One-Step Fabrication of Highly Sensitive Tris(2,2′-bipyridyl)ruthenium(II) Electrogenerated Chemiluminescence Sensor Based on Graphene-Titania-Nafion Composite Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrumentation
2.3. Preparation of ECL Sensor
2.4. ECL Experiments
3. Results and Discussion
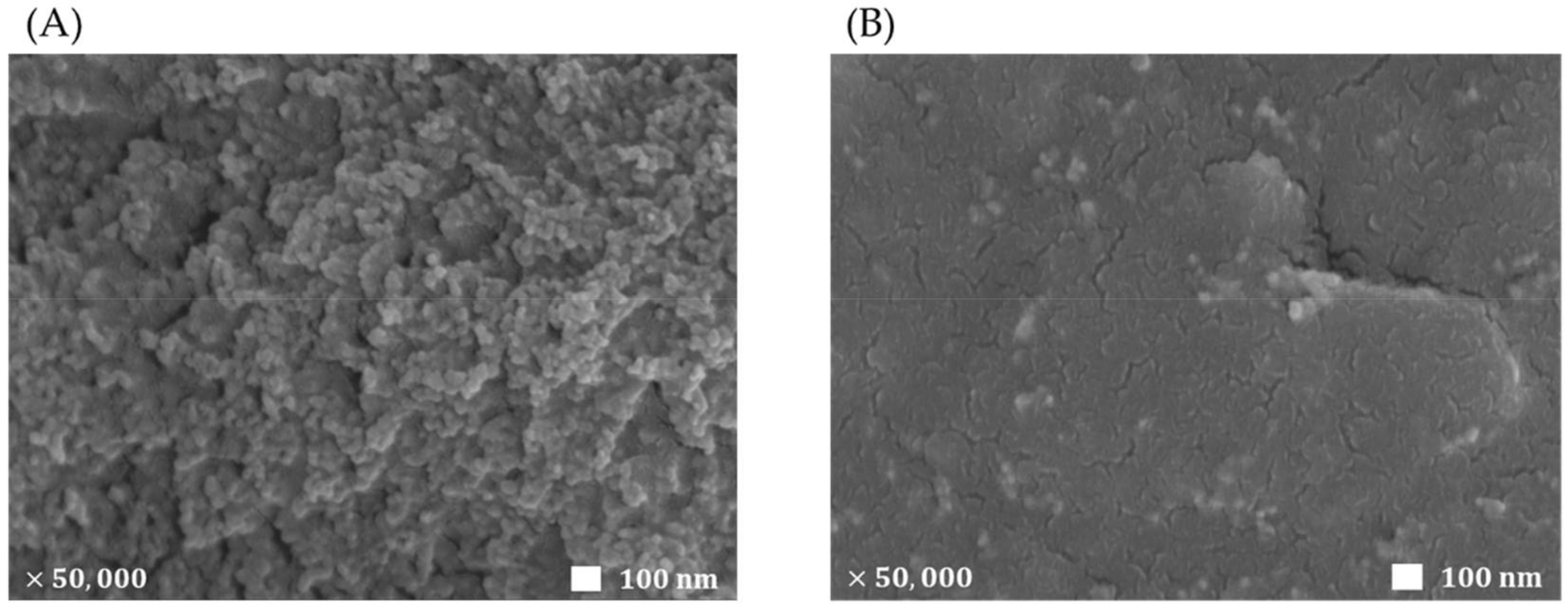
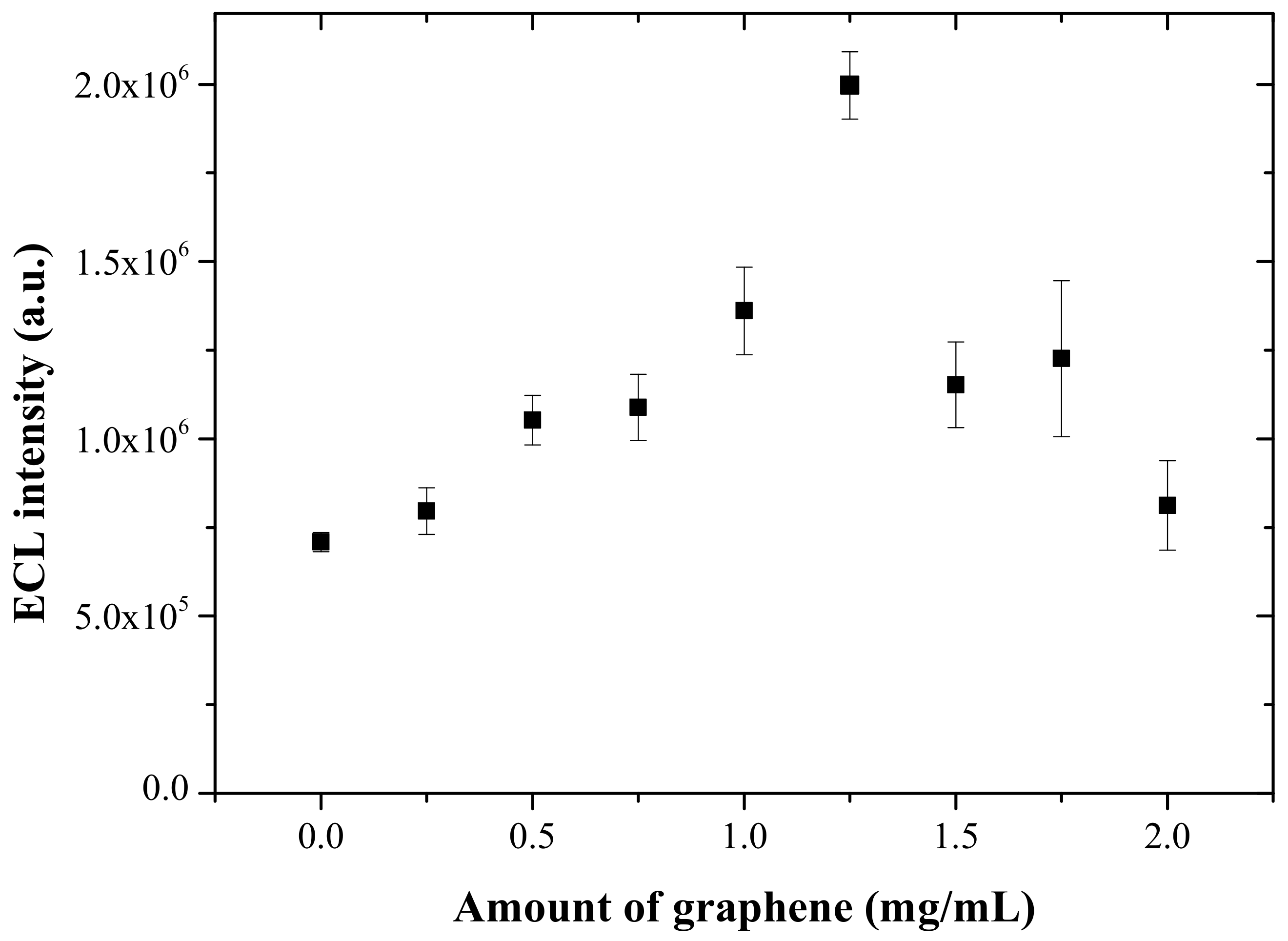
3.1. Characterization of the One-Step Prepared ECL Sensor
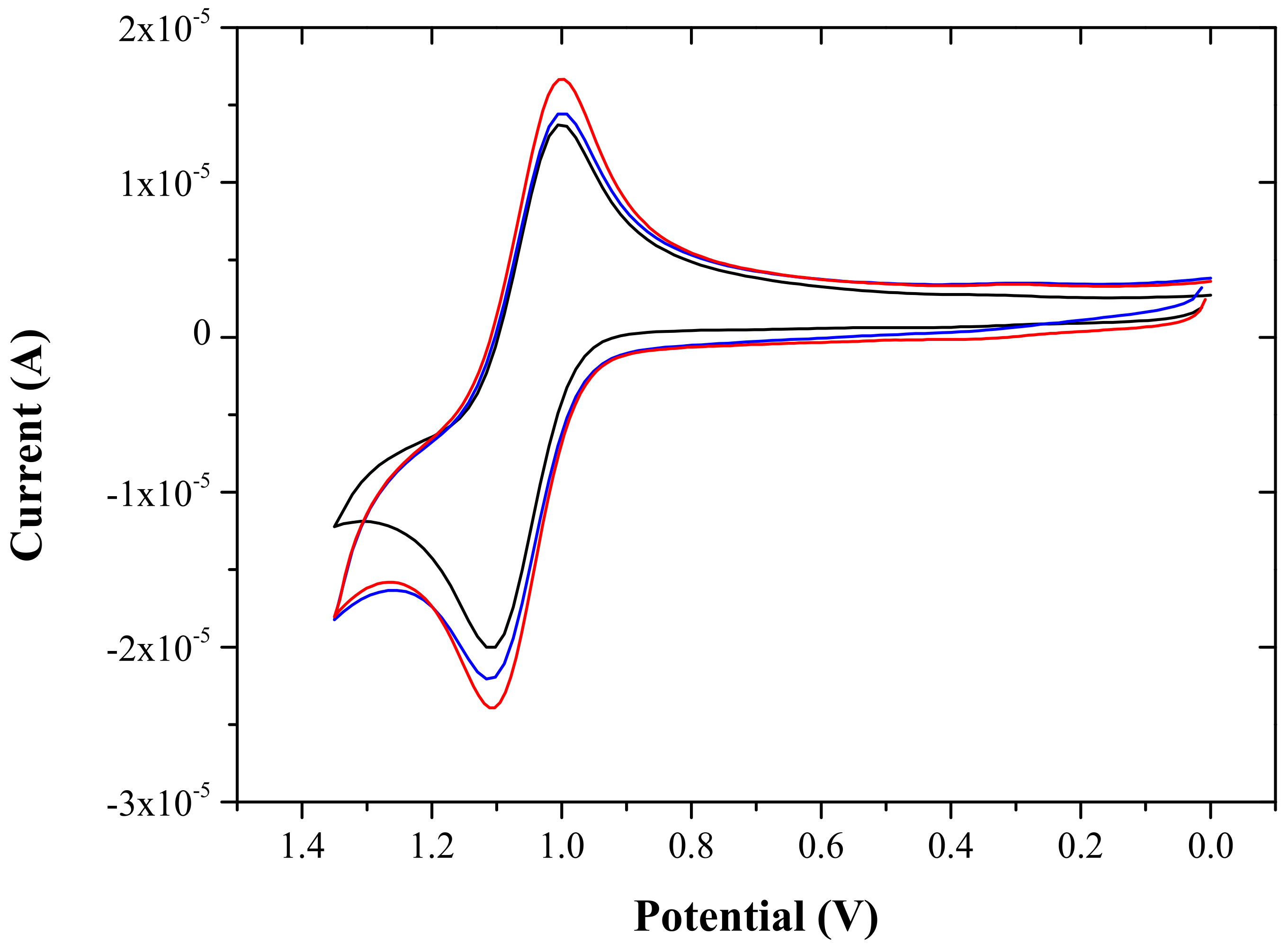
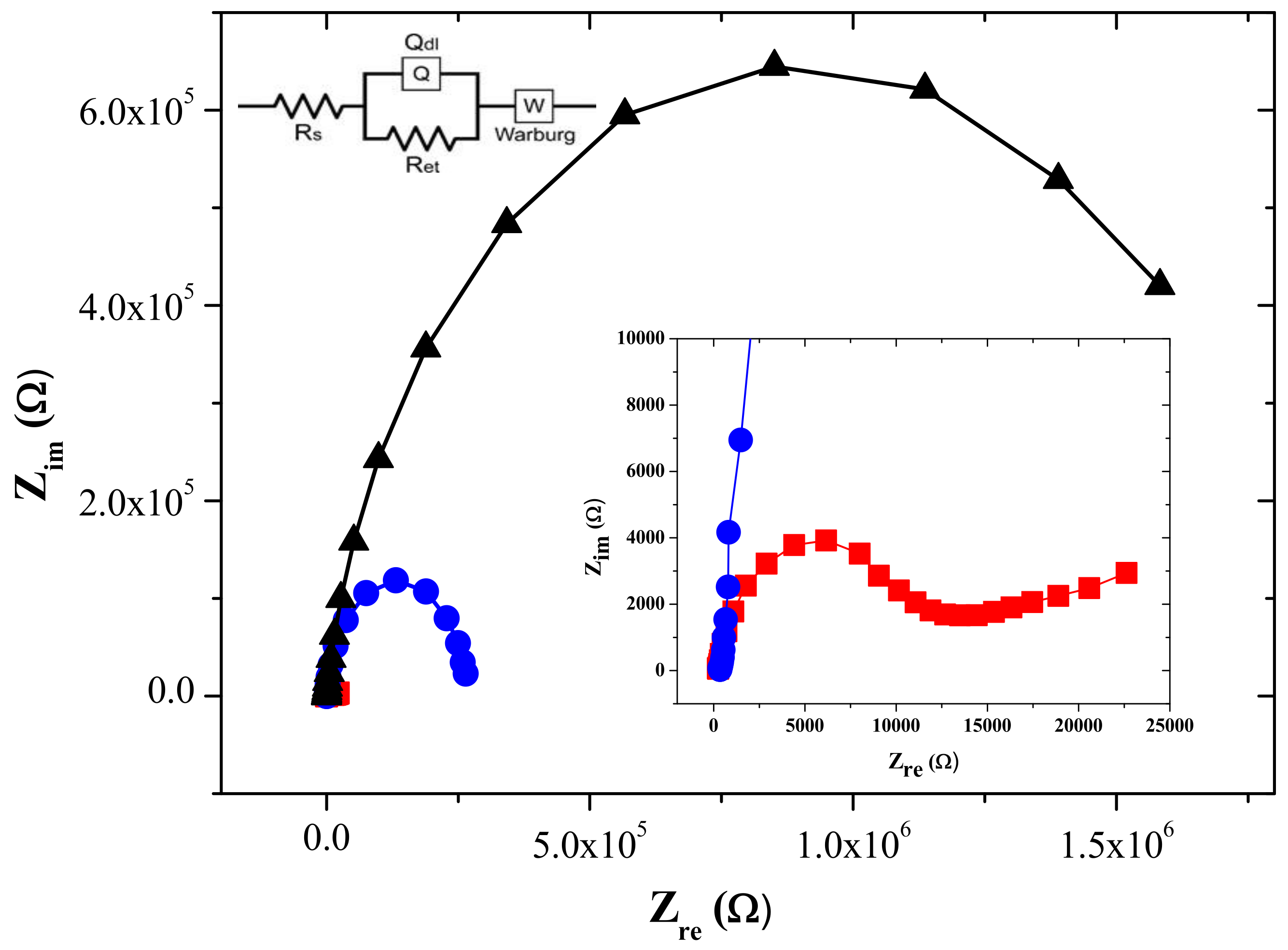
3.2. Electrochemical Behavior
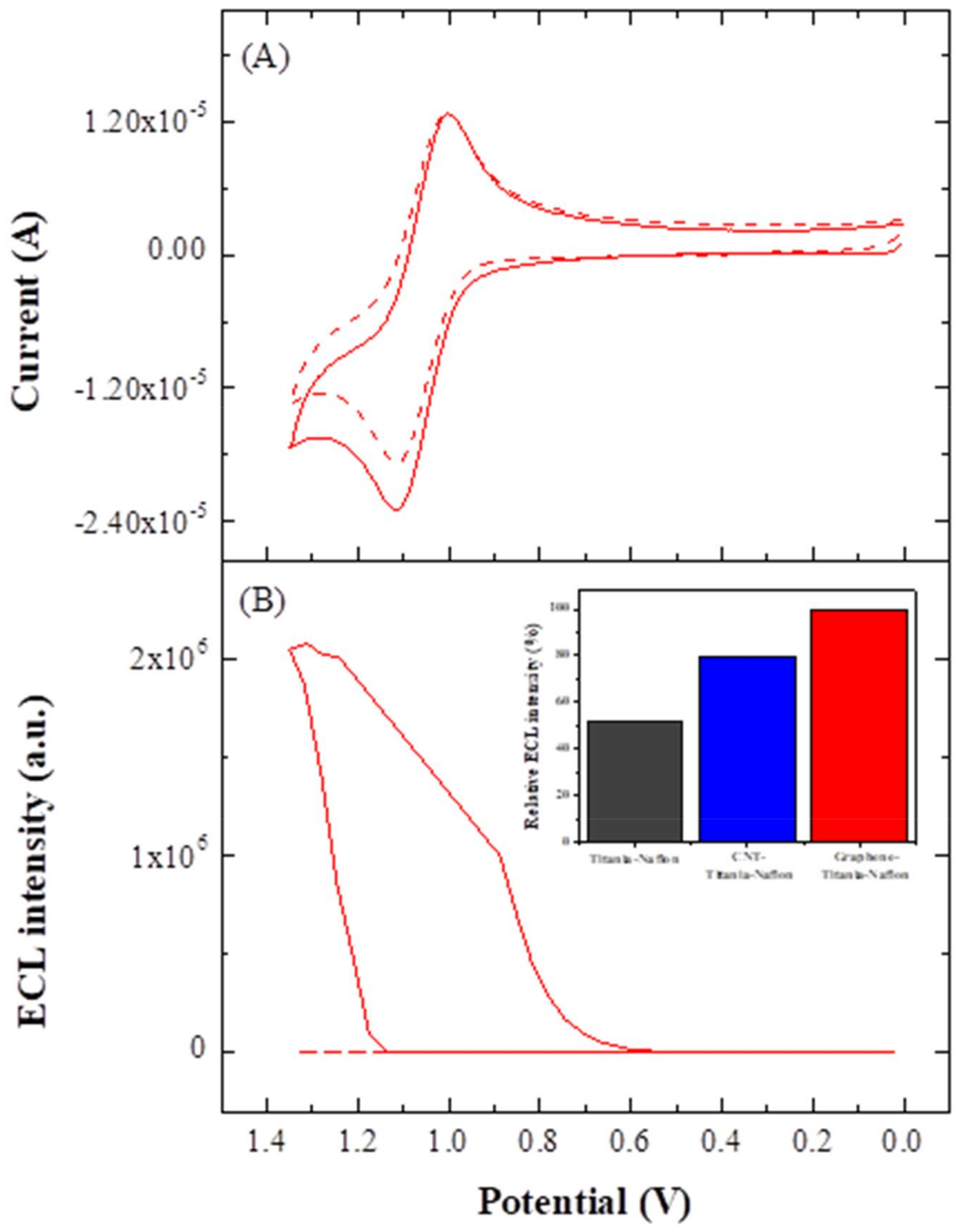
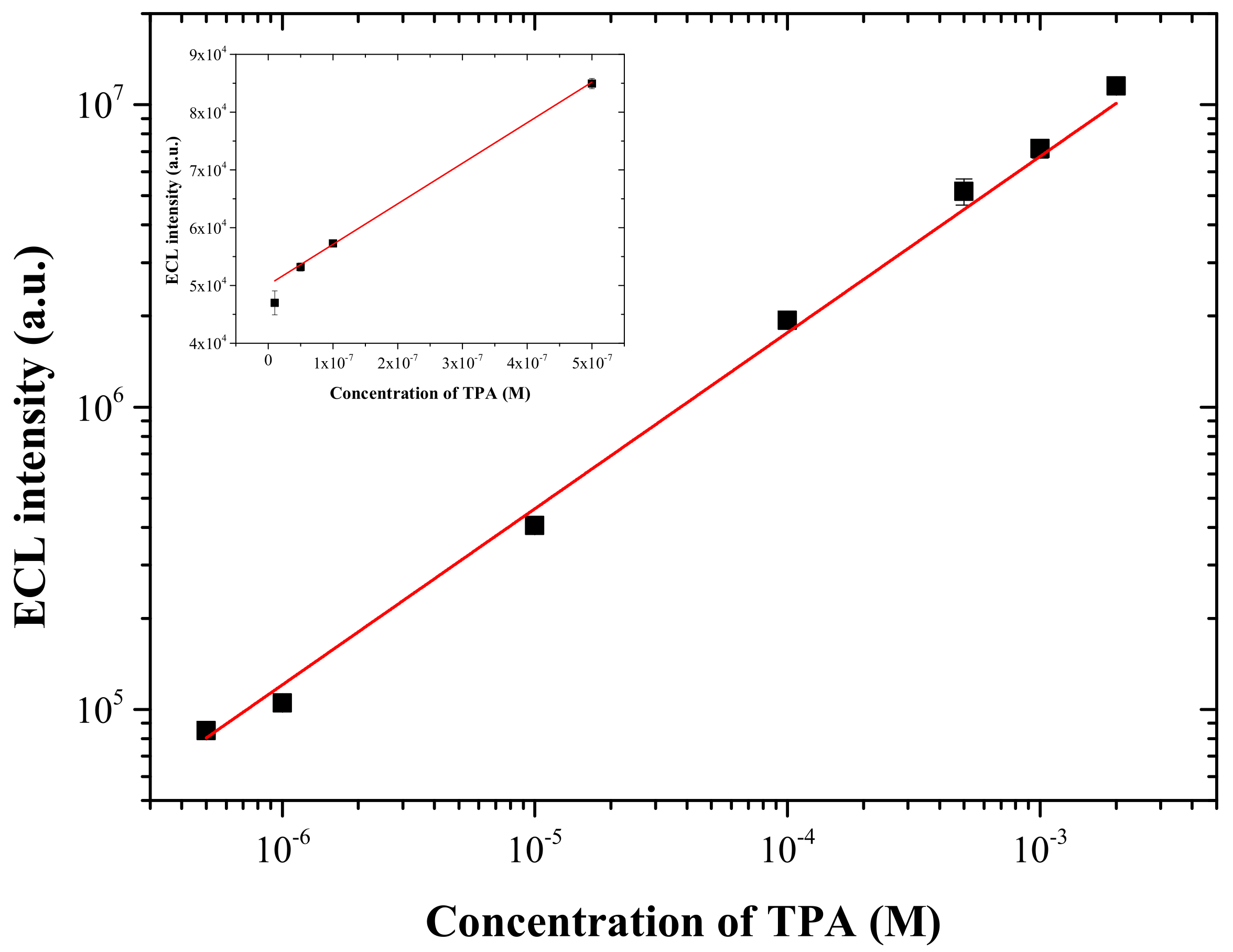
3.3. ECL Behavior
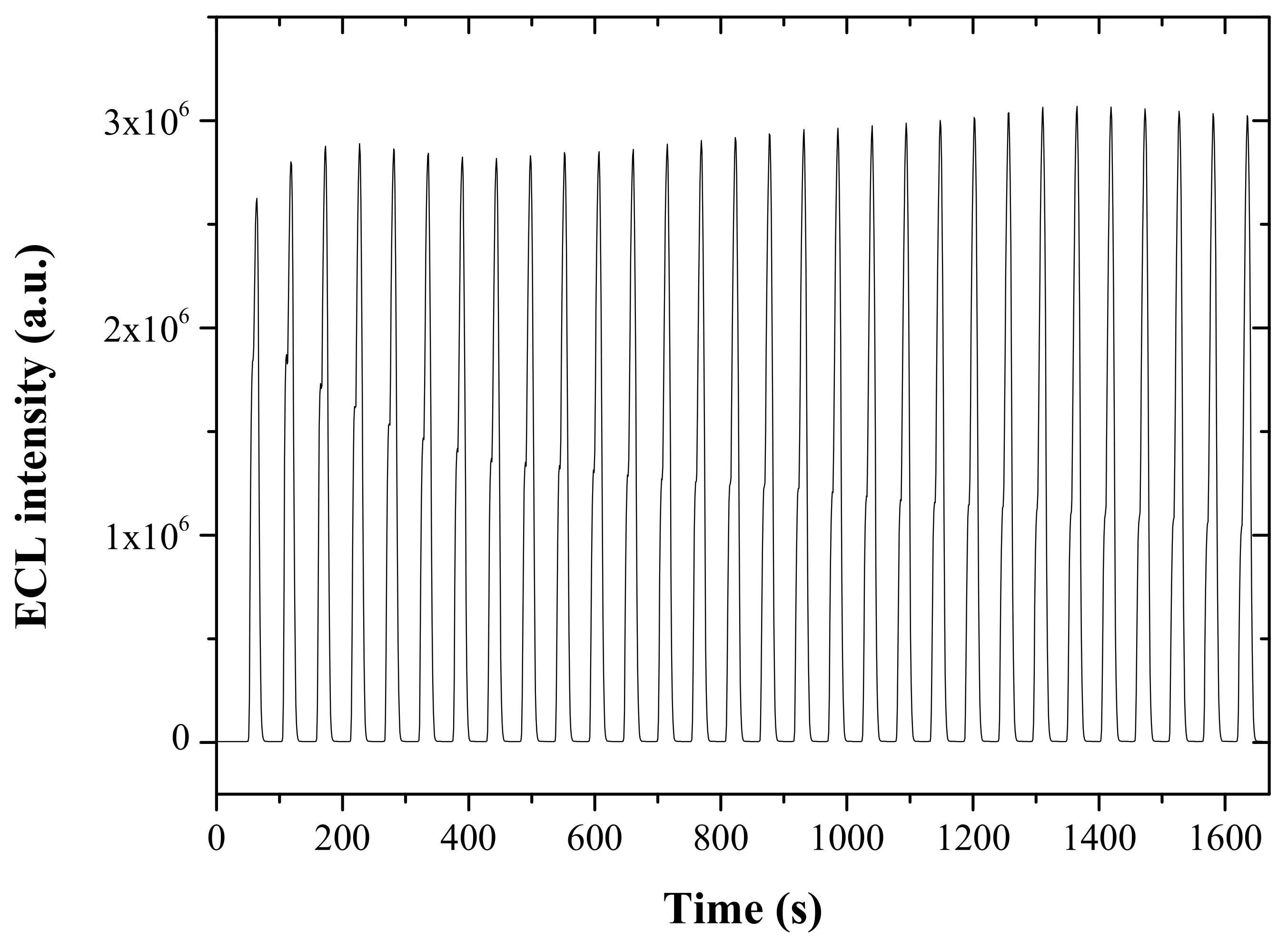
3.4. Selectivity and Stability
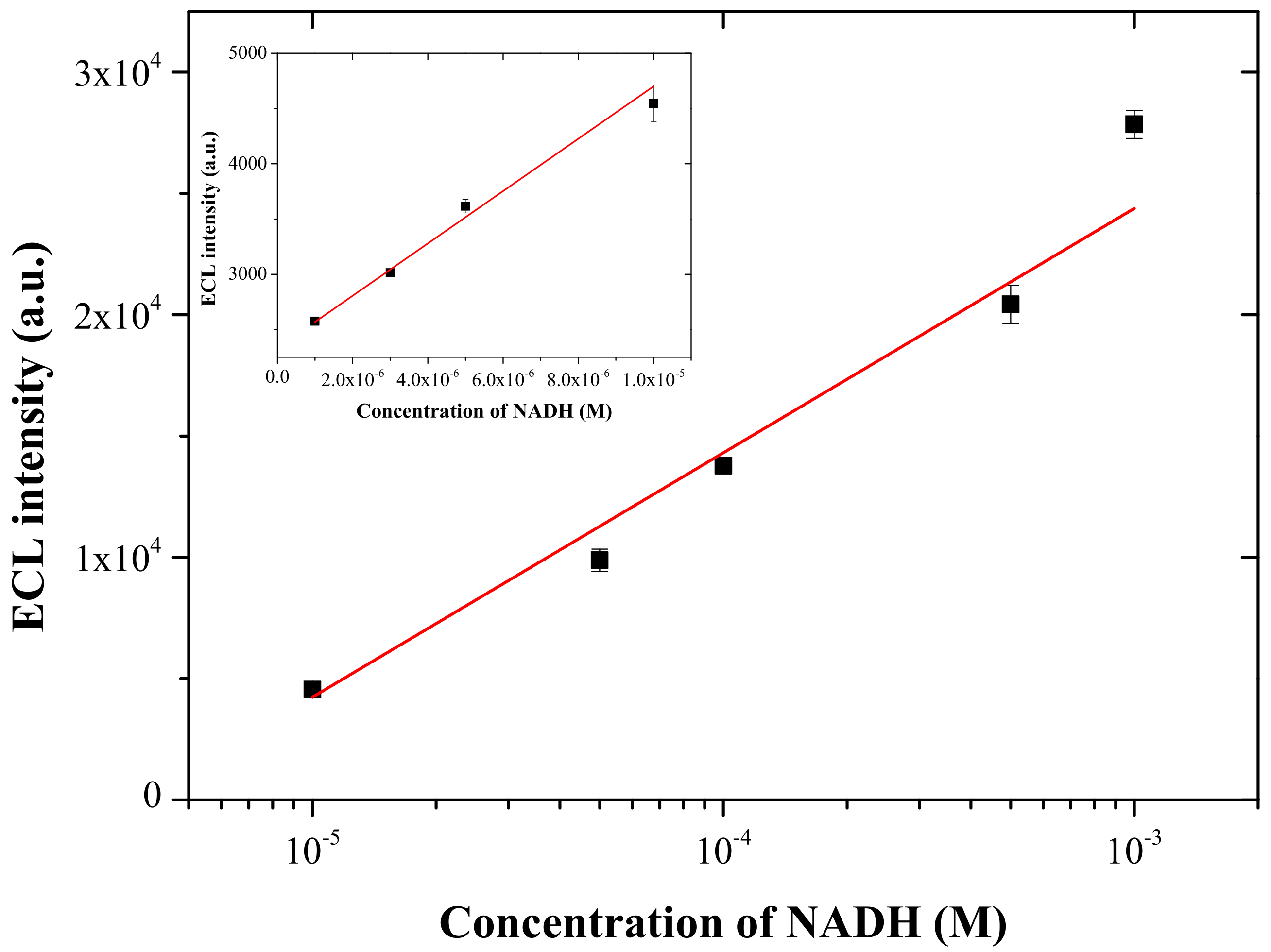
3.5. ECL Detection of NADH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richter, M.M. Electrochemiluminescence (ECL). Chem. Rev. 2004, 104, 3003–3036. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-Y. Tris(2,2′-bipyridyl)ruthenium (II) electrogenerated chemiluminescence in analytical science. Microchim. Acta 1997, 127, 19–39. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, W.; Xu, G. Recent advances in electrochemiluminescence. Chem. Soc. Rev. 2015, 44, 3117–3142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downey, T.M.; Nieman, T.A. Chemiluminescence detection using