Patterns in Dried Droplets to Detect Unfolded BSA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protein Solutions Preparation
2.2. Droplet Evaporation
2.3. Image Acquisition
2.4. Image Analysis
3. Results
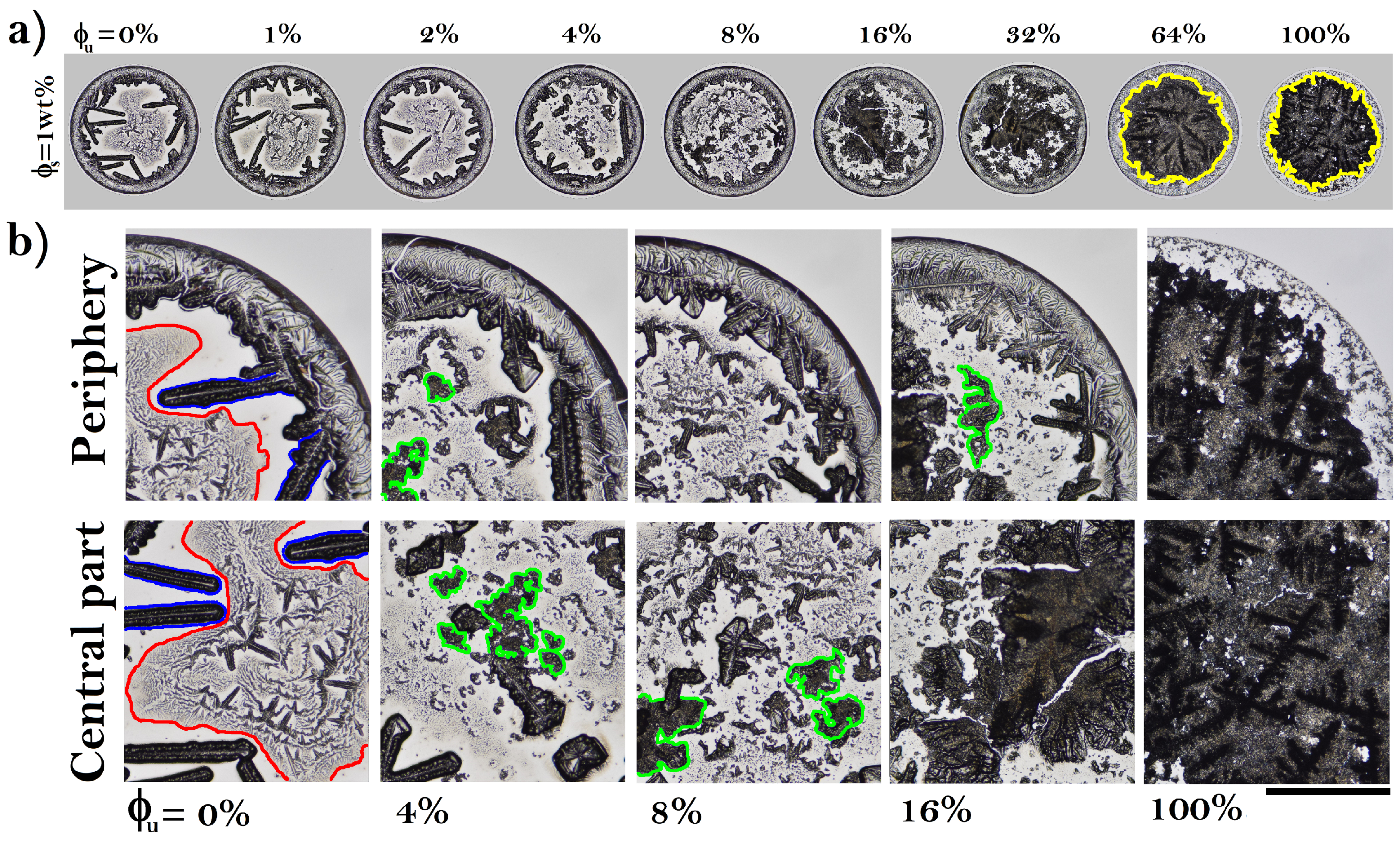
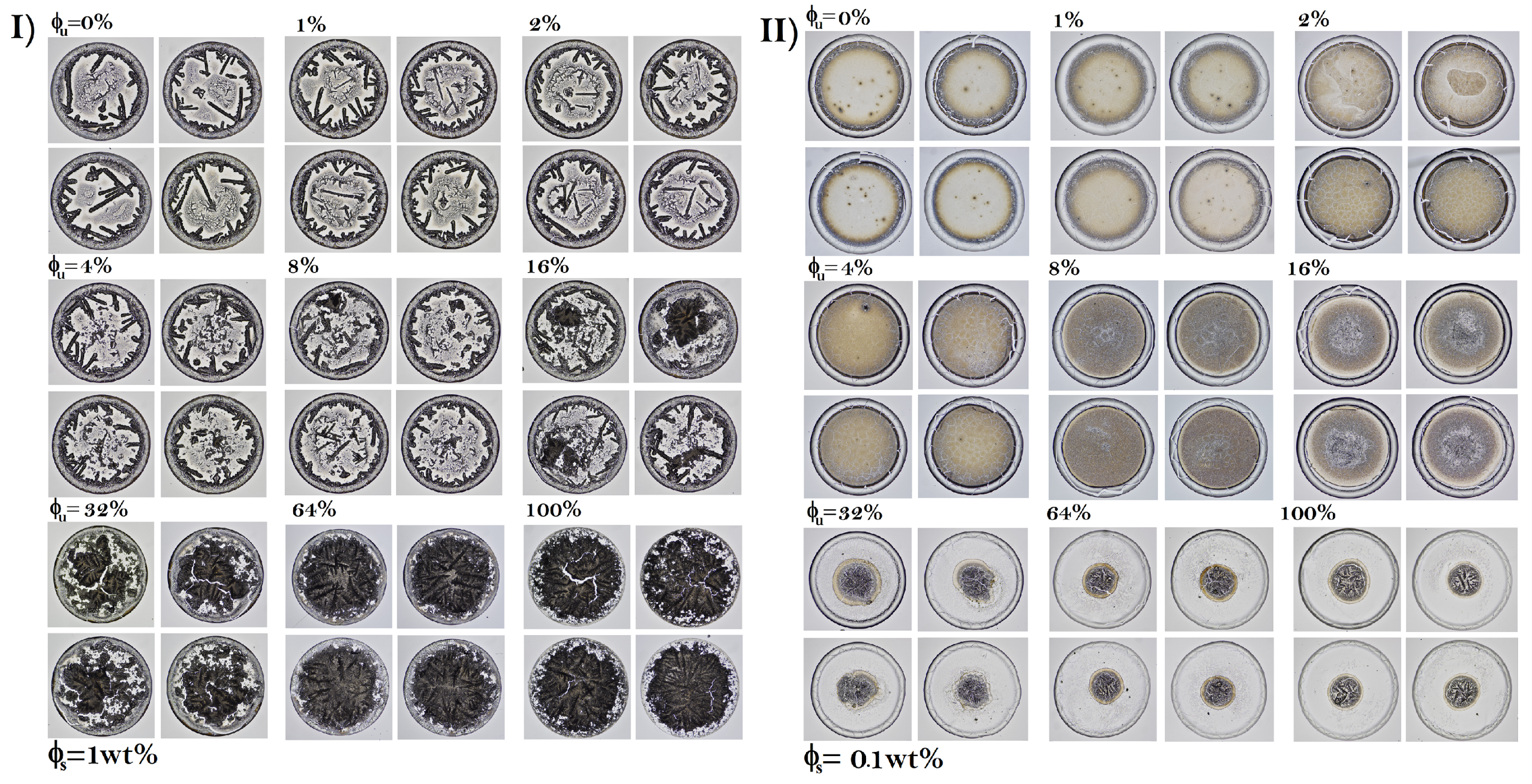
3.1. Patterns in Dried Droplets of Proteins
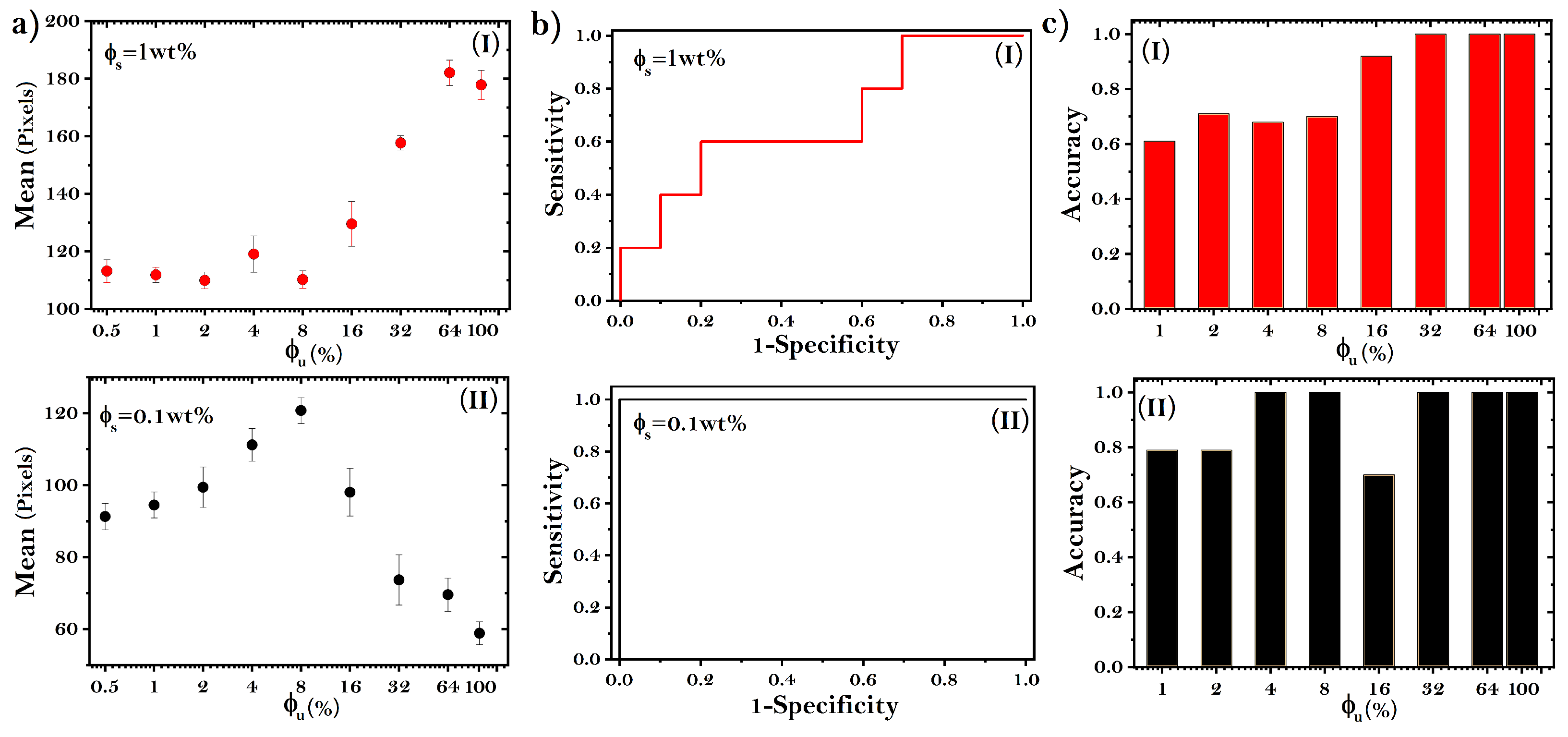
3.2. Accuracy to Identify Unfolded Proteins
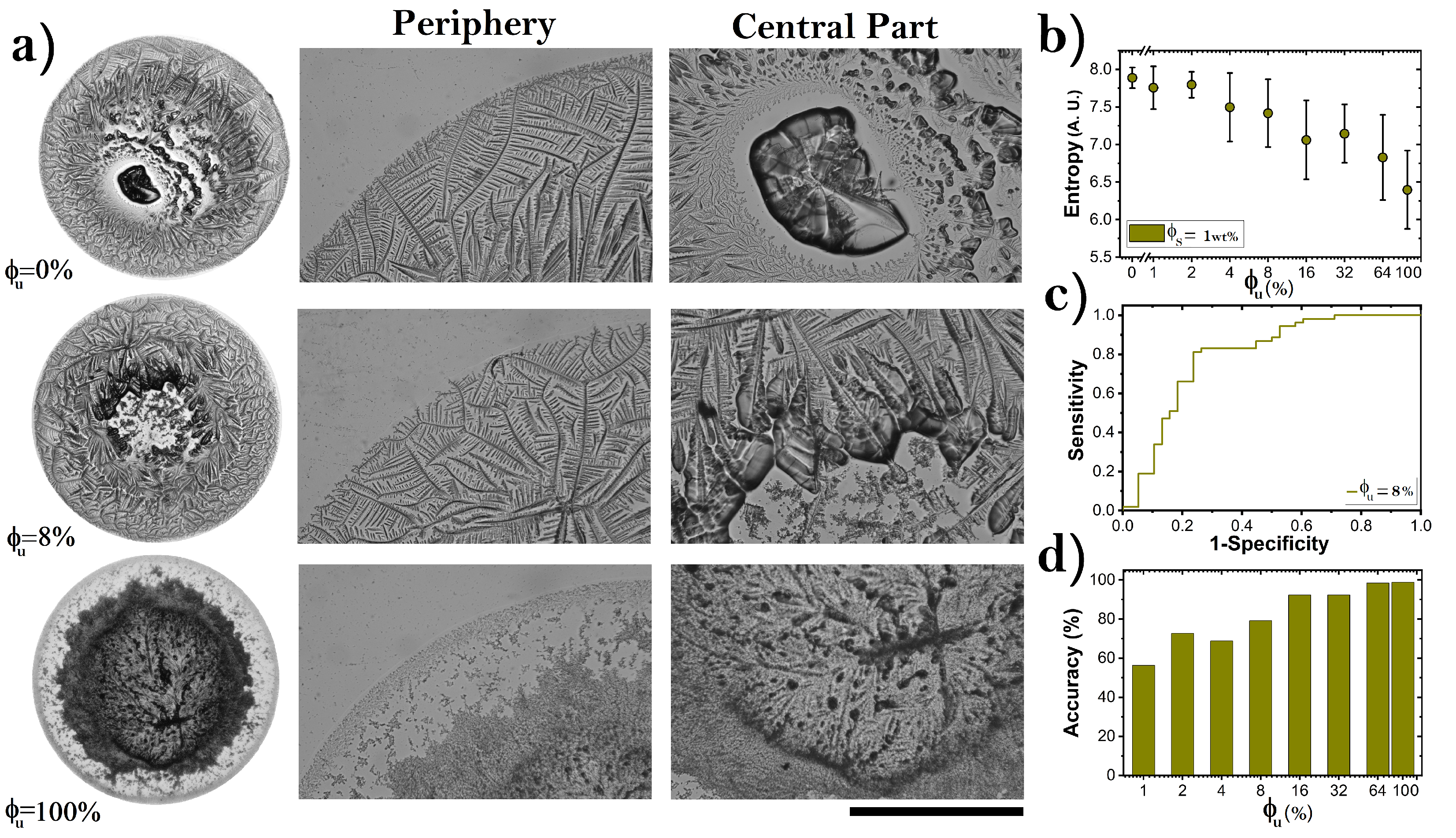
3.3. Pattern Formation in Dried Droplets of Folded and Unfolded Proteins
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aguzzi, A.; O’connor, T. Protein aggregation diseases: Pathogenicity and therapeutic perspectives. Nat. Rev. Drug Discov. 2010, 9, 237–248. [Google Scholar] [CrossRef]
- Wang, W.; Nema, S.; Teagarden, D. Protein aggregation—Pathways and influencing factors. Int. J. Pharm. 2010, 390, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Stefani, M.; Dobson, C.M. Protein aggregation and aggregate toxicity: New insights into protein folding, misfolding diseases and biological evolution. J. Mol. Med. 2003, 81, 678–699. [Google Scholar] [CrossRef]
- Thomas, P.J.; Qu, B.H.; Pedersen, P.L. Defective protein folding as a basis of human disease. Trends Biochem. Sci. 1995, 20, 456–459. [Google Scholar] [CrossRef]
- Merlini, G.; Bellotti, V.; Andreola, A.; Palladini, G.; Obici, L.; Casarini, S.; Perfetti, V. Protein aggregation. Clin. Chem. Lab. Med. 2001. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.L.; Westermark, G.T.; Westermark, P.; Kahn, S.E. Islet amyloid: A critical entity in the pathogenesis of type 2 diabetes. J. Clin. Endocrinol. Metab. 