Efficacy of Robot-Assisted Gait Therapy Compared to Conventional Therapy or Treadmill Training in Children with Cerebral Palsy: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Source Data and Search Strategy
2.3. Study Screening: Inclusion and Exclusion Criteria
2.4. Data Extraction
2.5. Variables
2.6. Methodological Quality and Quality of Evidence Assessment
2.7. Statistical Analysis
3. Results
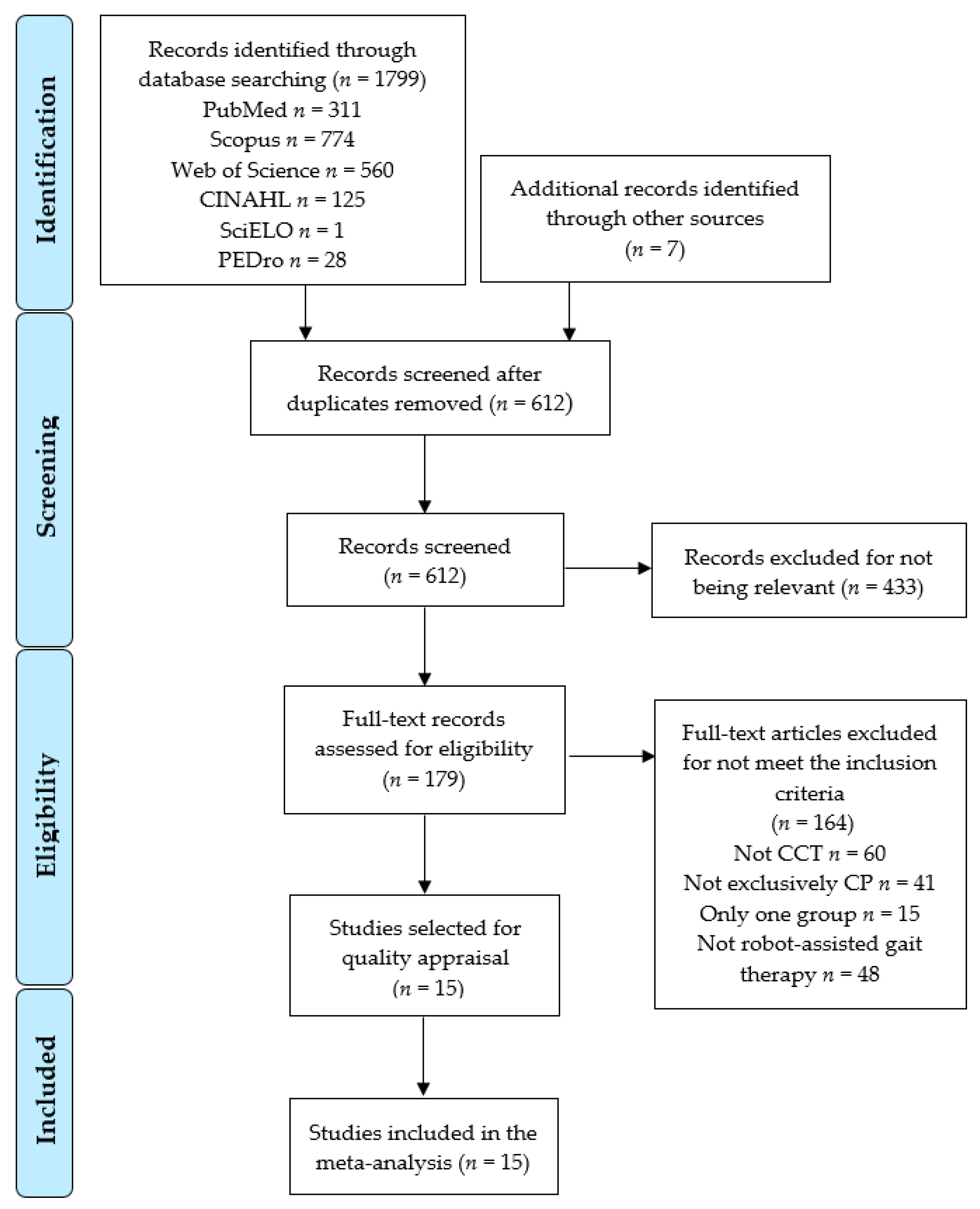
3.1. Search Results
3.2. Characteristics of the Included Studies
3.3. Methodological Quality of Included Studies
3.4. Quantitative Synthesis
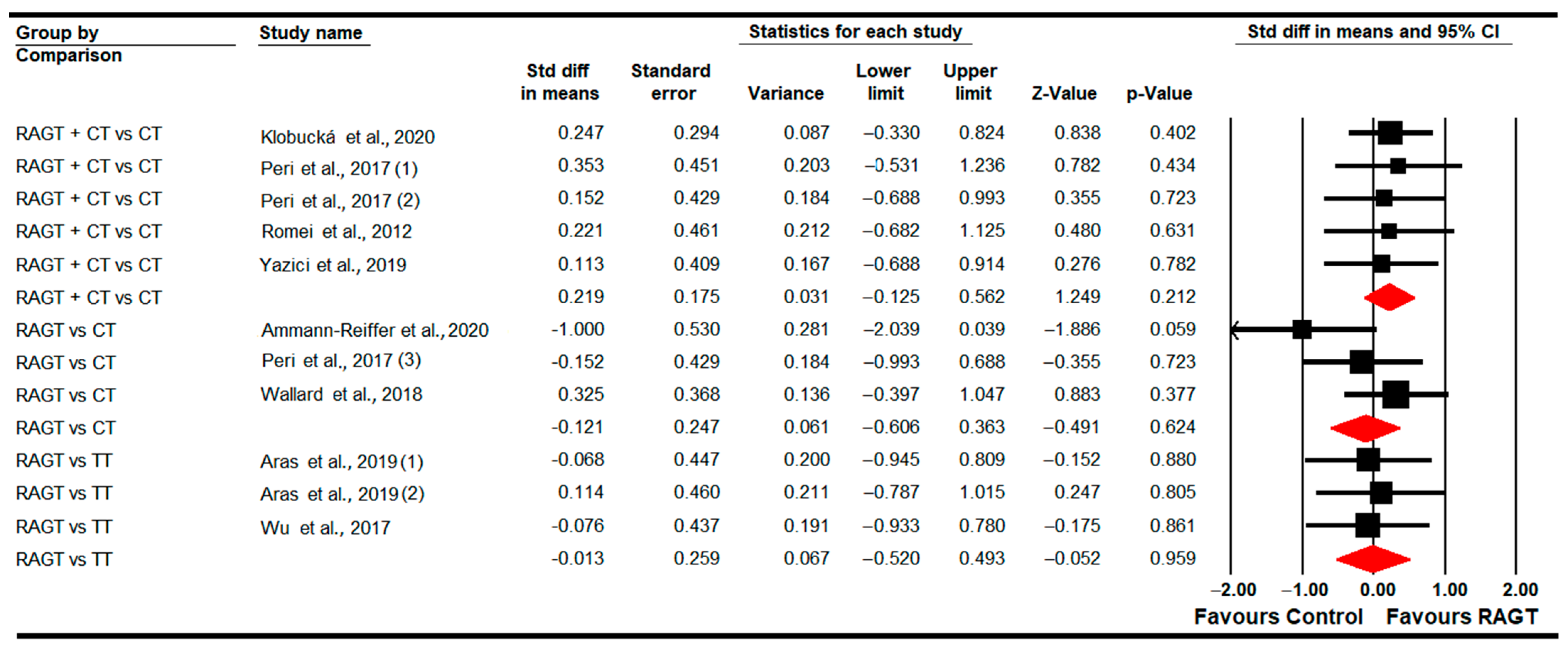
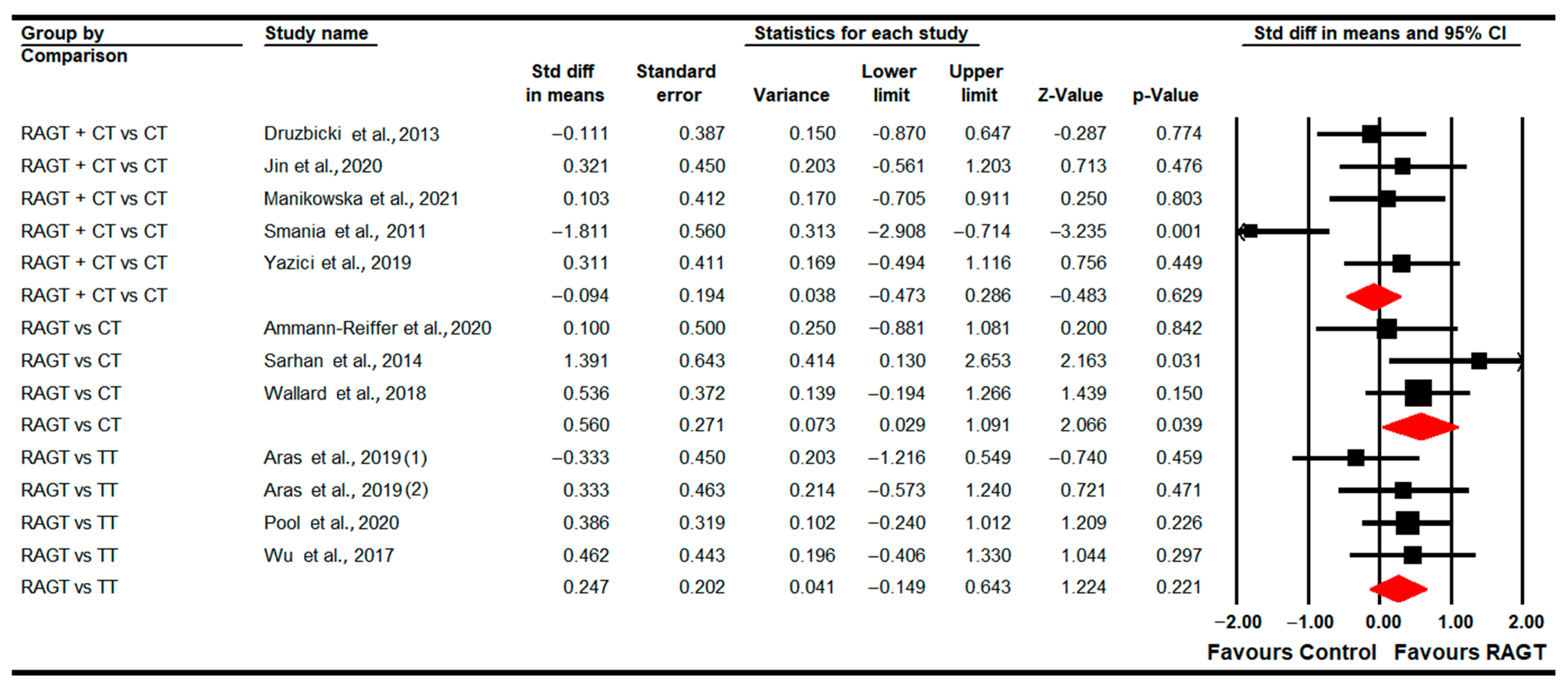
3.4.1. Gait Speed
3.4.2. Step Length
3.4.3. Step Width
3.4.4. Stride Length
3.4.5. Walking Distance
3.4.6. Cadence
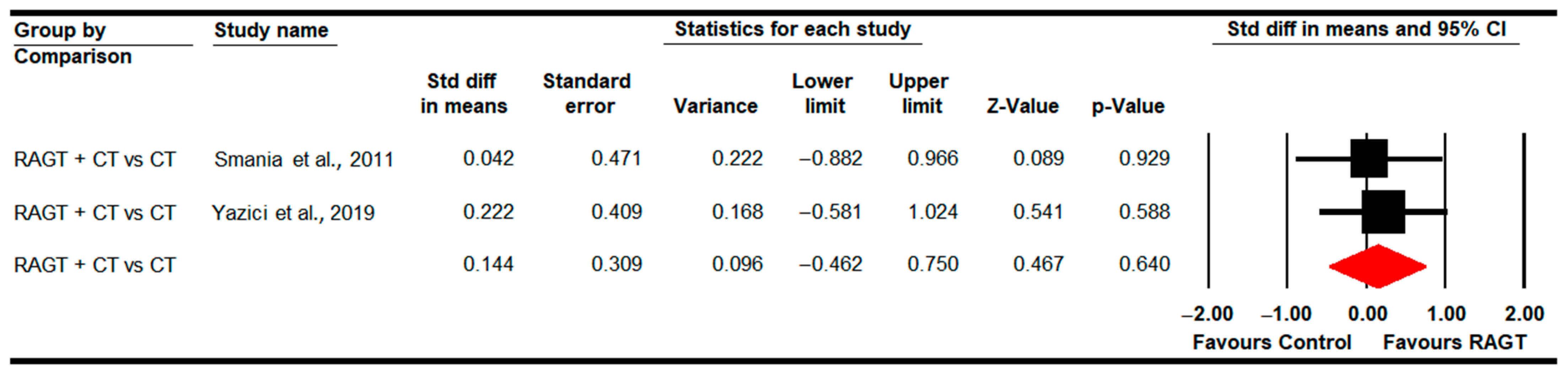
3.4.7. Standing Ability (GMFM-D Dimension)
3.4.8. Walking, Running and Jumping Ability (GMFM-E Dimension)
3.4.9. Gross Motor Function (Total Score)
3.4.10. Functional Independence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carr, L.J. Definition and classification of cerebral palsy. Dev. Med. Child Neurol. 2007, 47, 508. [Google Scholar] [CrossRef]
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. 2007, 49, 8–14. [Google Scholar]
- Michael-Asalu, A.; Taylor, G.; Campbell, H.; Lelea, L.L.; Kirby, R.S. Cerebral Palsy: Diagnosis, Epidemiology, Genetics, and Clinical Update. Adv. Pediatr. 2019, 66, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.; Noritz, G.; Maitre, N.L. Implementation of Early Diagnosis and Intervention Guidelines for Cerebral Palsy in a High-Risk Infant Follow-Up Clinic. Pediatr. Neurol. 2017, 76, 66–71. [Google Scholar] [CrossRef]
- Shih, S.T.F.; Tonmukayakul, U.; Imms, C.; Reddihough, D.; Graham, H.K.; Cox, L.; Carter, R. Economic evaluation and cost of interventions for cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2018, 60, 543–558. [Google Scholar] [CrossRef]
- Oskoui, M.; Coutinho, F.; Dykeman, J.; Jetté, N.; Pringsheim, T. An update on the prevalence of cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2013, 55, 509–519. [Google Scholar] [CrossRef]
- McIntyre, S.; Goldsmith, S.; Webb, A.; Ehlinger, V.; Hollung, S.J.; McConnell, K.; Arnaud, C.; Smithers-Sheedy, H.; Oskoui, M.; Khandaker, G.; et al. Global prevalence of cerebral palsy: A systematic analysis. Dev. Med. Child Neurol. 2022, 64, 1494–1506. [Google Scholar] [CrossRef]
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.C.; et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017, 171, 897–907. [Google Scholar] [CrossRef]
- Paneth, N.; Yeargin-Allsopp, M. Thinking about differences in the worldwide prevalence of cerebral palsy. Dev. Med. Child Neurol. 2022, 64, 1436–1437. [Google Scholar] [CrossRef]
- Khan, S.A.; Talat, S.; Malik, M.I. Risk factors, types, and neuroimaging findings in Children with Cerebral Palsy. Pak. J. Med. Sci. 2022, 38, 1738–1742. [Google Scholar] [CrossRef]
- Ellenberg, J.H.; Nelson, K.B. The association of cerebral palsy with birth asphyxia: A definitional quagmire. Dev. Med. Child Neurol. 2013, 55, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Forthun, I.; Wilcox, A.J.; Strandberg-Larsen, K.; Moster, D.; Nohr, E.A.; Lie, R.T.; Surén, P.; Tollånes, M.C. Maternal Prepregnancy BMI and Risk of Cerebral Palsy in Offspring. Pediatrics 2016, 138, e20160874. [Google Scholar] [CrossRef] [PubMed]
- Linsell, L.; Malouf, R.; Morris, J.; Kurinczuk, J.J.; Marlow, N. Prognostic factors for cerebral palsy and motor impairment in children born very preterm or very low birthweight: A systematic review. Dev. Med. Child Neurol. 2016, 58, 554–569. [Google Scholar] [CrossRef]
- Morgan, C.; Fetters, L.; Adde, L.; Badawi, N.; Bancale, A.; Boyd, R.N.; Chorna, O.; Cioni, G.; Damiano, D.L.; Darrah, J.; et al. Early Intervention for Children Aged 0 to 2 Years with or at High Risk of Cerebral Palsy: International Clinical Practice Guideline Based on Systematic Reviews. JAMA Pediatr. 2021, 175, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Paulson, A.; Vargus-Adams, J. Overview of Four Functional Classification Systems Commonly Used in Cerebral Palsy. Children 2017, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Sewell, M.D.; Eastwood, D.M.; Wimalasundera, N. Managing common symptoms of cerebral palsy in children. BMJ 2014, 349, g5474. [Google Scholar] [CrossRef]
- Asano, D.; Takeda, M.; Nobusako, S.; Morioka, S. Self-Rated Depressive Symptoms in Children and Youth with and without Cerebral Palsy: A Pilot Study. Behav. Sci. 2020, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Pizzighello, S.; Uliana, M.; Michielotto, M.; Pellegri, A.; Vascello, M.G.F.; Piccoli, S.; Martinuzzi, M.; Martinuzzi, A. Psychiatric symptoms in adult patients with cerebral palsy: A cohort study. Front. Neurol. 2022, 13, 998922. [Google Scholar] [CrossRef]