regenerable tris(2,2′-bipyridyl)ruthenium (II) immobilized in Nafion. Anal. Chem. 1992, 64, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-Y.; Nieman, T.A. Evaluation of Use of Tris(2,2′-bipyridyl)ruthenium (III) as a Chemiluminescent Reagent for Quantitation in Flowing Streams. Anal. Chem. 1995, 67, 1789–1796. [Google Scholar] [CrossRef]
- Khramov, A.N.; Collinson, M.M. Electrogenerated chemiluminescence of tris(2,2′-bipyridyl)ruthenium (II) ion-exchanged in Nafion-silica composite films. Anal. Chem. 2000, 72, 2943–2948. [Google Scholar] [CrossRef]
- Wang, H.; Xu, G.; Dong, S. Electrochemiluminescence of tris(2,2′-bipyridine)ruthenium(II) immobilized in poly (p-styrenesulfonate)-silica-Triton X-100 composite thin-films. Analyst 2001, 126, 1095–1099. [Google Scholar] [CrossRef]
- Choi, H.N.; Cho, S.-H.; Lee, W.-Y. Electrogenerated chemiluminescence from tris(2,2’-bipyridyl)ruthenium (II) immobilized in titania-perfluorosulfonated ionomer composite films. Anal. Chem. 2003, 75, 4250–4256. [Google Scholar] [CrossRef]
- Choi, H.N.; Lyu, Y.K.; Lee, W.-Y. Tris(2, 2′-bipyridyl)ruthenium (II) electrogenerated chemiluminescence sensor based on sol–gel-derived V2O5/Nafion composite films. Electroanalysis 2006, 18, 275–281. [Google Scholar] [CrossRef]