2004, 89, 3629–3643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobson, C.M. The structural basis of protein folding and its links with human disease. Philos. Trans. R. Soc. London. Ser. Biol. Sci. 2001, 356, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Rapis, E. A change in the physical state of a nonequilibrium blood plasma protein film in patients with carcinoma. Tech. Phys. 2002, 47, 510–512. [Google Scholar] [CrossRef]
- Gulka, C.P.; Swartz, J.D.; Trantum, J.R.; Davis, K.M.; Peak, C.M.; Denton, A.J.; Haselton, F.R.; Wright, D.W. Coffee rings as low-resource diagnostics: Detection of the malaria biomarker Plasmodium falciparum histidine-rich protein-II using a surface-coupled ring of Ni (II) NTA gold-plated polystyrene particles. ACS Appl. Mater. Interfaces 2014, 6, 6257–6263. [Google Scholar] [CrossRef]
- Choi, S.; Moon, S.W.; Shin, J.H.; Park, H.K.; Jin, K.H. Label-free biochemical analytic method for the early detection of adenoviral conjunctivitis using human tear biofluids. Anal. Chem. 2014, 86, 11093–11099. [Google Scholar] [CrossRef]
- Ríos-Ramírez, M.; Reyes-Figueroa, A.; Ruiz-Suárez, J.; González-Gutiérrez, J. Pattern formation of stains from dried drops to identify spermatozoa motility. Colloids Surf. B Biointerfaces 2018, 169, 486–493. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Ray, R.; Ayushman, M.; Sood, P.; Bhattacharyya, M.; Sarkar, D.; DasGupta, S. Interfacial energy driven distinctive pattern formation during the drying of blood droplets. J. Colloid Interface Sci. 2020, 573, 307–316. [Google Scholar] [CrossRef]
- Bahmani, L.; Neysari, M.; Maleki, M. The study of drying and pattern formation of whole human blood drops and the effect of thalassaemia and neonatal jaundice on the patterns. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 66–75. [Google Scholar] [CrossRef]
