A Multi-Modal Analysis of the Freezing of Gait Phenomenon in Parkinson’s Disease
Abstract
1. Introduction
- Electroencephalography (EEG) data were used to detect and predict FOG occurrence [22]. Coherence with EMG close and during FOG episodes has been revealed [23], along with bilateral cortical excessive synchronization during locomotion [23], an increase in the theta-band power in frontal and central areas [22,24], a decrease in power during the voluntary arrest compared to FOG [25] and a less complex cortical activity during the transition periods [26].
- Skin conductance (SC), encompassing selective information useful for FOG prediction and detection [27], even considering the heavy subject-dependency.
- Inertial sensors. Single or multiple sensors have been placed on several body segments (e.g., legs [28], wrist [29], waist [30]) for FOG detection [29,31] and prediction [28,29,32]. In particular, accelerometers are widely employed, due to their low energy consumption and cost (in particular, those embedded in smartphones [15,33]). Significant information is provided by the Freeze Index, defined as the ratio between the power contained in the so-called freeze band 3–8 Hz [34] and that in the locomotion band 0.5–3 Hz [29,35]. Entropy and statistical parameters such as mean value, standard deviation and variance are other sensible metrics [28,31]. Several features, extracted from both the time and frequency domain and different machine learning (ML) algorithms have been employed to classify FOG and pre-FOG events [36].
2. Materials and Methods
2.1. Dataset
- PD patients subject to FOG during OFF periods;
- PD patients able to walk independently during OFF periods;
- No severe vision or hearing impairment;
- No sign of dementia or other neurological/orthopedic disease.
- Task 1-
- When ready, the participants had to rise from a chair and walk up to a narrow space between the room and a corridor. Then, they turned right and walked into the corridor. After bypassing a first obstacle (e.g., a chair), they went straight along the narrow corridor, made a U-turn at the end of the corridor and went along in the opposite direction. They had to bypass three further obstacles, reach the left end of the corridor, make another U-turn, bypass two obstacles, reach the door of the room, enter the room, walk back to the chair and sit down.
- Task 2-
- Consisted of a repetition of Task 1.
- Task 3-