- Caramico-Favero, D.C.O.; Guedes, Z.C.F.; de Morais, M.B. Food intake, nutritional status and gastrointestinal symptoms in children with cerebral palsy. Arq. Gastroenterol. 2018, 55, 352–357. [Google Scholar] [CrossRef]
- van Gorp, M.; Dallmeijer, A.J.; van Wely, L.; de Groot, V.; Terwee, C.B.; Flens, G.; Stam, H.J.; van der Slot, W.; Roebroeck, M.E. Pain, fatigue, depressive symptoms and sleep disturbance in young adults with cerebral palsy. Disabil. Rehabil. 2021, 43, 2164–2171. [Google Scholar] [CrossRef]
- Johnston, M.V.; Hoon, A.H. Cerebral Palsy. NeuroMolecular Med. 2006, 8, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Nemanich, S.T.; Mueller, B.A.; Gillick, B.T. Neurite orientation dispersion and density imaging quantifies corticospinal tract microstructural organization in children with unilateral cerebral palsy. Hum. Brain Mapp. 2019, 40, 4888–4900. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.N.; Skuza, P.P.; Sandelance, M.; Flett, P. Upper limb impairments, process skills, and outcome in children with unilateral cerebral palsy. Dev. Med. Child Neurol. 2019, 61, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Straathof, E.J.M.; Hamer, E.G.; Hensens, K.J.; La Bastide-van Gemert, S.; Heineman, K.R.; Hadders-Algra, M. Development of muscle tone impairments in high-risk infants: Associations with cerebral palsy and cystic periventricular leukomalacia. Eur. J. Paediatr. Neurol. 2022, 37, 12–18. [Google Scholar] [CrossRef] [PubMed]
- MacWilliams, B.A.; Prasad, S.; Shuckra, A.L.; Schwartz, M.H. Causal factors affecting gross motor function in children diagnosed with cerebral palsy. PLoS ONE 2022, 17, e0270121. [Google Scholar] [CrossRef]
- Ouyang, R.-G.; Yang, C.-N.; Qu, Y.-L.; Koduri, M.P.; Chien, C.-W. Effectiveness of hand-arm bimanual intensive training on upper extremity function in children with cerebral palsy: A systematic review. Eur. J. Paediatr. Neurol. 2020, 25, 17–28. [Google Scholar] [CrossRef]
- O’Shea, T.M. Diagnosis, Treatment, and Prevention of Cerebral Palsy in Near-Term/Term Infants. Clin. Obstet. Gynecol. 2008, 51, 816. [Google Scholar] [CrossRef]
- Belizón-Bravo, N.; Romero-Galisteo, R.P.; Cano-Bravo, F.; Gonzalez-Medina, G.; Pinero-Pinto, E.; Luque-Moreno, C. Effects of Dynamic Suit Orthoses on the Spatio-Temporal Gait Parameters in Children with Cerebral Palsy: A Systematic Review. Children 2021, 8, 1016. [Google Scholar] [CrossRef]
- Barreira, C.C.; Forner-Cordero, A.; Grangeiro, P.M.; Moura, R.T. Kinect v2 based system for gait assessment of children with cerebral palsy in rehabilitation settings. J. Med. Eng. Technol. 2020, 44, 198–202. [Google Scholar] [CrossRef]
- Booth, A.T.C.; Buizer, A.I.; Meyns, P.; Oude Lansink, I.L.B.; Steenbrink, F.; van der Krogt, M.M. The efficacy of functional gait training in children and young adults with cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2018, 60, 866–883. [Google Scholar] [CrossRef]
- Dimakopoulos, R.; Syrogiannopoulos, G.; Grivea, I.; Dailiana, Z.; Youroukos, S.; Spinou, A. Kinematic and Temporospatial Changes in Children with Cerebral Palsy during the Initial Stages of Gait Development. Dev. Neurorehabilit. 2022, 25, 10–18. [Google Scholar] [CrossRef]
- Chakraborty, S.; Nandy, A.; Kesar, T.M. Gait deficits and dynamic stability in children and adolescents with cerebral palsy: A systematic review and meta-analysis. Clin. Biomech. 2020, 71, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, K.; Molinini, R.M.; Panibatla, S.T.; Chow, J.C.; Dusing, S.C. Physical therapy interventions to improve sitting ability in children with or at-risk for cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2021, 63, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Christy, J.B.; Chapman, C.G.; Murphy, P. The effect of intense physical therapy for children with cerebral palsy. J. Pediatr. Rehabil. Med. 2012, 5, 159–170. [Google Scholar] [CrossRef]
- Montoro-Cárdenas, D.; Cortés-Pérez, I.; Zagalaz-Anula, N.; Osuna-Pérez, M.C.; Obrero-Gaitán, E.; Lomas-Vega, R. Nintendo Wii Balance Board therapy for postural control in children with cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2021, 63, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Montoro-Cárdenas, D.; Cortés-Pérez, I.; Ibancos-Losada, M.d.R.; Zagalaz-Anula, N.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Nintendo® Wii Therapy Improves Upper Extremity Motor Function in Children with Cerebral Palsy: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 12343. [Google Scholar] [CrossRef]
