent pole ruffling in macrophages. J. Cell Biol. 2018, 217, 3873–3885. [CrossRef] [Green Version]
- GoTtfert, F.; Wurm, C.A.; Mueller, V.; Berning, S.; Cordes, V.C.; Honigmann, A.; Hell, S.W. Coaligned dual-channel STED nanoscopy and molecular diffusion analysis at 20 nm resolution. Biophys. J. 2013, 105, L01–L03. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, M.; Eggeling, C.; Jakobs, S.; Hell, S.W.; Hofmann, M.; Eggeling, C.; Jakobs, S.; Hell, S.W. Breaking the diffraction barrier in fluorescence microscopy at low light intensities by using reversibly photoswitchable proteins. Proc. Natl. Acad. Sci. USA 2006, 102, 17565–17569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brakemann, T.; Stiel, A.C.; Weber, G.; Andresen, M.; Testa, I.; Grotjohann, T.; Leutenegger, M.; Plessmann, U.; Urlaub, H.; Eggeling, C. A reversibly photoswitchable GFP-like protein with fluorescence excitation decoupled from switching. Nat. Biotechnol. 2011, 29, 942–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balzarotti, F.; Eilers, Y.; Gwosch, K.C.; Gynna, A.H.; Westphal, V.; Stefani, F.D.; Elf, J.; Hell, S.W. Nanometer resolution imaging and tracking of fluorescent molecules with minimal photon fluxes. Science 2017, 355, 606. [Google Scholar] [CrossRef] [Green Version]
- Dempsey; Graham; Vaughan; Joshua; Chen; Kok; Hao; Bates Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging. Nat. Methods 2011, 8, 1027–1036. [CrossRef]
- Butkevich, A.N.; Mitronova, G.Y.; Sidenstein, S.C.; Klocke, J.L.; Kamin, D.; Meineke, D.; Este, E.D.; Kraemer, P.; Danzl, J.G.; Belov, V.N. Fluorescent rhodamines and fluorogenic carbopyronines for super-resolution STED microscopy in living cells. Angew. Chem. Int. Ed. 2016, 55, 3290–3294. [Google Scholar] [CrossRef] [Green Version]
- Bottanelli, F.; Kromann, E.B.; Allgeyer, E.S.; Erdmann, R.S.; Baguley, S.W.; Sirinakis, G.; Schepartz, A.; Baddeley, D.; Toomre, D.K.; Rothman, J.E. Two-colour live-cell nanoscale imaging of intracellular targets. Nat. Commun. 2016, 7, 10778. [Google Scholar] [CrossRef] [Green Version]
- Winter, F.R.; Loidolt, M.; Westphal, V.; Butkevich, A.N.; Gregor, C.; Sahl, S.J.; Hell, S.W. Multicolour nanoscopy of fixed and living cells with a single STED beam and hyperspectral detection. Sci. Rep. 2017, 7, 46492. [Google Scholar] [CrossRef] [Green Version]
- Gibson, T.J.; Seiler, M.; Veitia, R.A. The transience of transient overexpression. Nat. Methods 2013, 10, 715–721. [Google Scholar] [CrossRef]
- Gong, C.; Gong, Y.; Oo, M.K.; Wu, Y.; Rao, Y.; Tan, X.; Fan, X. Sensitive sulfide ion detection by optofluidic catalytic laser using horseradish peroxidase (HRP) enzyme. Biosens. Bioelectron. 2017, 96, 351. [Google Scholar] [CrossRef]
- Rivera, J.A.; Eden, J.G. Flavin mononucleotide biomolecular laser: Longitudinal mode structure, polarization, and temporal characteristics as probes of local chemical environment. Opt. Express 2016, 24, 10858–10868. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Gong, Y.; Zhao, X.; Luo, Y.; Chen, Q.; Tan, X.; Wu, Y.; Fan, X.; Peng, G.-D.; Rao, Y.-J. Distributed fibre optofluidic laser for chip-scale arrayed biochemical sensing. Lab Chip 2018, 18, 2741–2748. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, W.; Yan, Y.; Yi, J.; Dong, H.; Wang, K.; Yao, J.; Zhao, Y.S. Proton-controlled organic microlaser switch. Acs Nano 2018, 12, 5734–5740. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Xu, A.; Wang, L.; Zhang, L.; Liu, S.; Liu, Y.; Li, H. Detecting enzymatic reactions in penicillinase via liquid crystal microdroplet-based pH sensor. Sens. Actuators B Chem. 2018, 258, 1090–1098. [Google Scholar] [CrossRef]