- Patients were asked to perform a turn in a limited space and a square was drawn on the ground for this aim. When the patient was ready, they had to stand up from the chair, walk to the square mark, make a U-turn in the narrow square region and then walk straight back to the chair and sit down.
- Task 4-
- Consisted of a repetition of Task 3.
2.2. Data Processing
2.2.1. Filtering and Standardization
2.2.2. Labeling and Segmentation
2.2.3. Feature Extraction
2.2.4. Subject-Independent Algorithm
| Algorithm 1 Algorithm for model optimization and performance evaluation in the Subject-Independent case. |
|
2.2.5. Subject-Dependent Algorithm
3. Results and Discussion
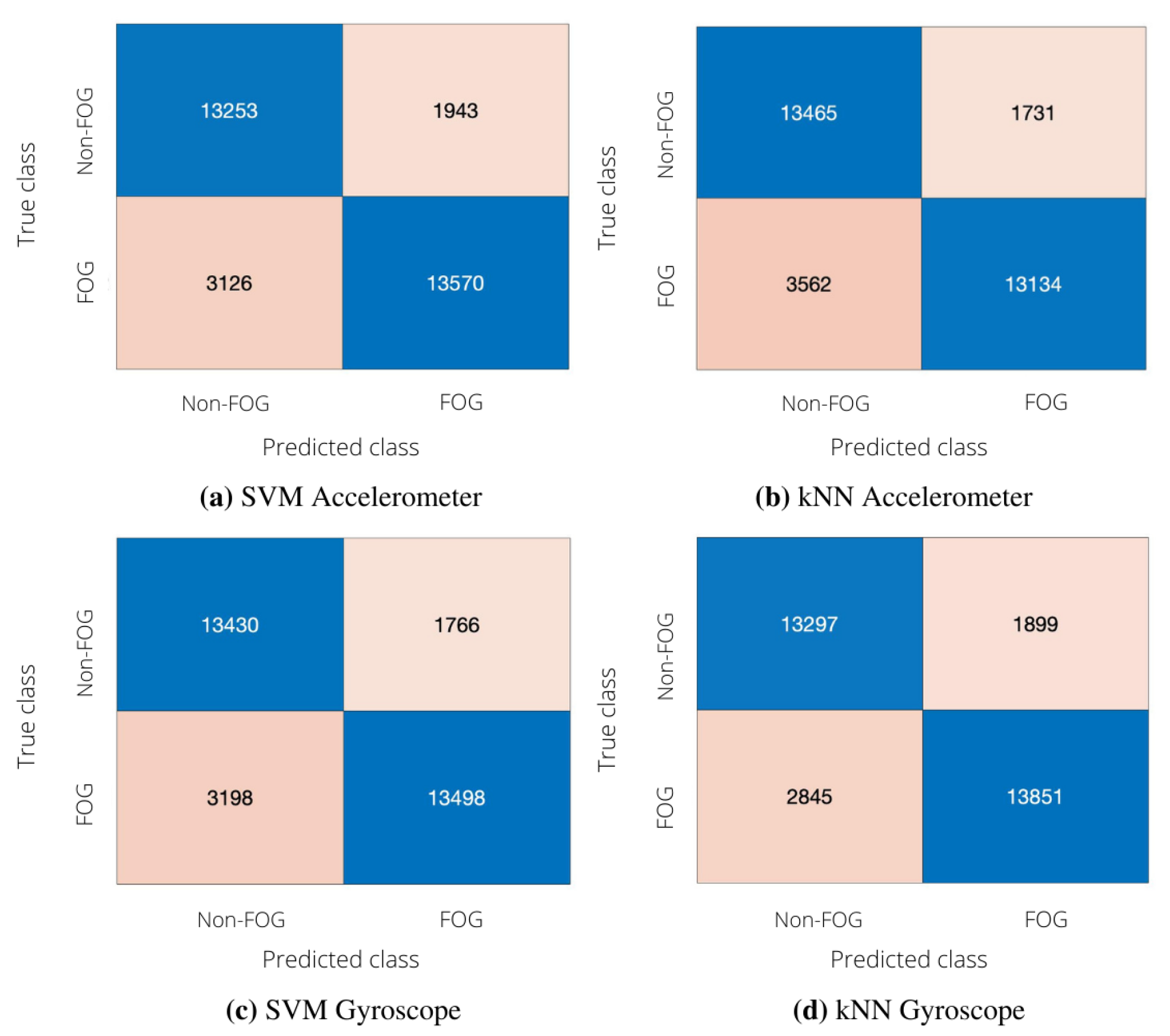
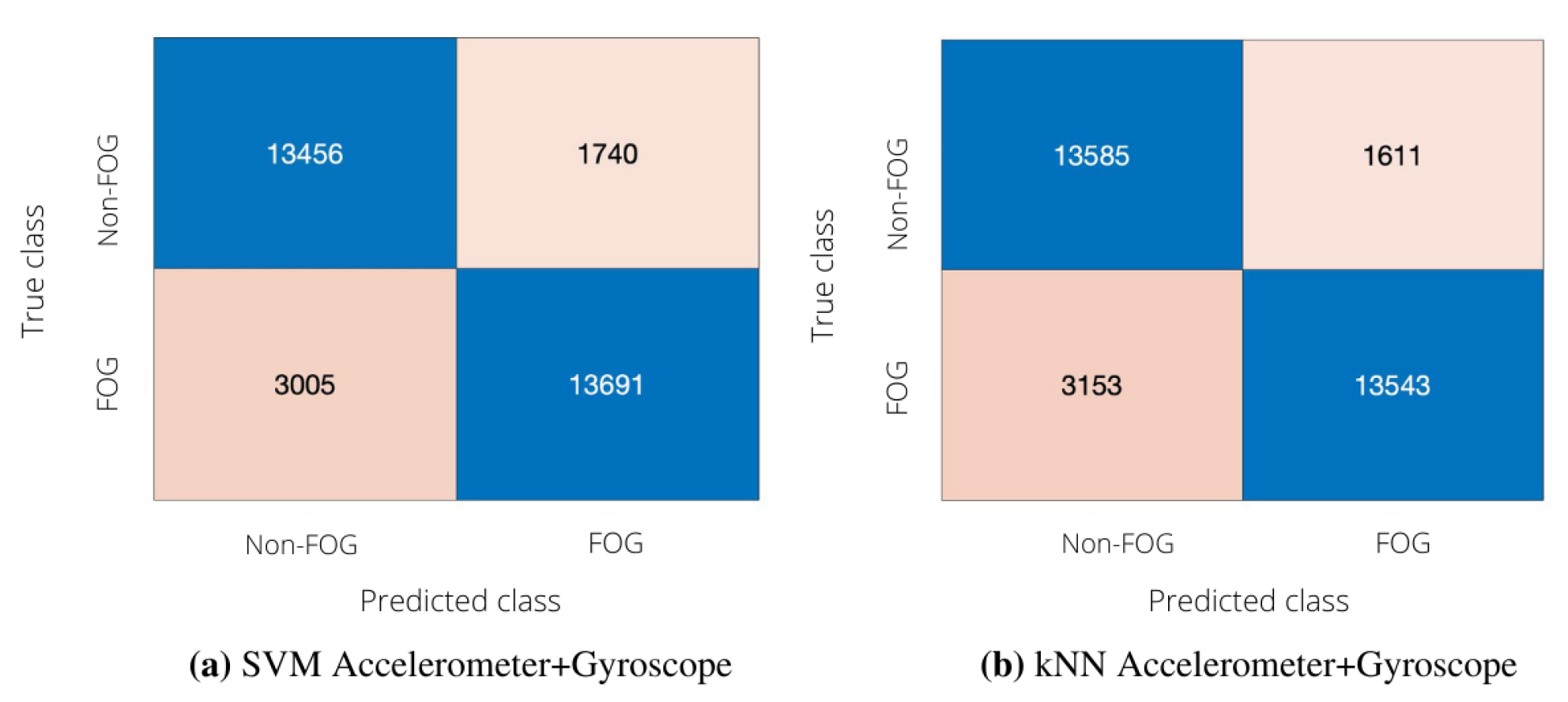
3.1. Subject-Independent Algorithm
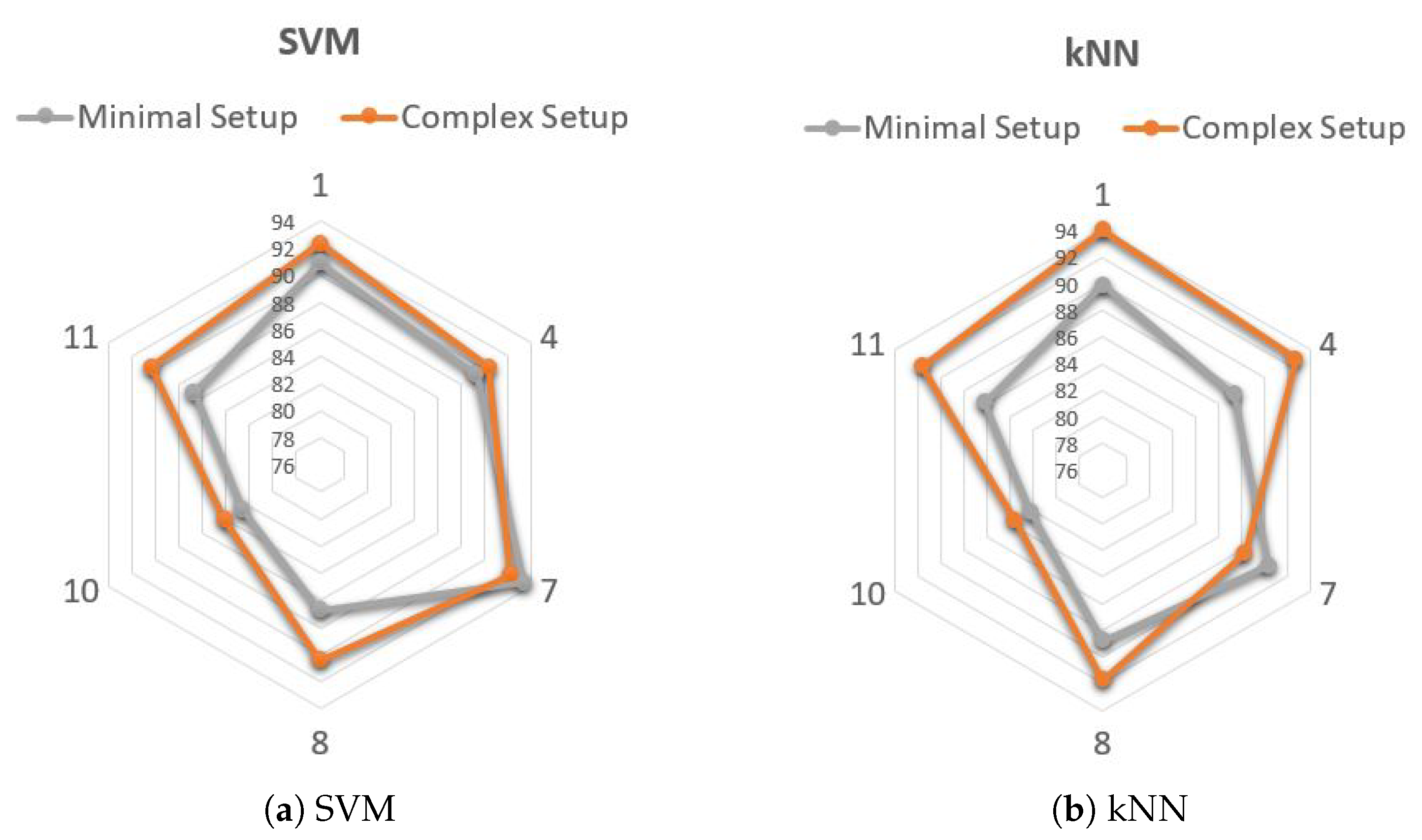
3.2. Subject-Dependent Algorithm
- Single-sensor classification, considering accelerometer data from the left tibial sensor only (Minimal Setup);
- Multi-sensor classification, using data from all sensors that provided significant features for each individual patients (Complex Setup), i.e., acceleration and angular velocity at tibial level, acceleration and angular velocity at wrist level and EEG.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADL | Activities of Daily Living |
| ECG | Electrocardiogram |
| EEG | Electroencephalogram |
| EMG | Electromiogram |
| EOC | Electrooculogram |
| FN | False Negative |
| FOG | Freezing Of Gait |
| FOG-Q | Freezing Of Gait Questionnaire |
| FP | False Positive |
| FS | Feauture Selection |
| kNN | k-Nearest Neighbor |
| LOSO | Leave-One-Subject-Out |
| LOTO | Leave-One-Task-Out |
| MDS | Movement Disorder Society |
| ML | Machine Learning |
| MMSE | Mini-Mental State Examination |
| MOCA | Montreal Cognitive Assessment |
| MSC | Magnitude Squared Coherence |
| MV | Majority Voting |
| PD | Parkinson disease |
| SC | Skin Conductance |
| SCR | Skin Conductance Response |
| SCL | Skin Conductance Level |
| SDA | Subject Dependent Algorithm |
| SIA | Subject Independent Algorithm |
| SVM | Support Vector Machine |
| TP | True Positive |
| TN | True Negative |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
References
- Garrett, E. Alexander, Biology of Parkinson’s disease: Pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin. Neurosci. 2004, 6, 259–280. [Google Scholar]