- Dominguez-Romero, J.G.; Molina-Aroca, A.; Moral-Munoz, J.A.; Luque-Moreno, C.; Lucena-Anton, D. Effectiveness of Mechanical Horse-Riding Simulators on Postural Balance in Neurological Rehabilitation: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Pérez, I.; Zagalaz-Anula, N.; Montoro-Cárdenas, D.; Lomas-Vega, R.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Leap Motion Controller Video Game-Based Therapy for Upper Extremity Motor Recovery in Patients with Central Nervous System Diseases. A Systematic Review with Meta-Analysis. Sensors 2021, 21, 2065. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Zhu, W. Dynamic Hand Gesture Recognition Based on a Leap Motion Controller and Two-Layer Bidirectional Recurrent Neural Network. Sensors 2020, 20, 2106. [Google Scholar] [CrossRef]
- Labruyère, R. Robot-assisted gait training: More randomized controlled trials are needed! Or maybe not? J. Neuroeng. Rehabil. 2022, 19, 58. [Google Scholar] [CrossRef]
- Scheidig, A.; Schütz, B.; Trinh, T.Q.; Vorndran, A.; Mayfarth, A.; Sternitzke, C.; Röhner, E.; Gross, H.-M. Robot-Assisted Gait Self-Training: Assessing the Level Achieved. Sensors 2021, 21, 6213. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Cacciola, A.; Bertè, F.; Manuli, A.; Leo, A.; Bramanti, A.; Naro, A.; Milardi, D.; Bramanti, P. Robotic gait rehabilitation and substitution devices in neurological disorders: Where are we now? Neurol. Sci. 2016, 37, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Beretta, E.; Storm, F.A.; Strazzer, S.; Frascarelli, F.; Petrarca, M.; Colazza, A.; Cordone, G.; Biffi, E.; Morganti, R.; Maghini, C.; et al. Effect of Robot-Assisted Gait Training in a Large Population of Children with Motor Impairment Due to Cerebral Palsy or Acquired Brain Injury. Arch. Phys. Med. Rehabil. 2020, 101, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Brütsch, K.; Schuler, T.; Koenig, A.; Zimmerli, L.; (-Koeneke), S.M.; Lünenburger, L.; Riener, R.; Jäncke, L.; Meyer-Heim, A. Influence of virtual reality soccer game on walking performance in robotic assisted gait training for children. J. Neuroeng. Rehabil. 2010, 7, 15. [Google Scholar] [CrossRef]
- Banz, R.; Bolliger, M.; Colombo, G.; Dietz, V.; Lünenburger, L. Computerized Visual Feedback: An Adjunct to Robotic-Assisted Gait Training. Phys. Ther. 2008, 88, 1135–1145. [Google Scholar] [CrossRef]
- Jamwal, P.K.; Hussain, S.; Ghayesh, M.H. Robotic orthoses for gait rehabilitation: An overview of mechanical design and control strategies. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2020, 234, 444–457. [Google Scholar] [CrossRef]
- Neckel, N.; Wisman, W.; Hidler, J. Limb Alignment and Kinematics Inside a Lokomat Robotic Orthosis. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 2698–2701. [Google Scholar]
- Chen, G.; Chan, C.K.; Guo, Z.; Yu, H. A Review of Lower Extremity Assistive Robotic Exoskeletons in Rehabilitation Therapy. Crit. Rev. Biomed. Eng. 2013, 41, 343–363. [Google Scholar] [CrossRef]
- Kuwahara, W.; Sasaki, S.; Yamamoto, R.; Kawakami, M.; Kaneko, F. The effects of robot-assisted gait training combined with non-invasive brain stimulation on lower limb function in patients with stroke and spinal cord injury: A systematic review and meta-analysis. Front. Hum. Neurosci. 2022, 16. [Google Scholar] [CrossRef]
- Son, S.; Lim, K.-B.; Kim, J.; Lee, C.; Cho, S.I.; Yoo, J. Comparing the Effects of Exoskeletal-Type Robot-Assisted Gait Training on Patients with Ataxic or Hemiplegic Stroke. Brain Sci. 2022, 12, 1261. [Google Scholar] [CrossRef]
- Picelli, A.; Capecci, M.; Filippetti, M.; Varalta, V.; Fonte, C.; Di Censo, R.; Zadra, A.; Chignola, I.; Scarpa, S.; Amico, A.P.; et al. Effects of robot-assisted gait training on postural instability in Parkinson’s disease: A systematic review. Eur. J. Phys. Rehabil. Med. 2021, 57, 472–477. [Google Scholar] [CrossRef]
- Pérez-De la Cruz, S. Use of Robotic Devices for Gait Training in Patients Diagnosed with Multiple Sclerosis: Current State of the Art. Sensors 2022, 22, 2580. [Google Scholar] [CrossRef] [PubMed]
- Sucuoglu, H. Effects of robot-assisted gait training alongside conventional therapy on the development of walking in children with cerebral palsy. J. Pediatr. Rehabil. Med. 2020, 13, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Žarkovic, D.; Šorfová, M.; Tufano, J.; Kutílek, P.; Vítecková, S.; Ravnik, D.; Groleger-srsen, K.; Cikajlo, I.; Otáhal, J. Gait Changes Following Robot-Assisted Gait Training in Children with Cerebral Palsy. Physiol. Res. 2021, 70 (Suppl. 3), S397–S408. [Google Scholar] [CrossRef]
- Bayón, C.; Martín-Lorenzo, T.; Moral-Saiz, B.; Ramírez, Ó.; Pérez-Somarriba, Á.; Lerma-Lara, S.; Martínez, I.; Rocon, E. A robot-based gait training therapy for pediatric population with cerebral palsy: Goal setting, proposal and preliminary clinical implementation. J. Neuroeng. Rehabil. 2018, 15, 69. [Google Scholar] [CrossRef]
- Cumplido, C.; Delgado, E.; Ramos, J.; Puyuelo, G.; Garcés, E.; Destarac, M.A.; Plaza, A.; Hernández, M.; Gutiérrez, A.; Garciá, E. Gait-assisted exoskeletons for children with cerebral palsy or spinal muscular atrophy: A systematic review. NeuroRehabilitation 2021, 49, 333–348. [Google Scholar] [CrossRef]