- Forster, R.J.; Hogan, C.F. Electrochemiluminescent metallopolymer coatings: Combined light and current detection in flow injection analysis. Anal. Chem. 2000, 72, 5576–5582. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, S.H.; Kim, M.; Kim, H.; Kim, D.H.; Lee, W.-Y. Organosilicate thin film containing Ru(bpy)32+ for an electrogenerated chemiluminescence (ECL) sensor. Chem. Commun. 2003, 9, 1602–1603. [Google Scholar] [CrossRef]
- Kang, C.H.; Choi, Y.B.; Kim, H.H.; Choi, H.N.; Lee, W.-Y. Electrogenerated chemiluminescence sensor based on a self-assembled monolayer of ruthenium(II)-bis(2,2’-bipyridyl)-(aminopropyl imidazole) on gold deposited screen printed electrode. Electroanalysis 2011, 23, 2131–2138. [Google Scholar] [CrossRef]
- Guo, Z.; Shen, Y.; Wang, M.; Zhao, F.; Dong, S. Electrochemistry and electrogenerated chemiluminescence of SiO2 nanoparticles/tris(2,2′-bipyridyl)ruthenium (ΙΙ) multilayer films on indium tin oxide electrodes. Anal. Chem. 2004, 76, 184–191. [Google Scholar] [CrossRef]
- Kim, D.J.; Lyu, Y.K.; Choi, H.N.; Min, I.H.; Lee, W.-Y. Nafion-stabilized magnetic nanoparticles (Fe3O4) for Ru(bpy)32+ (bpy=bipyridine) electrogenerated chemiluminescence sensor. Chem. Commun. 2005, 5, 2966–2968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, S. Electrogenerated chemiluminescence sensors using Ru(bpy)32+ doped in silica nanoparticles. Anal. Chem. 2006, 78, 5119–5123. [Google Scholar] [CrossRef]