- Mhyre, T.R.; Boyd, J.T.; Hamill, R.W.; Maguire-Zeiss, K.A. Parkinson’s disease. Subcell. Biochem. 2012, 65, 389–455. [Google Scholar] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368LP–376LP. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.G. Non-motor Symptoms of Parkinson’s Disease. Int. J. Gerontol. 2007, 1, 53–80. [Google Scholar] [CrossRef]
- Schapira, A.H. Treatment options in the modern management of Parkinson disease. Arch. Neurol. 2007, 64, 1083–1088. [Google Scholar] [CrossRef][Green Version]
- Regnault, A.; Boroojerdi, B.; Meunier, J.; Bani, M.; Morel, T.; Cano, S. Does the MDS-UPDRS provide the precision to assess progression in early Parkinson’s disease? Learnings from the Parkinson’s progression marker initiative cohort. J. Neurol. 2019, 266, 1927–1936. [Google Scholar] [CrossRef]
- Nutt, J.G.; Bloem, B.R.; Giladi, N.; Hallett, M.; Horak, F.B.; Nieuwboer, A. Freezing of gait: Moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011, 10, 734–744. [Google Scholar] [CrossRef]
- Bloem, B.R. Falls and freezing of gait in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov. Disord. 2004, 19, 871–884. [Google Scholar] [CrossRef]
- Pozzi, N.G. Freezing of gait in Parkinson’s disease reflects a sudden derangement of locomotor network dynamics. Brain 2008, 142, 2037–2050. [Google Scholar] [CrossRef]
- Schaafsma, J.D. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. Eur. J. Neurol. 2003, 4, 391–398. [Google Scholar] [CrossRef]
- Mancini, M. Clinical and methodological challenges for assessing freezing of gait: Future perspectives. Mov. Disord. 2019, 34, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Snijders, A.H. Clinimetrics of freezing of gait. Mov. Disord. 2008, 23, 1639–1640. [Google Scholar] [CrossRef]
- Barthel, C. The Practicalities of Assessing Freezing of Gait. J. Parkinsons. Dis. 2016, 6, 667–674. [Google Scholar] [CrossRef]
- Lindsay, R. The Timed Up and Go Test: Unable to predict falls on the acute medical ward. Aust. J. Physiother. 2004, 50, 249–251. [Google Scholar] [CrossRef]
- Borzì, L. Detection of Freezing of Gait in People with Parkinson’s Disease using Smartphones. In Proceedings of the IEEE 44th Annual Computers, Software, and Applications Conference (COMPSAC), Madrid, Spain, 13–17 July 2020; pp. 625–635. [Google Scholar]
- Mancini, M. Measuring freezing of gait during daily-life: An open-source, wearable sensors approach. J. Neuroeng. Rehabil. 2021, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, D. A Technological Review of Wearable Cueing Devices Addressing Freezing of Gait in Parkinson’s Disease. Sensors 2019, 19, 1277. [Google Scholar] [CrossRef] [PubMed]