- Volpini, M.; Aquino, M.; Holanda, A.C.; Emygdio, E.; Polese, J. Clinical effects of assisted robotic gait training in walking distance, speed, and functionality are maintained over the long term in individuals with cerebral palsy: A systematic review and meta-analysis. Disabil. Rehabil. 2022, 44, 5418–5428. [Google Scholar] [CrossRef]
- Conner, B.C.; Remec, N.M.; Lerner, Z.F. Is robotic gait training effective for individuals with cerebral palsy? A systematic review and meta-analysis of randomized controlled trials. Clin. Rehabil. 2022, 36, 873–882. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions 6.1; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 1, p. 728. [Google Scholar]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef] [PubMed]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef] [PubMed]
- Meader, N.; King, K.; Llewellyn, A.; Norman, G.; Brown, J.; Rodgers, M.; Moe-Byrne, T.; Higgins, J.P.; Sowden, A.; Stewart, G. A checklist designed to aid consistency and reproducibility of GRADE assessments: Development and pilot validation. Syst. Rev. 2014, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis Software, Version 3; Biostat Inc.: Englewood, NJ, USA, 2020. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Cooper, H.; Hedges, L.V.; Valentine, J.C. The Handbook of Research Synthesis and Meta-Analysis, 2nd ed.; Russell Sage Foundation: New York, NY, USA, 2009. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V. Interpreting estimates of treatment effects: Implications for managed care. Pharm. Ther. 2008, 33, 700–711. [Google Scholar]
- Rücker, G.; Schwarzer, G. Beyond the forest plot: The drapery plot. Res. Synth. Methods 2020, 12, 13–19. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test measures of funnel plot asymmetry. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Rothman, K.; Greenland, S.; Lash, T. Modern Epidemiology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Higgins, J.; Thompson, S.; Deeks, J.; Altman, D. Statistical heterogeneity in systematic reviews of clinical trials: A critical appraisal of guidelines and practice. J. Health Serv. Res. Policy 2002, 7, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Ammann-Reiffer, C.; Bastiaenen, C.H.G.; Meyer-Heim, A.D.; Van Hedel, H.J.A. Lessons learned from conducting a pragmatic, randomized, crossover trial on robot-assisted gait training in children with cerebral palsy (PeLoGAIT). J. Pediatr. Rehabil. Med. 2020, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Aras, B.; Yaşar, E.; Kesikburun, S.; Türker, D.; Tok, F.; Yılmaz, B. Comparison of the effectiveness of partial body weight-supported treadmill exercises, robotic-assisted treadmill exercises, and anti-gravity treadmill exercises in spastic cerebral palsy. Turk. J. Phys. Med. Rehabil. 2019, 65, 361. [Google Scholar] [CrossRef]
- Smania, N.; Bonetti, P.; Gandolfi, M.; Cosentino, A.; Waldner, A.; Hesse, S.; Werner, C.; Bisoffi, G.; Geroin, C.; Munari, D. Improved gait after repetitive locomotor training in children with cerebral palsy. Am. J. Phys. Med. Rehabil. 2011, 90, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Wallard, L.; Dietrich, G.; Kerlirzin, Y.; Bredin, J. Robotic-assisted gait training improves walking abilities in diplegic children with cerebral palsy. Eur. J. Paediatr. Neurol. 2017, 21, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Wallard, L.; Dietrich, G.; Kerlirzin, Y.; Bredin, J. Effect of robotic-assisted gait rehabilitation on dynamic equilibrium control in the gait of children with cerebral palsy. Gait Posture 2018, 60, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Kim, J.; Arora, P.; Gaebler-Spira, D.J.; Zhang, Y. The effects of the integration of dynamic weight shifting training into treadmill training on walking function of children with cerebral palsy– a randomized controlled study. Am. J. Phys. Med. Rehabil. 2017, 96, 765. [Google Scholar] [CrossRef]
- Yazıcı, M.; Livanelioğlu, A.; Gücüyener, K.; Tekin, L.; Sümer, E.; Yakut, Y. Effects of robotic rehabilitation on walking and balance in pediatric patients with hemiparetic cerebral palsy. Gait Posture 2019, 70, 397–402. [Google Scholar] [CrossRef]
- Druzbicki, M.; Rusek, W.; Snela, S.; Dudek, J.; Szczepanik, M.; Zak, E.; Durmala, J.; Czernuszenko, A.; Bonikowski, M.; Sobota, G. Functional effects of robotic-assisted locomotor treadmill thearapy in children with cerebral palsy. J. Rehabil. Med. 2013, 45, 358–363. [Google Scholar] [CrossRef]
- Jin, L.H.; Yang, S.S.; Choi, J.Y.; Sohn, M.K. The Effect of Robot-Assisted Gait Training on Locomotor Function and Functional Capability for Daily Activities in Children with Cerebral Palsy: A Single-Blinded, Randomized Cross-Over Trial. Brain Sci. 2020, 10, 801. [Google Scholar] [CrossRef]