- Guo, Z.; Dong, S. Electrogenerated chemiluminescence from Ru(bpy)32+ ion-exchanged in carbon nanotube/perfluorosulfonated ionomer composite films. Anal. Chem. 2004, 76, 2683–2688. [Google Scholar] [CrossRef]
- Choi, H.N.; Lee, J.Y.; Lyu, Y.-K.; Lee, W.-Y. Tris(2,2′-bipyridyl)ruthenium(II) electrogenerated chemiluminescence sensor based on carbon nanotube dispersed in sol-gel-derived titania-Nafion composite films. Anal. Chim. Acta 2006, 565, 48–55. [Google Scholar] [CrossRef]
- Yoon, S.H.; Han, J.H.; Kim, B.K.; Choi, H.N.; Lee, W.-Y. Tris (2, 2′-bipyridyl) ruthenium (II) electrogenerated chemiluminescence sensor based on platinized carbon nanotube-zirconia-Nafion composite films. Electroanalysis 2010, 22, 1349–1356. [Google Scholar] [CrossRef]
- Li, H.; Chen, J.; Han, S.; Niu, W.; Liu, X.; Xu, G. Electrochemiluminescence from tris(2,2’-bipyridyl)ruthenium(II)-graphene-Nafion modified electrode. Talanta 2009, 79, 165–170. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Xi, J.; Zhang, A.; Chen, Y.; Lu, F.; Chen, Z. A novel electrochemiluminescence sensor based on Ru(bpy)32+ immobilized by graphene on glassy carbon electrode surface via in situ wet-chemical reaction. Sens. Actuators B Chem. 2012, 171–172, 1159–1165. [Google Scholar] [CrossRef]