- Ginis, P. Cueing for people with Parkinson’s disease with freezing of gait: A narrative review of the state-of-the-art and novel perspectives. Ann. Phys. Rehabil. Med. 2018, 61, 407–413. [Google Scholar] [CrossRef]
- Kugler, P. Automatic recognition of Parkinson’s disease using surface electromyography during standardized gait tests. In Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 5781–5784. [Google Scholar]
- Cantú, H. Abnormal Muscle Activity and Variability Before, During, and After the Occurrence of Freezing in Parkinson’s Disease. Front. Neurol. 2019, 10, 951. [Google Scholar] [CrossRef]
- Mazzetta, I.; Zampogna, A.; Suppa, A.; Gumiero, A.; Pessione, M.; Irrera, F. Wearable Sensors System for an Improved Analysis of Freezing of Gait in Parkinson’s Disease Using Electromyography and Inertial Signals. Sensors 2019, 19, 948. [Google Scholar] [CrossRef]
- Handojoseno, A.M.A. Prediction of Freezing of Gait in Patients with Parkinson’s Disease Using EEG Signals. Stud. Health Technol. Inform. 2018, 246, 124–131. [Google Scholar]
- Günther, M. Coupling Between Leg Muscle Activation and EEG During Normal Walking, Intentional Stops, and Freezing of Gait in Parkinson’s Disease. Front. Physiol. 2019, 10, 870. [Google Scholar] [CrossRef]
- Shine, J.M. Abnormal patterns of theta frequency oscillations during the temporal evolution of freezing of gait in Parkinson’s disease. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2014, 125, 569–576. [Google Scholar] [CrossRef]
- Wang, Y. Characterizing and Detecting Freezing of Gait using Multi-modal Physiological Signals. arXiv 2020, arXiv:2009.12660. [Google Scholar]
- Handojoseno, A. Analysis and Prediction of the Freezing of Gait Using EEG Brain Dynamics. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 23, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Mazilu, S. Prediction of Freezing of Gait in Parkinson’s from Physiological Wearables: An Exploratory Study. IEEE J. Biomed. Heal. Inform. 2015, 19, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Borzì, L.; Mazzetta, I.; Zampogna, A.; Suppa, A.; Olmo, G.; Irrera, F. Prediction of Freezing of Gait in Parkinson’s Disease Using Wearables and Machine Learning. Sensors 2021, 21, 614. [Google Scholar] [CrossRef]