- Brutin, D.; Sobac, B.; Loquet, B.; Sampol, J. Pattern formation in drying drops of blood. J. Fluid Mech. 2011, 667, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, M.; Nahidi, F.; Majdfar, Z. Comparison of fern and evaporation tests for detection of ruptured fetal membranes. EMHJ-East. Mediterr. Health J. 2007, 13, 197–200. [Google Scholar]
- González-Gutiérrez, J.; Pérez-Isidoro, R.; Pérez-Camacho, M.; Ruiz-Suárez, J. The calorimetric properties of liposomes determine the morphology of dried droplets. Colloids Surf. B Biointerfaces 2017, 155, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Thokchom, A.K.; Swaminathan, R.; Singh, A. Fluid flow and particle dynamics inside an evaporating droplet containing live bacteria displaying chemotaxis. Langmuir 2014, 30, 12144–12153. [Google Scholar] [CrossRef]
- Kasyap, T.; Koch, D.L.; Wu, M. Bacterial collective motion near the contact line of an evaporating sessile drop. Phys. Fluids 2014, 26, 111703. [Google Scholar] [CrossRef]
- Keya, J.J.; Kudoh, H.; Kabir, A.M.R.; Inoue, D.; Miyamoto, N.; Tani, T.; Kakugo, A.; Shikinaka, K. Radial alignment of microtubules through tubulin polymerization in an evaporating droplet. PLoS ONE 2020, 15, e0231352. [Google Scholar] [CrossRef]
- Seo, H.W.; Jung, N.; Yoo, C.S. Oscillation dynamics of colloidal particles caused by surfactant in an evaporating droplet. J. Mech. Sci. Technol. 2020, 34, 801–808. [Google Scholar] [CrossRef]
- Tayeb, R.; Zhang, Y. Evaporation induced self-assembly of rough colloids: A multiscale simulation study. Int. J. Heat Mass Transf. 2021, 179, 121681. [Google Scholar] [CrossRef]