- Xu, Y.; Lou, B.; Lv, Z.; Zhou, Z.; Zhang, L.; Wang, E. Paper-based solid-state electrochemiluminescence sensor using poly(sodium 4-styrenesulfonate) functionalized graphene/nafion composite film. Anal. Chim. Acta 2013, 763, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Xu, Y.; Lou, B.; Lyu, Z.; Wang, E. One-step process for fabricating paper-based solid-state electrochemiluminescence sensor based on functionalized graphene. Electrochem. Commun. 2014, 38, 57–60. [Google Scholar] [CrossRef]
- Lin, J.; Wu, H.; Lu, L.; Sun, Z.; Zhang, Y.; Dang, F.; Qian, L. Porous graphene containing immobilized Ru(II) tris-bipyridyl for use in electrochemiluminescence sensing of tripropylamine. Microchim. Acta 2016, 183, 1211–1217. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Xi, J.; Lin, S.; Chen, Y.; Lin, Y.; Chen, Z. A novel electrochemiluminescence ethanol biosensor based on tris(2,2′-bipyridine)ruthenium(II) and alcohol dehydrogenase immobilized in graphene/bovine serum albumin composite film. Biosens. Bioelectron. 2013, 41, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.-N.; Wang, M.; Wang, F.-B.; Xia, X.-H. Graphene-ruthenium(II) complex composites for sensitive ECL immunosensors. Small 2014, 10, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Lv, Y. Graphene and graphene oxides: Recent advances in chemiluminescence and electrochemiluminescence. RSC Adv. 2014, 4, 29324–29339. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, W.-Y. Highly sensitive electrochemical capsaicin sensor based on graphene-titania-Nafion composite film. J. Electroanal. Chem. 2016, 776, 74–81. [Google Scholar] [CrossRef]