- Mazilu, S. The role of wrist-mounted inertial sensors in detecting gait freeze episodes in Parkinson’s disease. Pervasive Mob. Comput. 2016, 33, 1–16. [Google Scholar] [CrossRef]
- Rodríguez-Martín, D. Home detection of freezing of gait using support vector machines through a single waist-worn triaxial accelerometer. PLoS ONE 2017, 12.2, e0171764. [Google Scholar] [CrossRef]
- Xia, Y. A Machine Learning Approach to Detecting of Freezing of Gait in Parkinson’s Disease Patients. J. Med. Imaging Heal. Inform. 2018, 8, 647–654. [Google Scholar] [CrossRef]
- Mazilu, S. Gait, wrist, and sensors: Detecting freezing of gait in Parkinson’s disease from wrist movement. In Proceedings of the IEEE International Conference on Pervasive Computing and Communication Workshops (PerCom Workshops), St. Louis, MO, USA, 23–27 March 2015; pp. 579–584. [Google Scholar]
- Capecci, M. A smartphone-based architecture to detect and quantify freezing of gait in Parkinson’s disease. Gait Posture 2016, 50, 28–33. [Google Scholar] [CrossRef]
- Gokul, H. Gait Recovery System for Parkinson’s Disease using Machine Learning on Embedded Platforms. In Proceedings of the 2020 IEEE International Systems Conference (SysCon), Montreal, QC, Canada, 24 August–20 September 2020; pp. 1–8. [Google Scholar]
- Wang, Y. Freezing of gait detection in Parkinson’s disease via multimodal analysis of EEG and accelerometer signals. In Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 847–850. [Google Scholar]
- Ponciano, V.; Pires, I.M.; Reinaldo Ribeiro, F.; Marques, G.; Villasana, M.V.; Garcia, N.M.; Zdravevski, E.; Spinsante, S. Identification of Diseases Based on the Use of Inertial Sensors: A Systematic Review. Electronics 2020, 9, 778. [Google Scholar]
- Shalin, G. Prediction of Freezing of Gait in Parkinson’s Disease from Foot Plantar-Pressure Arrays using a Convolutional Neural Network. In Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 244–247. [Google Scholar]
- Li, H. Multimodal Dataset of Freezing of Gait in Parkinson’s Disease. Mendeley Data 2021, V3. [Google Scholar] [CrossRef]
- Gomez, G. Automatic Artifact Removal (AAR) Toolbox v 1.3 (Release 09.12.2007) for MATLAB; Tampere University of Technology: Tampere, Finland, 2007. [Google Scholar]
- Grigore, O. Psycho-Physiological Signal Processing and Algorithm for Adaptive Light Control. In Proceedings of the The Second International Symposium on Electrical and Electronics Engineering – ISEEE-2008, Galati, Romania, 12–13 September 2008. [Google Scholar]