- Klobucká, S.; Klobucký, R.; Kollár, B. Effect of robot-assisted gait training on motor functions in adolescent and young adult patients with bilateral spastic cerebral palsy: A randomized controlled trial. NeuroRehabilitation 2020, 47, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Manikowska, F.; Brazevic, S.; Krzyżańska, A.; Jóźwiak, M. Effects of Robot-Assisted Therapy on Gait Parameters in Pediatric Patients with Spastic Cerebral Palsy. Front. Neurol. 2021, 12, 724009. [Google Scholar] [CrossRef] [PubMed]
- Peri, E.; Turconi, A.C.; Biffi, E.; Maghini, C.; Panzeri, D.; Morganti, R.; Pedrocchi, A.; Gagliardi, C. Effects of dose and duration of Robot-Assisted Gait Training on walking ability of children affected by cerebral palsy. Technol. Health Care 2017, 25, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Pool, D.; Valentine, J.; Taylor, N.F.; Bear, N.; Elliott, C. Locomotor and robotic assistive gait training for children with cerebral palsy. Dev. Med. Child Neurol. 2021, 63, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Romei, M.; Montinaro, A.; Piccinini, L.; Maghini, C.; Germiniasi, C.; Bo, I.; Molteni, F.; Turconi, A.C. Efficacy of robotic-assisted gait training compared with intensive task-oriented physiotherapy for children with Cerebral Palsy. In Proceedings of the 2012 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24–27 June 2012; pp. 1890–1894. [Google Scholar] [CrossRef]
- Sarhan, R.S.M.; Chevidikunnan, M.F.; Gaowgzeh, R.A.M. Locomotor treadmill training program using driven gait orthosis versus manual treadmill therapy on motor output in spastic diplegic cerebral palsy children. J. Health Allied Sci. NU 2014, 04, 010–017. [Google Scholar] [CrossRef]
- Opheim, A.; Jahnsen, R.; Olsson, E.; Stanghelle, J.K. Walking function, pain, and fatigue in adults with cerebral palsy: A 7-year follow-up study. Dev. Med. Child Neurol. 2009, 51, 381–388. [Google Scholar] [CrossRef]
- Raposo, M.R.; Ricardo, D.; Teles, J.; Veloso, A.P.; João, F. Gait Analysis in Children with Cerebral Palsy: Are Plantar Pressure Insoles a Reliable Tool? Sensors 2022, 22, 5234. [Google Scholar] [CrossRef]
- Jung, Y.G.; Chang, H.J.; Jo, E.S.; Kim, D.H. The Effect of a Horse-Riding Simulator with Virtual Reality on Gross Motor Function and Body Composition of Children with Cerebral Palsy: Preliminary Study. Sensors 2022, 22, 2903. [Google Scholar] [CrossRef]
- Lee, K.; Oh, H.; Lee, G. Fully Immersive Virtual Reality Game-Based Training for an Adolescent with Spastic Diplegic Cerebral Palsy: A Case Report. Children 2022, 9, 1512. [Google Scholar] [CrossRef]
- Palomo-Carrión, R.; Romay-Barrero, H.; Pinero-Pinto, E.; Romero-Galisteo, R.-P.; López-Muñoz, P.; Martínez-Galán, I. Early Intervention in Unilateral Cerebral Palsy: Let’s Listen to the Families! What Are Their Desires and Perspectives? A Preliminary Family-Researcher Co-Design Study. Children 2021, 8, 750. [Google Scholar] [CrossRef]
- Carvalho, I.; Pinto, S.M.; Chagas, D.d.V.; Praxedes dos Santos, J.L.; de Sousa Oliveira, T.; Batista, L.A. Robotic Gait Training for Individuals with Cerebral Palsy: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2017, 98, 2332–2344. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.; Martinelli, L.; Cook, K.; Stoner, L.; Ryan-Stewart, H.; Paine, E.; Hobbs, H.; Lambrick, D. Effects of robotic-assisted gait training on the central vascular health of individuals with spinal cord injury: A pilot study. J. Spinal Cord Med. 2021, 44, 299–305. [Google Scholar] [CrossRef]
- Warken, B.; Graser, J.; Ulrich, T.; Borggraefe, I.; Heinen, F.; Meyer-Heim, A.; van Hedel, H.; Schroeder, A. Practical Recommendations for Robot-Assisted Treadmill Therapy (Lokomat) in Children with Cerebral Palsy: Indications, Goal Setting, and Clinical Implementation within the WHO-ICF Framework. Neuropediatrics 2015, 46, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-N.; Huang, S.-W.; Kuan, Y.-C.; Chen, H.-C.; Jian, W.-S.; Lin, L.-F. Hybrid robot-assisted gait training for motor function in subacute stroke: A single-blind randomized controlled trial. J. Neuroeng. Rehabil. 2022, 19, 99. [Google Scholar] [CrossRef]
- Krakauer, J.W. Motor learning: Its relevance to stroke recovery and neurorehabilitation. Curr. Opin. Neurol. 2006, 19, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, D.; Russo, E.F.; Cardone, D.; Palmieri, R.; Filippini, C.; Tritto, M.; Pellicano, F.; De Santis, G.P.; Calabrò, R.S.; Merla, A.; et al. Identification of Functional Cortical Plasticity in Children with Cerebral Palsy Associated to Robotic-Assisted Gait Training: An fNIRS Study. J. Clin. Med. 2022, 11, 6790. [Google Scholar] [CrossRef] [PubMed]
- Billinger, S.A.; Arena, R.; Bernhardt, J.; Eng, J.J.; Franklin, B.A.; Johnson, C.M.; MacKay-Lyons, M.; Macko, R.F.; Mead, G.E.; Roth, E.J.; et al. Physical Activity and Exercise Recommendations for Stroke Survivors. Stroke 2014, 45, 2532–2553. [Google Scholar] [CrossRef]
