UV-Light-Tunable p-/n-Type Chemiresistive Gas Sensors Based on Quasi-1D TiS3 Nanoribbons: Detection of Isopropanol at ppm Concentrations
Abstract
1. Introduction
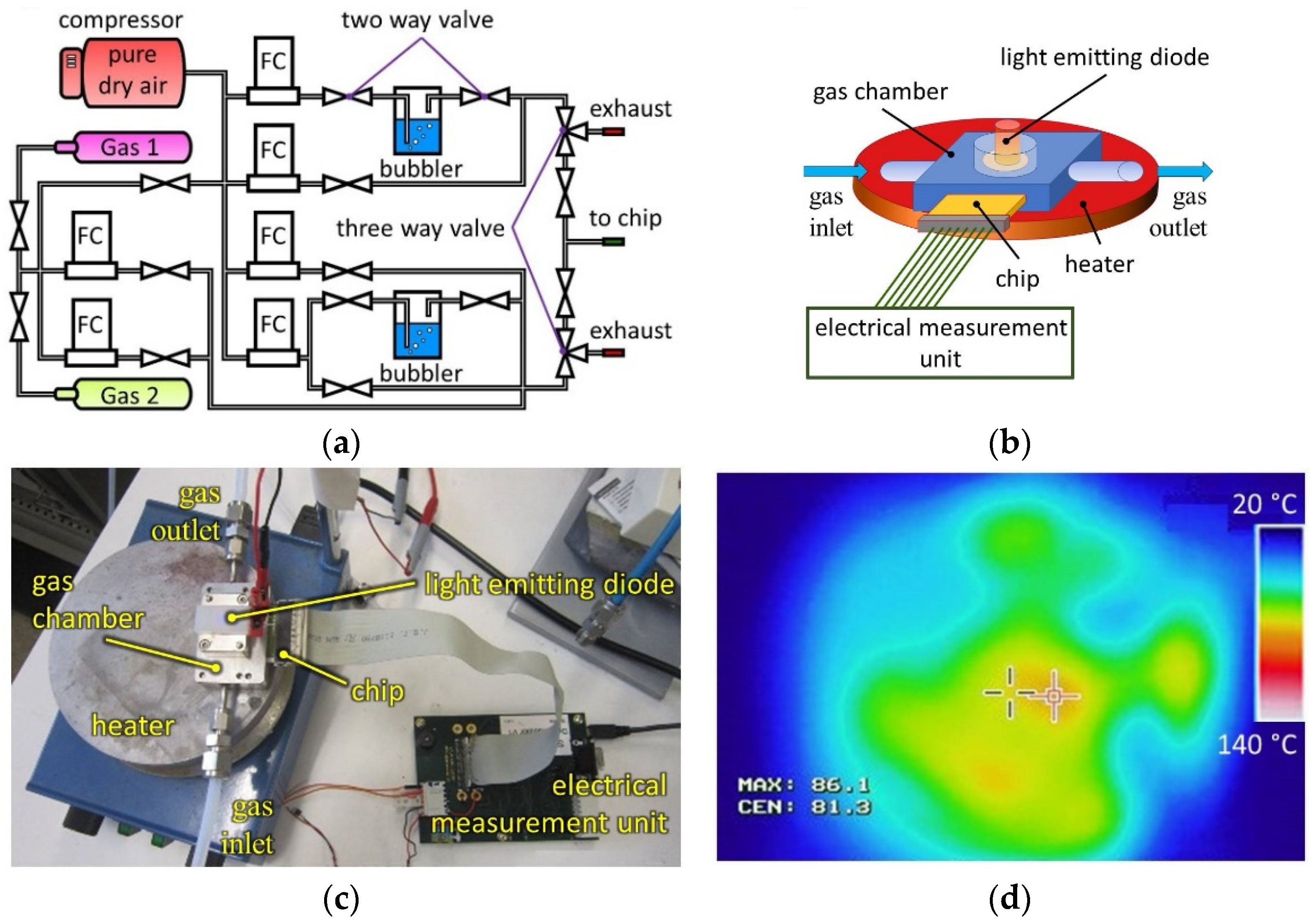