- Watanabe, K.; Kishi, Y.; Hachuda, S.; Watanabe, T.; Mai, S.; Nishijima, Y.; Baba, T. Simultaneous detection of refractive index and surface charges in nanolaser biosensors. Appl. Phys. Lett. 2015, 106, 021106. [Google Scholar] [CrossRef]
- Watanabe, T.; Saijo, Y.; Hasegawa, Y.; Watanabe, K.; Nishijima, Y.; Baba, T. Ion-sensitive photonic-crystal nanolaser sensors. Opt. Express 2017, 25, 24469–24479. [Google Scholar] [CrossRef]
- Martinez-Perdiguero, J.; Merino, S.; Morales-Vidal, M.; Boj, P.G.; Quintana, J.A.; Villalvilla, J.M.; Díaz-García, M. Organic distributed feedback laser for label-free biosensing of ErbB2 protein biomarker. Sens. Actuators B Chem. 2016, 223, 261–265. [Google Scholar]
- Gaio, M.; Caixeiro, S.; Marelli, B.; Omenetto, F.G.; Sapienza, R. Gain-based mechanism for pH sensing based on random lasing. Phys. Rev. Appl. 2017, 7, 034005. [Google Scholar] [CrossRef] [Green Version]
- Abegão, L.M.G.; Pagani, A.A.C.; Zílio, S.C.; Alencar, M.A.R.C.; Rodrigues, J.J. Measuring milk fat content by random laser emission. Sci. Rep. 2016, 6, 35119. [Google Scholar] [CrossRef] [Green Version]
- Wonsuk, L.; Xudong, F. Intracavity DNA melting analysis with optofluidic lasers. Anal. Chem. 2012, 84, 9558–9563. [Google Scholar]
- Yang, X.; Gong, C.; Wang, Y.; Luo, Y.; Rao, Y.J.; Peng, G.D.; Gong, Y. A sequentially bioconjugated optofluidic laser for wash-out-free and rapid biomolecular detection. Lab Chip 2021, 21, 1686–1693. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, Z.; Guan, P.; Wu, X.; Chen, Y. Lasing-encoded microsensor driven by interfacial cavity resonance energy transfer. Adv. Opt. Mater. 2020, 8, 1901596. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Gong, X.; Yuan, Z.; Chen, Y.C. Bio-electrostatic sensitive droplet lasers for molecular detection. Nanoscale Adv. 2020, 2, 2713–2719. [Google Scholar] [CrossRef]
- Sun, Y.; Shopova, S.I.; Wu, C.-S.; Stephen, A.; Fan, X. Bioinspired optofluidic FRET lasers via DNA scaffolds. Proc. Natl. Acad. Sci. USA 2010, 107, 16039–16042. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Chen, Q.; Fan, X.; Yoon, D.K. Digital DNA detection based on a compact optofluidic laser with ultra-low sample consumption. Lab Chip 2017, 16, 4770–4776. [Google Scholar] [CrossRef]
- Duan, R.; Hao, X.; Li, Y.; Li, H. Detection of acetylcholinesterase and its inhibitors by liquid crystal biosensor based on whispering gallery mode. Sens. Actuators B Chem. 2020, 308, 127672. [Google Scholar] [CrossRef]