- Evans, R.H. An Analysis of Criterion Variable Reliability in Conjoint Analysis. Percept. Mot. Ski. 1996, 82, 988–990. [Google Scholar] [CrossRef]


| Type | System | Number of Sensors | Location |
|---|---|---|---|
| 28D-EEG | Wireless MOVE | 28 | FP1 FP2 F3 F4 C3 C4 P3 P4 O1 O2 F7 F8 P7 P8 Fz Cz Pz FC1 FC2 CP1 CP2 FC5 FC6 CP5 CP6 TP9 TP10 *IO |
| 3D-Acc/Gyro | MPU6050 | 2 | Lateral tibia of left leg; Wrist |
| 1D-SC | LM324 | 1 | Second phalanx of the index finger/middle finger of the left hand |
| System | Range | Resolution | Sample Frequency |
|---|---|---|---|
| Wireless MOVE | 1000 Hz | ||
| MPU6050 | ± 2000 dps ± 16 g | 16.4 LSB/dps 2048 LSB/g | 100 Hz |
| LM324 | 100 Hz |
| Subjects | 12 PD |
|---|---|
| Age (years) | 69 ± 7.9 |
| Disease duration (years) | 9.3 ± 6.8 |
| ADL | 81.3 ± 16.0 |
| FOG-Q | 16.2 ± 4.2 |
| UPDRS-1 | 10.4 ± 5.5 |
| UPDRS-2 | 16.3 ± 10.6 |
| UPDRS-3 | 45.0 ± 16.0 |
| UPDRS-4 | 2.2 ± 2.9 |
| MMSE | 28.2 ± 1.5 |
| MOCA | 23.6 ± 3.6 |
| Domain | Acc/Gyro Lateral Left Tibia |
|---|---|
| Frequency | Total Power, Mean Power, Max Power, STD Power, Locomotion Band Power, Freeze Band Power, Locomotion Band Power STD, Freeze Band Power STD, Freeze Index, Freeze Ratio, Skewness, Kurtosis, Energy, Entropy, Dominant Frequency, Mean Frequency, Median Frequency |
| Time | RMS, Mean, STD, Number of zero-crossing, Zero-crossing rate, Number of peaks, Mean distance between peaks, Mean height of the peaks, Energy, Max Amplitude, Min Amplitude, Range, Integral, Axes correlation |
| Domain | Acc Wrist |
| Frequency | Signal magnitude: Total Power, Mean Power, STD power, Power [0–1, 1–2, …, 15–16 Hz], Locomotion Band Power, Freeze Band Power, Power 9–12 Hz, Power 13–16 Hz Signal components: Total Power, Mean Power, STD Power, Max Power, Dominant Frequency, Mean Frequency, Median Frequency |
| Time | Signal magnitude: RMS, Mean, STD, Axes correlation Signal components: Total Power, Mean Power, STD Power, Max Power, Dominant Frequency, Mean Frequency, Median Frequency |
| Domain | Gyro Wrist |
| Frequency | Signal magnitude: Total Power, Mean Power, STD Power, Locomotion Band Power, Freeze Band Power Signal components: Total Power, Mean Power, STD Power, Max Power, Dominant Frequency, Mean Frequency, Median Frequency |
| Time | Signal magnitude and components: RMS, Mean, STD |
| Domain | EEG |