- Misyura, S. Different modes of heat transfer and crystallization in a drop of NaCl solution: The influence of key factors on the crystallization rate and the heat transfer coefficient. Int. J. Therm. Sci. 2021, 159, 106602. [Google Scholar] [CrossRef]
- González-Gutiérrez, J.; Moctezuma, O.; Angeles, V.; Rios-Ramirez, M.; Zetina, S.; Marin, A.; Zenit, R. Pattern formation by droplet evaporation and imbibition in watercolor paintings. arXiv 2019, arXiv:1909.09098. [Google Scholar]
- Efstratiou, M.; Christy, J.; Bonn, D.; Sefiane, K. The Effect of Substrate Temperature on the Evaporative Behaviour and Desiccation Patterns of Foetal Bovine Serum Drops. Colloids Interfaces 2021, 5, 43. [Google Scholar] [CrossRef]
- Khatun, A.; Haque, S.; Tarafdar, S.; Dutta, T. Impact of varying preparation methods of colloidal suspensions on droplet desiccation patterns on hydrophobic and hydrophilic substrates. Phys. Scr. 2020, 96, 025211. [Google Scholar] [CrossRef]
- Hegde, O.; Chatterjee, R.; Rasheed, A.; Chakravortty, D.; Basu, S. Multiscale vapor-mediated dendritic pattern formation and bacterial aggregation in complex respiratory biofluid droplets. J. Colloid Interface Sci. 2022, 606, 2011–2023. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Hampton, M.A.; Nguyen, A.V. Evaporation of nanoparticle droplets on smooth hydrophobic surfaces: The inner coffee ring deposits. J. Phys. Chem. C 2013, 117, 4707–4716. [Google Scholar] [CrossRef]
- Kaya, D.; Belyi, V.; Muthukumar, M. Pattern formation in drying droplets of polyelectrolyte and salt. J. Chem. Phys. 2010, 133, 114905. [Google Scholar] [CrossRef]