- Ouyang, X.; Liu, T.; Zhang, Y.; He, J.; He, Z.; Zhang, A.P.; Tam, H.Y. Ultrasensitive optofluidic enzyme-linked immunosorbent assay by on-chip integrated polymer whispering-gallery-mode microlaser sensors. Lab Chip 2020, 20, 2438–2446. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, P.D.X. Distinguishing DNA by analog-to-digital-like conversion by using optofluidic lasers. Angew. Chem. 2012, 51, 1236–1239. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lee, W.; Fan, X. Bio-switchable optofluidic lasers based on DNA Holliday junctions. Lab Chip 2012, 12, 3673–3675. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Sun, Y.; Ritt, M.; Sivaramakrishnan, S.; Fan, X. Highly sensitive fluorescent protein FRET detection using optofluidic lasers. Lab Chip 2013, 13, 2679–2681. [Google Scholar] [CrossRef]
- Yuan, Z.; Cheng, X.; Zhou, Y.; Tan, X.; Chen, Y.C. Distinguishing small molecules in microcavity with molecular laser polarization. ACS Photonics 2020, 7, 1908–1914. [Google Scholar] [CrossRef]
- Tan, X.; Chen, Q.; Zhu, H.; Zhu, S.; Fan, X. A Fast and reproducible ELISA laser platform for ultrasensitive protein quantification. ACS Sens. 2019, 5, 110–117. [Google Scholar] [CrossRef]
- Duan, R.; Li, Y.; He, Y.; Yuan, Y.; Li, H. Quantitative and sensitive detection of lipase using a liquid crystal microfiber biosensor based on the whispering-gallery mode. Analyst 2020, 145, 7595–7602. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Fan, Y.; Liu, Z.; Zhao, Y.S. Smart protein-based biolasers: An alternative way to protein conformation detection. ACS Appl. Mater. Interfaces 2021, 13, 19187–19192. [Google Scholar] [CrossRef]
- Yang, X.; Shu, W.; Wang, Y.; Gong, Y.; Gong, C.; Chen, Q.; Tan, X.; Peng, G.D.; Fan, X.; Rao, Y.J. Turbidimetric inhibition immunoassay revisited to enhance its sensitivity via an optofluidic laser. Biosens. Bioelectron. 2019, 131, 60–66. [Google Scholar] [CrossRef]
- Wu, X.; Oo, M.; Reddy, K.; Chen, Q.; Sun, Y.; Fan, X. Optofluidic laser for dual-mode sensitive biomolecular detection with a large dynamic range. Nat. Commun. 2014, 5, 3779. [Google Scholar] [CrossRef] [Green Version]
- Toropovo, N.; Vollmer, F. Whispering-gallery microlasers for cell tagging and barcoding:the prospects for in vivo biosensing. Light Sci. Appl. 2021, 10, 77. [Google Scholar] [CrossRef]
- Nizamoglu, S.; Lee, K.B.; Gather, M.C.; Kim, K.S.; Jeon, M.; Kim, S.; Humar, M.; Yun, S.H. A simple approach to biological single-cell lasers via intracellular dyes. Adv. Opt. Mater. 2015, 3, 1197–1200. [Google Scholar] [CrossRef]
- Humar, M.; Gather, M.C.; Yun, S.H. Cellular dye lasers: Lasing thresholds and sensing in a planar resonator. Opt. Express 2015, 23, 27865–27879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGloin, D. Cellular lasers. Nat. Photonics 2015, 9, 559–560. [Google Scholar] [CrossRef]
- Humar, M.; Yun, S.H. Intracellular microlasers. Nat. Photonics 2015, 9, 572–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Man, Z.; Xu, Z.; Feng, C.; Yong, Y.; Liao, Q.; Xu, W.; Zheng, L.; Fu, H. Intracellular near-infrared microlaser probes based on organic microsphere-SiO2 Core-shell structures for cell tagging and tracking. ACS Appl. Mater. Interfaces 2018, 10, 32981–32987. [Google Scholar]