- Lopes, J.M.; Figueiredo, J.; Fonseca, P.; Cerqueira, J.J.; Vilas-Boas, J.P.; Santos, C.P. Deep Learning-Based Energy Expenditure Estimation in Assisted and Non-Assisted Gait Using Inertial, EMG, and Heart Rate Wearable Sensors. Sensors 2022, 22, 7913. [Google Scholar] [CrossRef]


| Database | Search Strategy |
|---|---|
| PubMed Medline | (cerebral palsy [mh] OR cerebral palsy [tiab]) AND (robot [tiab] OR robotic [tiab] OR exoskeleton [tiab] OR robotic gait assisted training [tiab]) |
| SCOPUS | TITLE-ABS-KEY (“cerebral palsy”) AND TITLE-ABS-KEY (“robotic” OR “exoskeleton” OR “robot gait assisted training”) |
| Web of Science | TOPIC: (*cerebral palsy*) AND TOPIC: (*robotic* OR *exoskeleton* OR *robot gait assisted training*) |
| CINAHL Complete | AB (cerebral palsy) AND AB (robotic OR exoskeleton OR robot gait assisted training) |
| PEDro | cerebral palsy AND robotic cerebral palsy AND robot |
| SciELO | cerebral palsy AND robotic |
| Study | Funding | N | F/M | CP Type | TC | GMFCS | Groups | Age (Years) | Evaluation | Outcomes | Test |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ammann–Reiffer, C et al., 2020 (Switzerland) [78] | Yes | 16 | 3/13 | Spastic | Bilateral | II n = 9 III n = 5 IV n = 2 | CG n = 8 EG n = 8 | 11.3 ± 2.3 | T1 post-intervention | Gait speed Gross motor function Walking distance | 10MWTS GMFM-88 D, E 6MWT |
| Aras, B et al., 2019 (Turkey) [79] | No | 29 | 11/18 | Spastic | Hemiplegic n = 9 Diplegic n = 20 | II n = 24 III n = 5 | CG1 n = 10 CG2 n = 9 EG n = 10 | 9.3 ± 2.3 | T1 post-intervention T2 Follow-up (2 months) | Gait speed Gross motor function Stride length Walking distance Cadence | 3D gait analysis GMFM-66 D, E m 6MWT Step/min |
| Druzbicki, M et al., 2013 (Poland) [85] | No | 35 | 19/16 | Spastic | Diplegic | II n = 23 III n = 12 | CG n = 9 EG n = 26 | 10.6 ± 2.3 | T1 post-intervention | Step length Gait speed Step width | M m/s m |
| Jin, LH et al., 2020 (Korea) [86] | Yes | 20 | 7/13 | Spastic n = 17 Dyskinetic n = 1 Mixed n = 2 | Hemiplegic n = 1 Diplegic n = 19 | II n = 5 III n = 9 IV n = 6 | CG n = 10 EG n = 10 | 6.8 ± 2.2 | T1 post-intervention | Gait speed | m/s |
| Klobucká, S et al., 2020 (Slovakia) [87] | Yes | 47 | 20/27 | Spastic | Diplegic | I n = 1 II n = 7 III n = 21 IV n = 18 | GC n = 26 GE n = 21 | 21.2 ± 5.3 | T1 post-intervention | Gross motor function | GMFM-88, D, E and total |
| Manikowska, F et al., 2021 (Poland) [88] | Yes | 26 | 10/16 | Spastic | Bilateral | I-II n = 17 III-IV n = 9 | CG n = 17 EG n = 9 | 14.8 ± 1.9 | T1 post-intervention T2 post-intervention (6 weeks) | Gait speed Cadence Step width Step length | m/s Step/min m m |
| Peri, E et al., 2017 (Italy) [89] | No | 44 | 22/22 | Spastic | Bilateral | I n = 14 II n = 16 III n = 14 III n = 16 | CG1 n = 10 EG1 n = 12 EG2 n = 10 EG3 n = 12 | 8.7 ± 1.7 | T1 post-intervention T2 post-intervention (3 months) | Gross motor function | GMFM-88 D, E and total |
| Walking distance | 6MWT | ||||||||||
| Pool, D et al., 2021 (Australia) [90] | Yes | 40 | 18/22 | NR | NR | III n = 16IV n = 10 V n = 14 | CG n = 20 EG n = 20 | 5–12 (range) | T1 post-intervention | Gross motor function Gait speed Functional Independence | GMFM-88 total 10MWTS WeeFIM |
| Romei, M et al., 2012 (Italy) [91] | No | 19 | 11/8 | Spastic | Bilateral | I n = 6 II n = 11 III n = 3 | CG n = 10 EG n = 9 | 8.1 ± 1.7 | T1 post-intervention T2 post-intervention (3 months) | Gross motor function | GMFM-88 D, E and total |
| Walking distance | 6MWT | ||||||||||
| Sarhan, RSM et al., 2014 (Saudi Arabia) [92] | Yes | 12 | 5/7 | Spastic | Diplegic | III-IV | CG n = 6 EG n = 6 | 4.2 ± 0.7 | T1 post-intervention | Cadence Gait speed Stride length | step/min m/s m |
| Smania, N et al., 2011 (Italy) [80] | No | 18 | 8/10 | Spastic | Diplegic n = 11 Tetraplegic n = 7 | I n = 6 II n = 2 III n = 3 IV n = 7 | CG n = 9 EG n = 9 | 12.5 ± 2.9 | T1 post-intervention T2 post-intervention (1 month) | Gait speed Walking distance Cadence Step length Functional Independence | m/s 6MWT step/min m WeeFIM |
| Wallard, L et al., 2017 (France) [81] | No | 30 | 15/15 | Spastic | Diplegic | II | CG n = 16 EG n = 14 | 8.9 ± 1.4 | T1 post-intervention | Gross Motor Function | GMFM-88 D, E |
| Wallard, L et al., 2018 (France) [82] | No | 30 | 15/15 | Spastic | Diplegic | II | CG n = 16 EG n = 14 | 8.9 ± 1.4 | T1 post-intervention | Gait speed Cadence Step length Step width | m/s step/min m m |
| Wu, M et al., 2017 (United States) [83] | No | 23 | 9/14 | Spastic | Diplegic n = 11 Triplegic n = 1 Tetraplegic n = 7 | I n = 3 II n = 9 III n = 8 IV n = 3 | CG n = 12 EG n = 11 | 10.9 ± 3.2 | T1 post-intervention T2 post-intervention (2 months) | Gait speed Gross Motor Function | m/s GMFM 88 D, E, total |