- Deegan, R.D. Pattern formation in drying drops. Phys. Rev. E 2000, 61, 475. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Huang, X.; Zhao, Y. Formation of wavy-ring crack in drying droplet of protein solutions. Sci. China Technol. Sci. 2018, 61, 949–958. [Google Scholar] [CrossRef] [Green Version]
- Mulka, R.; Kujawska, A.; Zajączkowski, B.; Mancin, S.; Buschmann, M. Drying silica-nanofluid droplets. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126730. [Google Scholar] [CrossRef]
- Yan, N.; Luo, H.; Yu, H.; Liu, Y.; Jing, G. Drying crack patterns of sessile drops with tuned contact line. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126780. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Z.; Lyu, P.; Ding, Z.; Man, X. Deposition pattern of drying droplets. Commun. Theor. Phys. 2021, 73, 047601. [Google Scholar] [CrossRef]
- Tarasevich, Y.Y. Mechanisms and models of the dehydration self-organization in biological fluids. Physics-Uspekhi 2004, 47, 717. [Google Scholar] [CrossRef] [Green Version]
- Takhistov, P.; Chang, H.C. Complex stain morphologies. Ind. Eng. Chem. Res. 2002, 41, 6256–6269. [Google Scholar] [CrossRef]
- Yakhno, T. Salt-induced protein phase transitions in drying drops. J. Colloid Interface Sci. 2008, 318, 225–230. [Google Scholar] [CrossRef]
- Gorr, H.M.; Zueger, J.M.; Barnard, J.A. Lysozyme pattern formation in evaporating drops. Langmuir 2012, 28, 4039–4042. [Google Scholar] [CrossRef]
- Gorr, H.M.; Xiong, Z.; Barnard, J.A. Pattern recognition for identification of lysozyme droplet solution chemistry. Colloids Surf. B Biointerfaces 2014, 115, 170–175. [Google Scholar] [CrossRef]
- Pal, A.; Gope, A.; Athair, A.S.; Iannacchione, G.S. A comparative study of the drying evolution and dried morphology of two globular proteins in de-ionized water solutions. RSC Adv. 2020, 10, 16906–16916. [Google Scholar] [CrossRef]