| Frequency | Total Power, Mean Power, STD Power, Skewness, Kurtosis, Energy, Entropy, Dominant Frequency, Median Frequency, Mean Frequency, Delta Band Power, Theta Band Power, Alpha Band Power, Beta1 Band Power, Beta2 Band Power, Magnitude Squared Coherence |
| Time | RMS, Mean, STD |
| Domain | Phasic Component SC |
| Frequency | Total Power, Mean Power, STD Power, Skewness, Kurtosis, Energy, Entropy, Dominant Frequency, Median Frequency, Mean Frequency |
| Time | RMS, Mean, STD, Median, Min, Max, Range, Number of local min, Number of local max |
| Domain | *Der1/Der2 Phasic Component SC |
| Frequency | – |
| Time | Mean, Median, STD, Min, Max, Range, Number of local min, Number of local max |
| Domain | Tonic Component SC |
| Frequency | Total Power, Mean Frequency, Median Frequency |
| Time | Slope |
| Signal | # Channels | # Feature |
|---|---|---|
| Inertial—Lateral Left Tibia | 6 | 186 |
| Inertial—Wrist | 6 | 168 |
| EEG | 18 | 1107 |
| SC | 1 | 39 |
| Model | SVM | k-NN | ||||
|---|---|---|---|---|---|---|
| Parameter | kernel function | kernel scale | cost | # neighbors | distance metric | distance weight |
| Value | linear quadratic cubic gaussian | 0.1–100 | 0.1–100 | 1–180 | cityblock euclidean squared-euclidean | equal inverse squared-inverse |
| Accelerometer | r (p-Value) | Gyroscope | r (p-Value) |
|---|---|---|---|
| Kurtosis-PSD x-axis | −0.35 (<0.0001) | Max power y-axis | −0.48 (<0.0001) |
| Median frequency x-axis | 0.37 (<0.0001) | Freeze ratio y-axis | 0.46 (<0.0001) |
| Locomotion band power x-axis | −0.49 (<0.0001) | Max amplitude y-axis | −0.36 (<0.0001) |
| Freeze ratio x-axis | 0.59 (<0.0001) | Skewness-PSD z-axis | −0.45 (<0.0001) |
| Median frequency y-axis | 0.38 (<0.0001) | Entropy-PSD z-axis | 0.58 (<0.0001) |
| Dominant frequency y-axis | 0.45 (<0.0001) | Dominant frequency z-axis | 0.40 (<0.0001) |
| Locomotion band power y-axis | −0.50 (<0.0001) | STD Locomotion band power z-axis | −0.50 (<0.0001) |
| Freeze index y-axis | 0.37 (<0.0001) | Freeze ratio z-axis | 0.64 (<0.0001) |
| Zero crossing rate y-axis | 0.48 (<0.0001) | RMS z-axis | −0.55 (<0.0001) |
| Freeze ratio z-axis | 0.40 (<0.0001) | P-max Max amplitude z-axis | −0.41 (<0.0001) |
| Locomotion band power z-axis | −0.36 (<0.0001) | Zero crossing rate z-axis | 0.61 (<0.0001) |
| Zero crossing rate z-axis | 0.35 (<0.0001) | – | – |
| (a) Lateral left tibial accelerometer. | ||
| Performance | SVM | kNN |
| Accuracy (%) | 84.11 | 83.40 |
| Precision (%) | 87.50 | 88.36 |
| Specificity (%) | 87.21 | 88.61 |
| Sensitivity (%) | 81.30 | 78.67 |