| Step length Walking distance | M 6MWT | ||||||||||
| Yazici, M et al., 2019 (Turkey) [84] | No | 24 | 12/12 | Spastic | Hemiplegic | I-II | CG n = 12 EG n = 12 | 8.5 ± 8.5 | T1 post-intervention T2 post-intervention (3 months) | Gait speed Gross Motor FunctionWalking distance Functional Independence | 10MWTS GMFM 88 D, E6MWT FAQ-WL |
| Study | Intervention | Type Robot | Session Time (min) | Number of Sessions | Frequency (ss/wk) | Duration of Treatment (wk) | Qualitative Findings |
|---|---|---|---|---|---|---|---|
| Ammann-Reiffer, C et al., 2020 [78] | CG UC (CT) EG RAGT | Lokomat® | 45 | 35 25 | 2/3/2 3/2 | 5/5/5 5/5 | No significant differences were found after the RAGT period in dimensions E (p = 0.91), D (p = 0.46) and gait speed. |
| Aras, B et al., 2019 [79] | CG1 PBWSTE (TT) CG2 ATE (TT) EG RAGT | Lokomat® | 45 | 20 | 5 | 4 | No statistically significant difference among the groups according to the GFMF-D, GMFM-E and 6MinWT (p > 0.05). |
| Druzbicki, M et al., 2013 [85] | CG IE (CT) EG RAGT + IE | Lokomat® | 45 | 20 | 5 | 4 | Improvement of both groups in gait speed with no significant difference between groups (p = 0.5909). Decrease in range of motion with no significant difference between groups (p = 0.8676). |
| Jin, LH et al., 2020 [86] | CG CT EG RAGT + CT | Walkbot-K system® | 30 | 36 54 | 3/3 3/3/3 | 12 18 | No significant differences were found after the RAGT period in gait speed (p = 0.223). |
| Klobucká, S et al., 2020 [87] | CG CT EG RAGT | Lokomat® | 45 | 20 | 3–5 | 4–6 | Statistically significant difference (p < 0.001) and large effect size in GMFM in favor of the RAGT group. |
| Manikowska, F et al., 2021 [88] | CG CT EG RAGT + CT | EksoGT® | 30–60 | 30 | 5 | 10 (2 wk work + 2 wk break) | Walking speed significantly improved (t2 vs. t3, p = 0.02) for group AS. |
| Peri, E. et al., 2017 [89] | CG1 TOP10 (CT) EG1 RAGT EG2 RAGT + TOP10EG3 RAGT + TOP4 | Lokomat® | 45 | 40 | 4 4 2 + 2 4 + 4 | 10 10 10 4 | No differences among the 4 groups. Only RAGT and TOP groups obtained significant improvement in gross motor function. |
| Pool, D. et al., 2021 [90] | CG LT (TT) EG RAGT+LT | RT600® | 60 | 18 | 3 | 6 | No significant differences between the groups. |
| Romei, M. et al., 2012 [91] | CG TOP (CT) EG RAGT + TOP | Lokomat® | 30 | 40 | 4 2 + 2 | 10 | Both groups improved GMFM scores with no statistically significant differences. No improvement in their 6MinTW scores. |
| Sarham, RSM et al., 2014 [92] | CG CT EG RAGT | Lokomat Pro Version 4® | 30–40 | 30 | 3 | 10 | EG significantly improves stride length, cadence and gait speed (p < 0.001). CG does not show significant improvement. |
| Smania, N et al., 2011 [80] | CG CT EG RAGT + CT | Gait Trainer GT I® | 40 30 + 10 | 10 | 5 | 2 | Comparison between the groups shows statistically significant differences favoring the EG in gait speed (p < 0.001), 6MinWT (p = 0.015) and step length (p = 0.004). |
| Wallard, L. et al., 2017 [81] | CG CT EG RAGT | Lokomat® | 40 | 20 | 5 | 4 | Statistically significant differences favoring the EG in dimension D (p = 0.048) and dimension E (p = 0.026) |
| Wallard, L. et al., 2018 [82] | CG CT EG RAGT | Lokomat® | 40 | 20 | 5 | 4 | Significant differences were also found for the intergroup comparison in gait speed (p = 0.031), cadence (p = 0.043), step length (p = 0.042), step width (p = 0.022) and step width (p = 0.029). |
| Wu, M. et al., 2017 [83] | CG TT EG RAGT | 3DCaLT® | 30–40 | 18 | 3 | 6 | RT significantly increases walking speed (p = 0.03) and a greater increase in 6MinWT over TT (p = 0.01). |
| Yazici, M et al., 201 [84] | CG CT EG RAGT + CT | Innowalk Pro® | 30 | 36 | 3 | 12 | No between-group analysis is performed but the within-group analysis of the EG shows significant changes in GMFM-88 (p < 0.001), GMFM-D (p = 0.003) and GMFM-E (p = 0.000) scores in the short term, and the first two are maintained in the long term. |
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ammann-Reiffer, C. et al., 2020 [78] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 |
| Aras, B. et al., 2019 [79] | Yes | Yes | Yes | Yes | No | No | No | Yes | No | Yes | Yes | 6 |
| Druzbicki, M. et al., 2013 [85] | y | Yes | No | Yes | No | No | Yes | No | No | Yes | Yes | 5 |
| Jin, LH. et al., 2020 [86] | Yes | Yes | No | Yes | No | No | Yes | Yes | No | Yes | Yes | 6 |
| Klobucká, S. et al., 2020 [87] | No | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 |
| Manikowska, F. et al., 2021 [88] | Yes | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes | 5 |
| Peri, E. et al., 2017 [89] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 |
| Pool, D. et al., 2021 [90] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 8 |
| Romei, M. et al., 2012 [91] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 |
| Sarham, RSM. et al., 2014 [92] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 |