- Himmelhaus, M.; Francois, A. In-vitro sensing of biomechanical forces in live cells by a whispering gallery mode biosensor. Biosens. Bioelectron. 2010, 25, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Steude, A.; Liehm, P.; Kronenberg, N.M.; Karl, M.; Campbell, E.C.; Powis, S.J.; Gather, M.C. Lasing within live cells containing intracellular optical microresonators for barcode-type cell tagging and tracking. Nano Lett. 2015, 15, 5647–5652. [Google Scholar] [CrossRef] [Green Version]
- Yan, R.; Park, J.H.; Choi, Y.; Heo, C.J.; Yang, S.M.; Lee, L.P.; Yang, P. Nanowire-based single-cell endoscopy. Nat. Nanotechnol. 2011, 7, 191–196. [Google Scholar] [CrossRef]
- Fikouras, A.H.; Schubert, M.; Karl, M.; Kumar, J.D.; Powis, S.J.; Falco, A.D.; Gather, M.C. Non-obstructive intracellular nanolasers. Nat. Commun. 2018, 9, 4817. [Google Scholar] [CrossRef] [Green Version]
- Septiadi, D.; Barna, V.; Saxena, D.; Sapienza, R.; Cola, L.D. Biolasing from individual cells in a low-Q resonator enables spectral fingerprinting. Adv. Opt. Mater. 2020, 8, 1901573. [Google Scholar] [CrossRef] [Green Version]
- Gather, M.C.; Yun, S.H. Single-cell biological lasers. Nat. Photonics 2011, 5, 406–410. [Google Scholar] [CrossRef]
- Shambat, G. Single-cell photonic nanocavity probes. Nano Lett. 2014, 13, 4999–5005. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Chen, Q.; Xu, P.; Chen, Y.C.; Wu, B.; Coleman, R.M.; Tong, L.; Fan, X. Nanowire lasers as intracellular probes. Nanoscale 2018, 10, 9729–9735. [Google Scholar] [CrossRef]
- Humar, M.; Upadhya, A.; Yun, S.H. Spectral reading of optical resonance-encoded cells in microfluidics. Lab Chip 2017, 17, 2777–2784. [Google Scholar] [CrossRef]
- Schubert, M.; Volckaert, K.; Karl, M.; Morton, A.; Gather, M.C. Lasing in live mitotic and non-phagocytic cells by efficient delivery of microresonators. Sci. Rep. 2017, 7, 40877. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Chen, Y.C.; Zhang, Z.; Wu, B.; Coleman, R.; Fan, X. An integrated microwell array platform for cell lasing analysis. Lab Chip 2017, 17, 2814–2820. [Google Scholar] [CrossRef]
- Ta, V.D.; Caixeiro, S.; Fernandes, F.M.; Sapienza, R. Microsphere solid-state biolasers. Adv. Opt. Mater. 2017, 5, 8. [Google Scholar] [CrossRef]
- Tang, S.J.; Dannenberg, P.H.; Liapis, A.C.; Martino, N.; Yun, S.H. Laser particles with omnidirectional emission for cell tracking. Light Sci. Appl. 2021, 10, 23. [Google Scholar] [CrossRef]
- Chen, Y.C.; Li, X.; Zhu, H.; Weng, W.H.; Fan, X. Monitoring neuron activities and interactions with laser emissions. ACS Photonics 2020, 7, 2182–2189. [Google Scholar] [CrossRef]
- Humar, M.; Dobravec, A.; Zhao, X.; Yun, S.H. Biomaterial microlasers implantable in the cornea, skin, and blood. Optica 2017, 4, 1080–1085. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, Q.; Tan, X.; Chen, G.; Bergin, I.; Aslam, M.N.; Fan, X. Chromatin laser imaging reveals abnormal nuclear changes for early cancer detection. Biomed. Opt. Express 2019, 10, 838–854. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tan, X.; Sun, Q.; Chen, Q.; Fan, X. Laser-emission imaging of nuclear biomarkers for high-contrast cancer screening and immunodiagnosis. Nat. Biomed. Eng. 2017, 1, 724–735. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Chen, Q.; Fan, X. Lasing in blood. Optica 2016, 3, 809–815. [Google Scholar] [CrossRef]