- Yu, X.; Dai, J.; Yang, L.; Xiao, D. 1-Butyl-3-methylimidazolium based ionic liquid as solvent for determination of hydrophobic naphthol with the electrogenerated chemiluminescence of tris(2,2′-bipyridine) ruthenium(II). Analyst 2010, 135, 630–635. [Google Scholar] [CrossRef]
- Radoi, A.; Compagnone, D. Recent advances in NADH electrochemical sensing design. Bioelectrochemistry 2009, 76, 126–134. [Google Scholar] [CrossRef]
- Chen, X.-M.; Su, B.-Y.; Song, X.-H.; Chen, Q.-A.; Chen, X.; Wang, X.-R. Recent advances in electrochemiluminescent enzyme biosensors. Trends Anal. Chem. 2011, 30, 665–676. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, X.-Y.; Shan, D.; Yuan, P.-X.; Zhang, X.-J. Sensitive electrochemical detection of NADH and ethanol at low potential based on pyrocatechol violet electrodeposited on single walled carbon nanotubes-modified pencil graphite electrode. Talanta 2014, 130, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Bilgi, M.; Sahin, E.M.; Ayranci, E. Sensor and biosensor application of a new redox mediator: Rosmarinic acid modified screen-printed carbon electrode for electrochemical determination of NADH and ethanol. J. Electroanal. Chem. 2018, 813, 67–74. [Google Scholar] [CrossRef]