| F-score (%) | 84.26 | 83.23 |
| (b) Lateral left tibial gyroscope. | ||
| Performance | SVM | kNN |
| Accuracy (%) | 84.44 | 85.13 |
| Precision (%) | 88.43 | 87.94 |
| Specificity (%) | 88.38 | 87.53 |
| Sensitivity (%) | 80.85 | 82.96 |
| F-score (%) | 84.47 | 85.38 |
| Performance | SVM | kNN |
|---|---|---|
| Accuracy (%) | 85.12 | 85.06 |
| Precision (%) | 88.72 | 89.37 |
| Specificity (%) | 88.55 | 89.40 |
| Sensitivity (%) | 82.20 | 81.11 |
| F-score (%) | 85.23 | 85.04 |
| Performance | Minimal Setup | Complex Setup | ||
|---|---|---|---|---|
| kNN | SVM | kNN | SVM | |
| Accuracy (%) | 84.59 | 85.71 | 87.65 | 88 |
| Sensitivity (%) | 82.65 | 81.76 | 86.04 | 85.14 |
| Precision (%) | 86.18 | 84.49 | 88.86 | 87.71 |
| Specificity (%) | 82.60 | 87.23 | 86.13 | 88.38 |
| F-score (%) | 82.63 | 84.41 | 86.08 | 86.73 |
| Subject | Episodes | Length (Range) (s) | Episodes Detected with SIA | Episodes Detected with SDA |
|---|---|---|---|---|
| 1 | 22 | 12.12 (3.3–35.4) | 22 | 19 |
| 2 | 1 | 3.3 | 1 | – |
| 3 | 33 | 52.33 (3.3–238.5) | 32 | 32 |
| 4 | 15 | 9.22 (4.5–25.20) | 15 | 8 |
| 6 | 22 | 16.5 (5.4–32.4) | 22 | 21 |
| 7 | 28 | 12.02 (3.3–43.5)) | 27 | 27 |
| 8 | 44 | 19.98 (3.3–58.20) | 39 | 37 |
| 9 | 22 | 4.25 (3.3–8.4) | 7 | 0 |
| 10 | 30 | 25.48 (4.2–64.20) | 30 | 30 |
| 11 | 36 | 12.58 (3.3–45) | 26 | 34 |
| 12 | 11 | 22.42 (4.5–46.5) | 11 | 11 |
| Tot | 264 | 17.29 (3.79–54.6) | 232 | 219 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesin, L.; Porcu, P.; Russu, D.; Farina, G.; Borzì, L.; Zhang, W.; Guo, Y.; Olmo, G. A Multi-Modal Analysis of the Freezing of Gait Phenomenon in Parkinson’s Disease. Sensors 2022, 22, 2613. https://doi.org/10.3390/s22072613
Mesin L, Porcu P, Russu D, Farina G, Borzì L, Zhang W, Guo Y, Olmo G. A Multi-Modal Analysis of the Freezing of Gait Phenomenon in Parkinson’s Disease. Sensors. 2022; 22(7):2613. https://doi.org/10.3390/s22072613
Chicago/Turabian StyleMesin, Luca, Paola Porcu, Debora Russu, Gabriele Farina, Luigi Borzì, Wei Zhang, Yuzhu Guo, and Gabriella Olmo. 2022. "A Multi-Modal Analysis of the Freezing of Gait Phenomenon in Parkinson’s Disease" Sensors 22, no. 7: 2613. https://doi.org/10.3390/s22072613
APA StyleMesin, L., Porcu, P., Russu, D., Farina, G., Borzì, L., Zhang, W., Guo, Y., & Olmo, G. (2022). A Multi-Modal Analysis of the Freezing of Gait Phenomenon in Parkinson’s Disease. Sensors, 22(7), 2613. https://doi.org/10.3390/s22072613