- Carreón, Y.J.; González-Gutiérrez, J.; Pérez-Camacho, M.; Mercado-Uribe, H. Patterns produced by dried droplets of protein binary mixtures suspended in water. Colloids Surf. B Biointerfaces 2018, 161, 103–110. [Google Scholar] [CrossRef]
- Noh, H.; Vogler, E.A. Volumetric interpretation of protein adsorption: Competition from mixtures and the Vroman effect. Biomaterials 2007, 28, 405–422. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Mohamed, G.J. Complex protein patterns formation via salt-induced self-assembly and droplet evaporation. Eur. Phys. J. E 2010, 33, 19–26. [Google Scholar] [CrossRef]
- Yakhno, T.A. Complex pattern formation in sessile droplets of protein-salt solutions with low protein content. What substance fabricates these patterns. Phys. Chem. 2011, 1, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Annarelli, C.; Fornazero, J.; Bert, J.; Colombani, J. Crack patterns in drying protein solution drops. Eur. Phys. J. E 2001, 5, 599–603. [Google Scholar] [CrossRef] [Green Version]
- Gorr, H.M.; Zueger, J.M.; Barnard, J.A. Characteristic size for onset of coffee-ring effect in evaporating lysozyme-water solution droplets. J. Phys. Chem. B 2012, 116, 12213–12220. [Google Scholar] [CrossRef]
- Pauchard, L.; Parisse, F.; Allain, C. Influence of salt content on crack patterns formed through colloidal suspension desiccation. Phys. Rev. E 1999, 59, 3737. [Google Scholar] [CrossRef]
- Gorr, H.M.; Zueger, J.M.; McAdams, D.R.; Barnard, J.A. Salt-induced pattern formation in evaporating droplets of lysozyme solutions. Colloids Surf. B Biointerfaces 2013, 103, 59–66. [Google Scholar] [CrossRef]