- Yang, Y.; Nam, S.; Lee, W.-Y. Tris(2,2′-bipyridyl)ruthenium(II) electrogenerated chemiluminescence ethanol biosensor based on ionic liquid doped titania-Nafion composite film. Microchem. J. 2018, 142, 62–69. [Google Scholar] [CrossRef]
| Immobilization Matrix | Linear Range (M) | LOD (M) | Reference |
|---|---|---|---|
| Nafion | 1.0 × 10−6–1.0 × 10−3 | 1.0 × 10−6 | [5] |
| Titania-Nafion | 1.0 × 10−7–1.0 × 10−3 | 1.0 × 10−7 | [8] |
| CNT-Nafion | 1.0 × 10−7–1.0 × 10−3 | 5.0 × 10−8 | [16] |
| CNT-titania-Nafion | 5.0 × 10−8–1.0 × 10−3 | 1.0 × 10−8 | [17] |
| Graphene-Nafion | 1.0 × 10−7–1.0 × 10−4 | 5.0 × 10−8 | [19] |
| Graphene oxide | 2.5 × 10−7–1.5 × 10−4 | 5.0 × 10−9 | [20] |
| Graphene NS-PSS-C paste | 5.0 × 10−7–1.0 × 10−4 | 4.0 × 10−10 | [21,22] |
| Porous graphene-Nafion | 5.0 × 10−7–1.0 × 10−4 | 4.0 × 10−10 | [23] |
| Graphene-titania-Nafion | 1.0 × 10−8–2.0 × 10−3 | 8.0 × 10−10 | This study |
| Analytes (a) | Background-Corrected ECL Intensity (a.u.) (b) | Relative ECL Response (%) |
|---|---|---|
| Dopamine | 6.80 × 102 | 0.04 |
| Tryptophan | 1.71 × 103 | 0.11 |
| Ascorbic acid | 1.79 × 103 | 0.11 |
| Sodium oxalate | 3.82 × 103 | 0.24 |
| Ethanol | 3.84 × 103 | 0.24 |
| Histidine | 6.17 × 103 | 0.39 |
| Proline | 8.24 × 104 | 5.2 |
| NADH | 1.38 × 105 | 8.7 |
| Promazine | 1.65 × 105 | 10.4 |