| Smania, N. et al., 2011 [80] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | 7 |
| Wallard, L. et al., 2017 [81] | Yes | Yes | No | Yes | No | No | Yes | No | No | Yes | Yes | 5 |
| Wallard, L. et al., 2018 [82] | Yes | Yes | No | Yes | No | No | No | No | No | Yes | No | 3 |
| Wu, M. et al., 2017 [83] | Yes | Yes | Yes | Yes | No | No | No | Yes | No | Yes | Yes | 6 |
| Yazici, M. et al., 201 [84] | Yes | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 5 |
| Findings Summary | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect Size | Heterogeneity | Publication Bias | ||||||||||
| Variable (Post-Intervention Assessment) | Specific Comparison | K | N | Ns | SMD | 95% CI | p | Q (df) | I2 (p) | Egger p | Trim and Fill | |
| Adj SMD | % var | |||||||||||
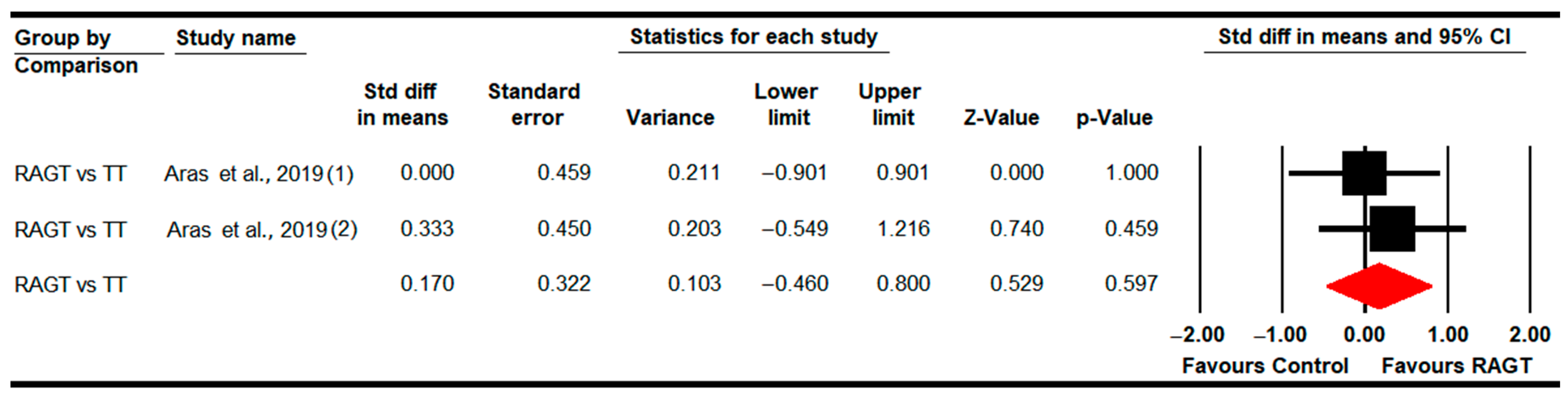
| Gait Speed | RAGT vs. TT | 4 | 121 | 30.3 | 0.25 | −0.15 to 0.64 | 0.22 | 11.5 (4) | 51.3% (0.02) | 0.3 | 0.19 | 24% |
| RAGT vs. CT | 3 | 58 | 19.3 | 0.56 | 0.03 to 1.1 | 0.04 | 2.52 (2) | 20.6% (0.28) | 0.65 | 0.56 | 0% | |
| RAGT + CT vs. CT | 5 | 123 | 24.6 | −0.1 | −0.47 to 0.29 | 0.63 | 2.12 (3) | 0% (0.55) | 0.05 | −0.18 | 100% | |
| Step Length | RAGT vs. TT | 3 | 81 | 27 | 0.1 | −0.41 to 0.6 | 0.71 | 0.08 (2) | 0% (0.96) | 0.43 | 0.1 | 0% |
| RAGT + CT vs. CT | 3 | 79 | 26.3 | 0.2 | −0.28 to 0.67 | 0.43 | 0.27 (2) | 0% (0.87) | 0.52 | 0.2 | 0% | |
| Step width | RAGT + CT vs. CT | 2 | 61 | 30.5 | −0.28 | −0.83 to 0.28 | 0.33 | 0.86 (1) | 0% (0.35) | NP | NP | NP |
| Stride length | RAGT vs. TT | 2 | 58 | 29 | 0.17 | −0.46 to 0.8 | 0.6 | 0.27 (1) | 0% (0.61) | NP | NP | NP |
| Walking distance | RAGT vs. TT | 3 | 81 | 27 | 0.1 | −1 to 1.2 | 0.86 | 0.96 (2) | 0% (0.62) | 0.14 | 0.1 | 0% |
| RAGT vs. CT | 2 | 60 | 30 | 2 | 0.36 to 3.65 | 0.017 | 3.79 (1) | 32.3% (0.05) | NP | NP | NP | |
| RAGT + CT vs. CT | 5 | 149 | 29.8 | 0.35 | −0.51 to 1.2 | 0.43 | 0.05 (2) | 0% (0.97) | 0.12 | 0.42 | 20% | |
| Cadence | RAGT vs. TT | 2 | 58 | 29 | 0.09 | −0.54 to 0.72 | 0.79 | 0.01 (1) | 0% (0.92) | NP | NP | NP |
| RAGT vs. CT | 2 | 42 | 21 | 0.21 | −0.4 to 0.82 | 0.5 | 0.01 (1) | 0% (0.92) | NP | NP | NP | |
| RAGT + CT vs. CT | 2 | 44 | 22 | 0.3 | −0.31 to 0.92 | 0.33 | 0.3 (1) | 0% (0.59) | NP | NP | NP | |
| Standing ability | RAGT vs. TT | 3 | 81 | 27 | −0.01 | −0.52 to 0.5 | 0.96 | 0.11 (2) | 0% (0.95) | 0.27 | −0.01 | 0% |
| RAGT vs. CT | 3 | 90 | 30 | −0.12 | −0.61 to 0.36 | 0.62 | 4.22 (2) | 52% (0.12) | 0.01 | 0.32 | 100% | |
| RAGT + CT vs. CT | 5 | 131 | 26.2 | 0.22 | −0.13 to 0.56 | 0.21 | 0.19 (4) | 0% (0.99) | 0.88 | 0.22 | 0% | |
| Walking, running and jumping abilty | RAGT vs. TT | 3 | 81 | 27 | 0.11 | −0.49 to 0.71 | 0.72 | 0.01 (2) | 0% (0.99) | 0.27 | 0.11 | 0% |
| RAGT vs. CT | 3 | 90 | 30 | 0.7 | 0.09 to 1.4 | 0.035 | 4.72 (2) | 47% (0.05) | 0.33 | 0.7 | 0% | |
| RAGT + CT vs. CT | 5 | 131 | 26.2 | 0.11 | −0.31 to 0.54 | 0.61 | 0.48 (4) | 0% (0.97) | 0.09 | 0.22 | 100% | |
| Gross motor function | RAGT vs. TT | 2 | 63 | 31.5 | 0.15 | −0.36 to 0.65 | 0.57 | 0.05 (1) | 0% (0.82) | NP | NP | NP |
| RAGT + CT vs. CT | 4 | 154 | 38.5 | 0.18 | −0.2 to 0.56 | 0.36 | 0.42 (3) | 0% (0.93) | 0.2 | 0.23 | 22% | |
| Funct. indep. | RAGT + CT vs. CT | 2 | 42 | 21 | 0.14 | −0.46 to 0.75 | 0.64 | 0.08 (1) | 0% (0.77) | NP | NP | NP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Pérez, I.; González-González, N.; Peinado-Rubia, A.B.; Nieto-Escamez, F.A.; Obrero-Gaitán, E.; García-López, H. Efficacy of Robot-Assisted Gait Therapy Compared to Conventional Therapy or Treadmill Training in Children with Cerebral Palsy: A Systematic Review with Meta-Analysis. Sensors 2022, 22, 9910. https://doi.org/10.3390/s22249910
Cortés-Pérez I, González-González N, Peinado-Rubia AB, Nieto-Escamez FA, Obrero-Gaitán E, García-López H. Efficacy of Robot-Assisted Gait Therapy Compared to Conventional Therapy or Treadmill Training in Children with Cerebral Palsy: A Systematic Review with Meta-Analysis. Sensors. 2022; 22(24):9910. https://doi.org/10.3390/s22249910
Chicago/Turabian StyleCortés-Pérez, Irene, Noelia González-González, Ana Belén Peinado-Rubia, Francisco Antonio Nieto-Escamez, Esteban Obrero-Gaitán, and Héctor García-López. 2022. "Efficacy of Robot-Assisted Gait Therapy Compared to Conventional Therapy or Treadmill Training in Children with Cerebral Palsy: A Systematic Review with Meta-Analysis" Sensors 22, no. 24: 9910. https://doi.org/10.3390/s22249910
APA StyleCortés-Pérez, I., González-González, N., Peinado-Rubia, A. B., Nieto-Escamez, F. A., Obrero-Gaitán, E., & García-López, H. (2022). Efficacy of Robot-Assisted Gait Therapy Compared to Conventional Therapy or Treadmill Training in Children with Cerebral Palsy: A Systematic Review with Meta-Analysis. Sensors, 22(24), 9910. https://doi.org/10.3390/s22249910