- Carreon, Y.J.; Ríos-Ramírez, M.; Moctezuma, R.; González-Gutiérrez, J. Texture analysis of protein deposits produced by droplet evaporation. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chhasatia, V.H.; Joshi, A.S.; Sun, Y. Effect of relative humidity on contact angle and particle deposition morphology of an evaporating colloidal drop. Appl. Phys. Lett. 2010, 97, 231909. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, M.D.; Dutta, T.; Tarafdar, S. Growth kinetics of NaCl crystals in a drying drop of gelatin: Transition from faceted to dendritic growth. Soft Matter 2015, 11, 6938–6947. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.; Gope, A.; Iannacchione, G. Temperature and Concentration Dependence of Human Whole Blood and Protein Drying Droplets. Biomolecules 2021, 231, 2021. [Google Scholar] [CrossRef]
- Gatapova, E.Y.; Semenov, A.A.; Zaitsev, D.V.; Kabov, O.A. Evaporation of a sessile water drop on a heated surface with controlled wettability. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 776–785. [Google Scholar] [CrossRef]
- Carreón, Y.J.; Ríos-Ramírez, M.; Vázquez-Vergara, P.; Salinas-Almaguer, S.; Cipriano-Urbano, I.; Briones-Aranda, A.; Díaz-Hernández, O.; Santos, G.J.E.; González-Gutiérrez, J. Effects of substrate temperature on patterns produced by dried droplets of proteins. Colloids Surf. B Biointerfaces 2021, 203, 111763. [Google Scholar] [CrossRef]
- Zang, D.; Tarafdar, S.; Tarasevich, Y.Y.; Choudhury, M.D.; Dutta, T. Evaporation of a droplet: From physics to applications. Phys. Rep. 2019, 804, 1–56. [Google Scholar] [CrossRef]
- Carreón, Y.J.; Díaz-Hernández, O.; Escalera Santos, G.J.; Cipriano-Urbano, I.; Solorio-Ordaz, F.J.; González-Gutiérrez, J.; Zenit, R. Texture Analysis of Dried Droplets for the Quality Control of Medicines. Sensors 2021, 21, 4048. [Google Scholar] [CrossRef]