- Song, Q.; Xiao, S.; Xu, Z.; Liu, J.; Sun, X.; Drachev, V.; Shalaev, V.M.; Akkus, O.; Kim, Y.L. Random lasing in bone tissue. Opt. Lett. 2010, 35, 1425–1427. [Google Scholar] [CrossRef]
- Polson, R.C.; Vardeny, Z.V. Random lasing in human tissues. Appl. Phys. Lett. 2004, 85, 1289–1291. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, Z.; Qiu, Z.; Zhang, P.; Wu, J.; Zhang, D.; Xiang, T. Random lasing in human tissues embedded with organic dyes for cancer diagnosis. Sci. Rep. 2017, 7, 8385. [Google Scholar] [CrossRef]
- Li, X.; Qin, Y.; Tan, X.; Chen, Y.C.; Chen, Q.; Weng, W.H.; Wang, X.; Fan, X. Ultrasound modulated droplet lasers. ACS Photonics 2019, 6, 531–537. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, Q.; Zhang, T.; Wang, W.; Fan, X. Versatile tissue lasers based on high-Q Fabry–Pérot microcavities. Lab Chip 2017, 17, 538–548. [Google Scholar] [CrossRef] [Green Version]
- Nadkarni, S.K. Characterization of atherosclerotic plaques by laser speckle imaging. Circulation 2005, 112, 885–892. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Hu, S.; Ren, J.; Cheng, X.; Hu, Z.; Wang, N.; Zhang, H.; Lam, R.H.W.; Tam, H.-Y. Biofluidic random laser cytometer for biophysical phenotyping of cell suspensions. ACS Sens. 2019, 4, 832–840. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Bravo, A.; Yao, K.; Barnard, E.S.; Borys, N.J.; Levy, E.S.; Tian, B.; Tajon, C.A.; Moretti, L.; Altoe, M.; Aloni, S.; et al. Continuous-wave upconverting nanoparticle microlasers. Nat. Nanotechnol. 2018, 13, 572–577. [Google Scholar] [CrossRef] [Green Version]
- Martino, N.; Kwok, S.; Liapis, A.C.; Forward, S.; Yun, S.H. Wavelength-encoded laser particles for massively-multiplexed cell tagging. Nat. Photonics 2019, 13, 720–727. [Google Scholar] [CrossRef]
- Lee, Y.J.; Yeh, T.W.; Yang, Z.P.; Yao, Y.C.; Chang, C.Y.; Tsai, M.T.; Sheu, J.K. A curvature-tunable random laser. Nanoscale 2019, 11, 3534–3545. [Google Scholar] [CrossRef]
- He, L.; Zdemir, A.K.; Zhu, J.; Kim, W.; Lan, Y. Detecting single viruses and nanoparticles using whispering gallery microlasers. Nat. Nanotechnol. 2011, 6, 428–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hales, J.E.; Matmon, G.; Dalby, P.A.; Ward, J.M.; Aeppli, G. Virus lasers for biological detection. Nat. Commun. 2019, 10, 3594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, X.; Feng, S.; Qiao, Z.; Chen, Y.C. Imaging-based optofluidic biolaser array encapsulated with dynamic living organisms. Anal. Chem. 2021, 93, 5823–5830. [Google Scholar] [CrossRef] [PubMed]
- Gather, M.C.; Yun, S.H. Lasing from Escherichia coli bacteria genetically programmed to express green fluorescent protein. Opt. Lett. 2011, 36, 3299–3301. [Google Scholar] [CrossRef]
- Galanzha, E.I.; Weingold, R.; Nedosekin, D.A.; Sarimollaoglu, M.; Nolan, J.; Harrington, W.; Kuchyanov, A.S.; Parkhomenko, R.G.; Watanabe, F.; Nima, Z. Spaser as a biological probe. Nat. Commun. 2017, 8, 15528. [Google Scholar] [CrossRef] [Green Version]
- Schubert, M.; Woolfson, L.; Barnard, I.; Dorward, A.M.; Casement, B.; Morton, A.; Robertson, G.B.; Appleton, P.L.; Miles, G.B.; Tucker, C.S. Monitoring contractility in cardiac tissue with cellular resolution using biointegrated microlasers. Nat. Photonics 2020, 14, 452–458. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, X.; Yan, S.; Zhang, X.; Zhai, T. Single-mode lasing in plasmonic-enhanced woven microfibers for multifunctional sensing. ACS Sens. 2021, 6, 3416–3423. [Google Scholar] [CrossRef]