| Erythromycin | 2.82 × 105 | 17.8 |
| TPA | 1.58 × 106 | 100 |
| Sample (a) | Added (µM) | Found (µM) | Recovery (%) (b) |
|---|---|---|---|
| 1 | 0 | 2.4 ± 1.7 | - |
| 2 | 100 | 104.2 ± 6.3 | 104.2 ± 6.3 |
| 3 | 400 | 409.7 ± 5.2 | 102.4 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.J.; Lee, D.H.; Lee, W.-Y. One-Step Fabrication of Highly Sensitive Tris(2,2′-bipyridyl)ruthenium(II) Electrogenerated Chemiluminescence Sensor Based on Graphene-Titania-Nafion Composite Film. Sensors 2022, 22, 3064. https://doi.org/10.3390/s22083064
Lee SJ, Lee DH, Lee W-Y. One-Step Fabrication of Highly Sensitive Tris(2,2′-bipyridyl)ruthenium(II) Electrogenerated Chemiluminescence Sensor Based on Graphene-Titania-Nafion Composite Film. Sensors. 2022; 22(8):3064. https://doi.org/10.3390/s22083064
Chicago/Turabian StyleLee, Sang Jung, Don Hui Lee, and Won-Yong Lee. 2022. "One-Step Fabrication of Highly Sensitive Tris(2,2′-bipyridyl)ruthenium(II) Electrogenerated Chemiluminescence Sensor Based on Graphene-Titania-Nafion Composite Film" Sensors 22, no. 8: 3064. https://doi.org/10.3390/s22083064
APA StyleLee, S. J., Lee, D. H., & Lee, W.-Y. (2022). One-Step Fabrication of Highly Sensitive Tris(2,2′-bipyridyl)ruthenium(II) Electrogenerated Chemiluminescence Sensor Based on Graphene-Titania-Nafion Composite Film. Sensors, 22(8), 3064. https://doi.org/10.3390/s22083064