- Pal, A.; Gope, A.; Iannacchione, G.S. Statistical Image Analysis of Drying Bovine Serum Albumin Droplets in Phosphate Buffered Saline. arXiv 2021, arXiv:2105.05922. [Google Scholar]
- Hospelhorn, V.D.; Jensen, E.V. Sulfhydryl-Dependent Aggregation Accompanying the Thermal Denaturation of Bovine Plasma Albumin1, 2. J. Am. Chem. Soc. 1954, 76, 2830–2832. [Google Scholar] [CrossRef]
- González-Gutiérrez, J.; Pérez-Isidoro, R.; Ruiz-Suárez, J. A technique based on droplet evaporation to recognize alcoholic drinks. Rev. Sci. Instrum. 2017, 88, 074101. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreón, Y.J.P.; Gómez-López, M.L.; Díaz-Hernández, O.; Vazquez-Vergara, P.; Moctezuma, R.E.; Saniger, J.M.; González-Gutiérrez, J. Patterns in Dried Droplets to Detect Unfolded BSA. Sensors 2022, 22, 1156. https://doi.org/10.3390/s22031156
Carreón YJP, Gómez-López ML, Díaz-Hernández O, Vazquez-Vergara P, Moctezuma RE, Saniger JM, González-Gutiérrez J. Patterns in Dried Droplets to Detect Unfolded BSA. Sensors. 2022; 22(3):1156. https://doi.org/10.3390/s22031156
Chicago/Turabian StyleCarreón, Yojana J. P., Mary Luz Gómez-López, Orlando Díaz-Hernández, Pamela Vazquez-Vergara, Rosario E. Moctezuma, José M. Saniger, and Jorge González-Gutiérrez. 2022. "Patterns in Dried Droplets to Detect Unfolded BSA" Sensors 22, no. 3: 1156. https://doi.org/10.3390/s22031156
APA StyleCarreón, Y. J. P., Gómez-López, M. L., Díaz-Hernández, O., Vazquez-Vergara, P., Moctezuma, R. E., Saniger, J. M., & González-Gutiérrez, J. (2022). Patterns in Dried Droplets to Detect Unfolded BSA. Sensors, 22(3), 1156. https://doi.org/10.3390/s22031156