- Gong, C.; Qiao, Z.; Yuan, Z.; Huang, S.-H.; Wang, W.; Wu, P.C.; Chen, Y.-C. Topological encoded vector beams for monitoring amyloid-lipid interactions in microcavity. Adv. Sci. 2021, 8, 2100096. [Google Scholar] [CrossRef]
- Li, Z.; Psaltis, D. Optofluidic dye lasers. Microfluid. Nanofluidics 2008, 4, 145–158. [Google Scholar] [CrossRef]
- Lee, W.; Hao, L.; Suter, J.D.; Reddy, K.; Sun, Y.; Fan, X. Tunable single mode lasing from an on-chip optofluidic ring resonator laser. Appl. Phys. Lett. 2011, 98, 106–171. [Google Scholar]
- Yang, A.H.; Erickson, D. Optofluidic ring resonator switch for optical particle transport. Lab Chip 2010, 10, 769–774. [Google Scholar] [CrossRef]
- Gong, C.; Gong, Y.; Yang, X.; Peng, G.D.; Rao, Y.J. Pseudo whispering gallery mode optofluidic lasing based on air-clad optical fiber. J. Lightwave Technol. 2018, 37, 2623–2627. [Google Scholar] [CrossRef]
- Hs, A.; Hg, A.; Jie, Y.B.; Zhi, W.A.; Xl, A.; Ygl, A. A multi-sample analysis method with spatial resolution based on a single-longitudinal-mode fiber optofluidic microring laser. Opt. Laser Technol. 2021, 138, 106835. [Google Scholar]
- Chen, Q.; Kiraz, A.; Fan, X. Optofluidic FRET lasers using aqueous quantum dots as donors. Lab Chip 2016, 16, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Lewisgb, B.J. Nucleic acid analysis. Anal. Chem. 1999, 71, 425–438. [Google Scholar]
- Veer, L.J.V.T.; Bernards, R. Enabling personalized cancer medicine through analysis of gene-expression patterns. Nature 2008, 452, 564–570. [Google Scholar] [CrossRef]
- Jones, P.A.; Baylin, S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002, 3, 415–428. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, X.; Yuan, Z.; Wang, W.; Chen, Y.-C. DNA self-switchable microlaser. ACS Nano 2020, 14, 16122–16130. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Gong, C.; Mao, J.; Gong, Y. DC-biased optofluidic biolaser for uric acid detection. J. Lightwave Technol. 2019, 38, 1557–1563. [Google Scholar] [CrossRef]
- Hutchinson, M.R.; Goldys, E.M.; Gong, C.; Gong, Y.; Oo, M.; Wu, Y.; Rao, Y.; Fan, X. Enzyme catalyzed optofluidic biolaser for sensitive ion concentration detection. SPIE BioPhotonics Australas. 2016, 10013, 100131S. [Google Scholar]
- Aubry, G.; Kou, Q.; Soto-Velasco, J.; Wang, C.; Mean, S.; He, J.J.; Haghiri-Gosnet, A.M. A multicolor microfluidic droplet dye laser with single mode emission. Appl. Phys. Lett. 2011, 98, 111111. [Google Scholar] [CrossRef]
- Matsko, A.B.; Ilchenko, V.S. Optical resonators with whispering-gallery modes-part I: Basics. IEEE J. Sel. Top. Quantum Electron. 2006, 12, 3–14. [Google Scholar] [CrossRef]
- Jiang, X.; Qavi, A.J.; Huang, S.H.; Yang, L. Whispering-gallery sensors. Matter 2020, 3, 371–392. [Google Scholar] [CrossRef]
- Ward, J.; Benson, O. WGM microresonators: Sensing, lasing and fundamental optics with microspheres. Laser Photonics Rev. 2011, 5, 553–570. [Google Scholar] [CrossRef]
- Yang, J.; Guo, L.J. Optical sensors based on active microcavities. IEEE J. Sel. Top. Quantum Electron. 2006, 12, 143–147. [Google Scholar] [CrossRef]
- Mai, H.H.; Trong, T.N.; Giang, K.M.; Xuan, T.D.; Ta, D. Chicken albumen based whispering gallery mode microlasers. Soft Matter 2020, 16, 9069–9073. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Mai, H.H.; Nguyen, T.V.; Duong, D.C.; Ta, V.D. Egg white based biological microlasers. J. Phys. D Appl. Phys. 2020, 53, 445104. [Google Scholar] [CrossRef]
- Nizamoglu, S.; Gather, M.C.; Yun, S.H. Biomaterial laser: All-biomaterial laser using vitamin and biopolymers. Adv. Mater. 2013, 25, 5988. [Google Scholar] [CrossRef]
- Wei, Y.; Lin, X.; Wei, C.; Zhang, W.; Yan, Y.; Zhao, Y.S. Starch-based biological microlasers. Acs Nano 2016, 11, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Ta, V.D.; Caixeiro, S.; Saxena, D.; Sapienza, R. Biocompatible polymer and protein microspheres with inverse photonic glass structure for random micro-biolasers. Adv. Photonics Res. 2021, 2, 2100036. [Google Scholar] [CrossRef]
- Lahoz, F.; Acebes, A.; Gonzalez-Hernandez, T.; Armas-Rillo, S.D.; Soler-Carracedo, K.; Cuesto, G.; Mesa-Infante, V. Random lasing in brain tissues. Org. Electron. 2019, 75, 105389. [Google Scholar] [CrossRef]
- Wang, L.; Da, L.; He, N.; Jacques, S.L.; Thomsen, S.L. Biological laser action. Appl. Opt. 1996, 35, 1775–1779. [Google Scholar] [CrossRef]
- Briones-Herrera, J.C.; Cuando-Espitia, N.; Sanchez-Arevalo, F.M.; Hernandez-Cordero, J. Evaluation of mechanical behavior of soft tissue by means of random laser emission. Rev. Sci. Instrum. 2013, 84, 104301. [Google Scholar] [CrossRef]
- Lahoz, F.; Martín, I.R.; Urgellés, M.; Marrero-Alonso, J.; Marín, R.; Saavedra, C.J.; Boto, A.; Díaz, M. Random laser in biological tissues impregnated with a fluorescent anticancer drug. Laser Phys. Lett. 2015, 12, 045805. [Google Scholar] [CrossRef]
- Ignesti, E.; Tommasi, F.; Fini, L.; Martelli, F.; Azzali, N.; Cavalieri, S. A new class of optical sensors: A random laser based device. Sci. Rep. 2016, 6, 35225. [Google Scholar] [CrossRef] [Green Version]
- Kavčič, A.; Garvas, M.; Marinčič, M.; Unger, K.; Coclite, A.M.; Majaron, B.; Humar, M. Deep tissue localization and sensing using optical microcavity probes. Nat. Commun. 2022, 13, 1269. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, H.; Dai, H.; Chen, X. Monitoring Various Bioactivities at the Molecular, Cellular, Tissue, and Organism Levels via Biological Lasers. Sensors 2022, 22, 3149. https://doi.org/10.3390/s22093149
Shan H, Dai H, Chen X. Monitoring Various Bioactivities at the Molecular, Cellular, Tissue, and Organism Levels via Biological Lasers. Sensors. 2022; 22(9):3149. https://doi.org/10.3390/s22093149
Chicago/Turabian StyleShan, Hongrui, Hailang Dai, and Xianfeng Chen. 2022. "Monitoring Various Bioactivities at the Molecular, Cellular, Tissue, and Organism Levels via Biological Lasers" Sensors 22, no. 9: 3149. https://doi.org/10.3390/s22093149
APA StyleShan, H., Dai, H., & Chen, X. (2022). Monitoring Various Bioactivities at the Molecular, Cellular, Tissue, and Organism Levels via Biological Lasers. Sensors, 22(9), 3149. https://doi.org/10.3390/s22093149