2. Materials and Methods
3. Results and Discussion
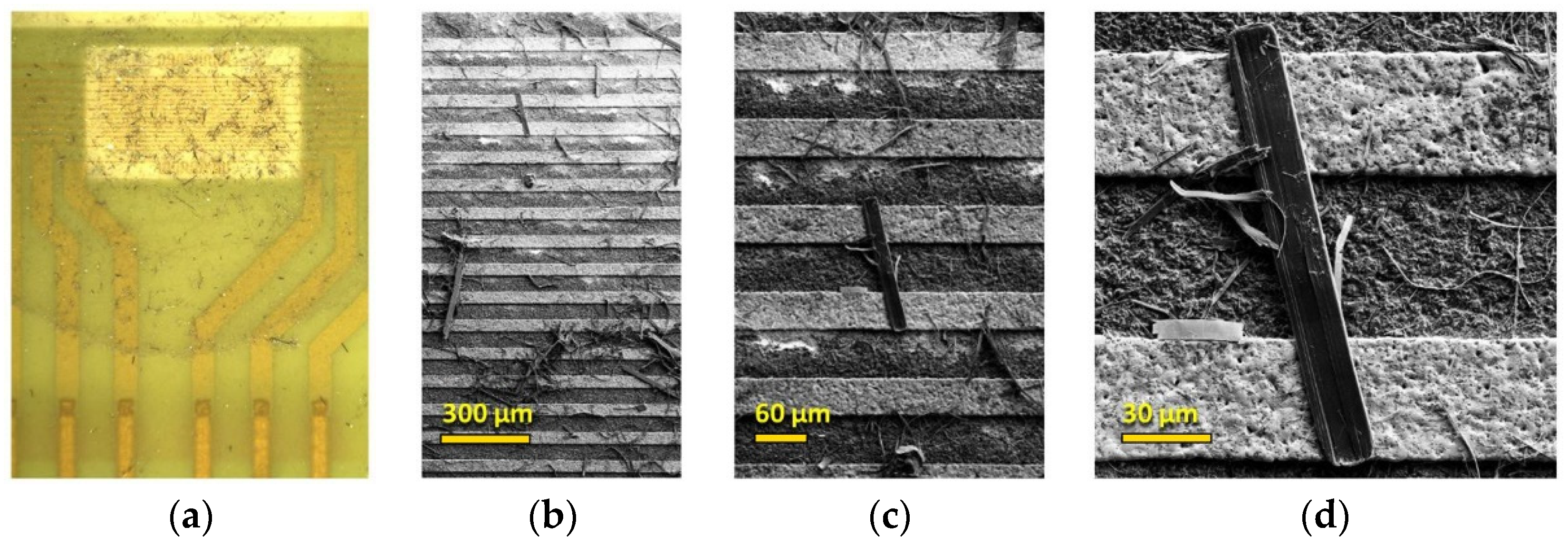
3.1. Physical Characterization of TiS3 NWs
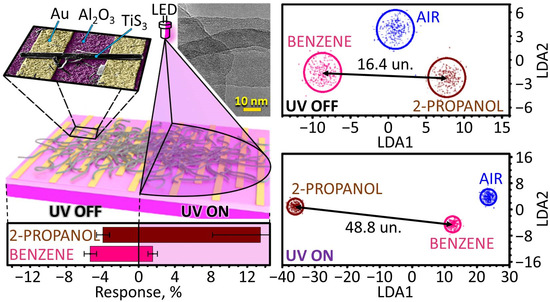
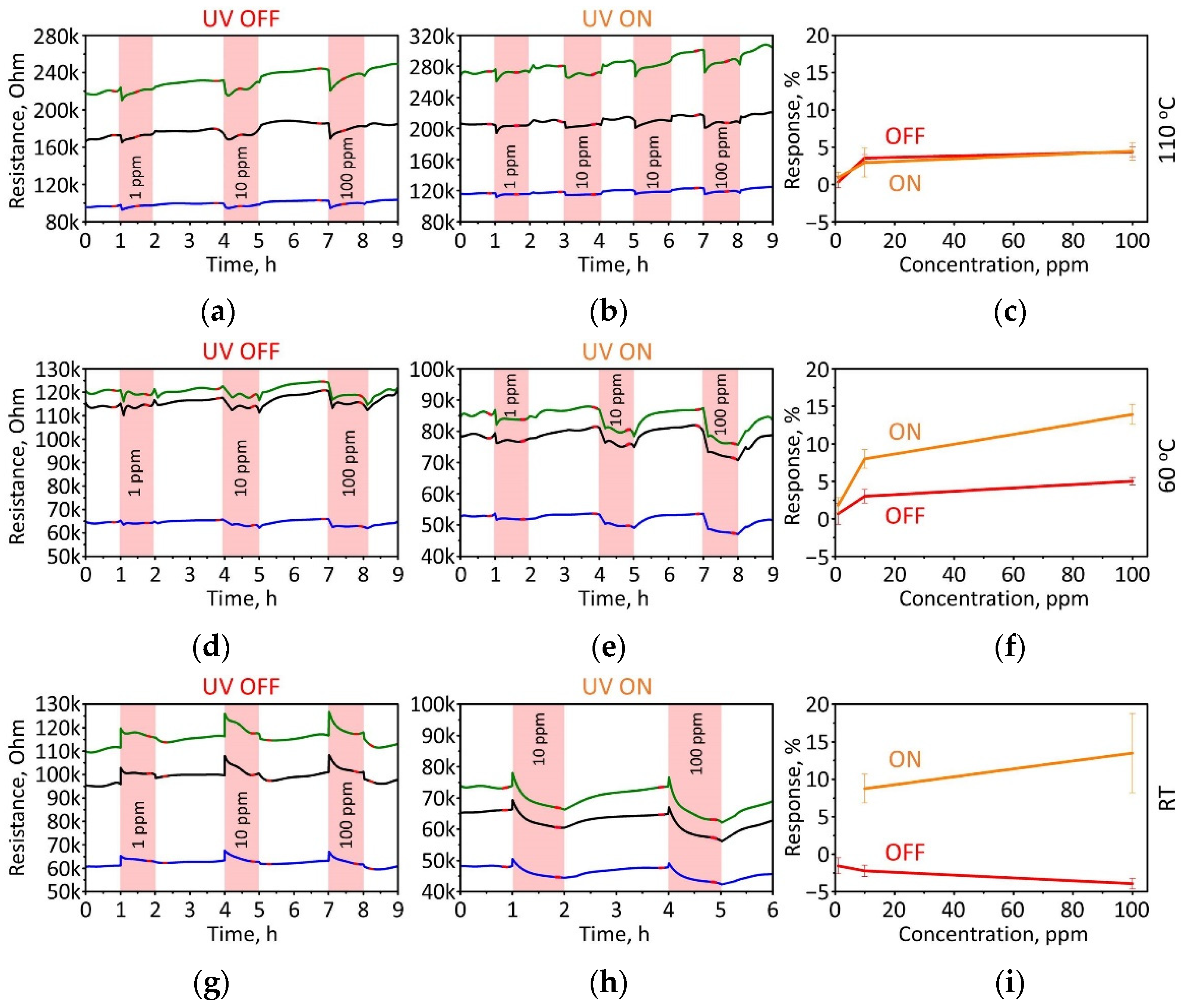
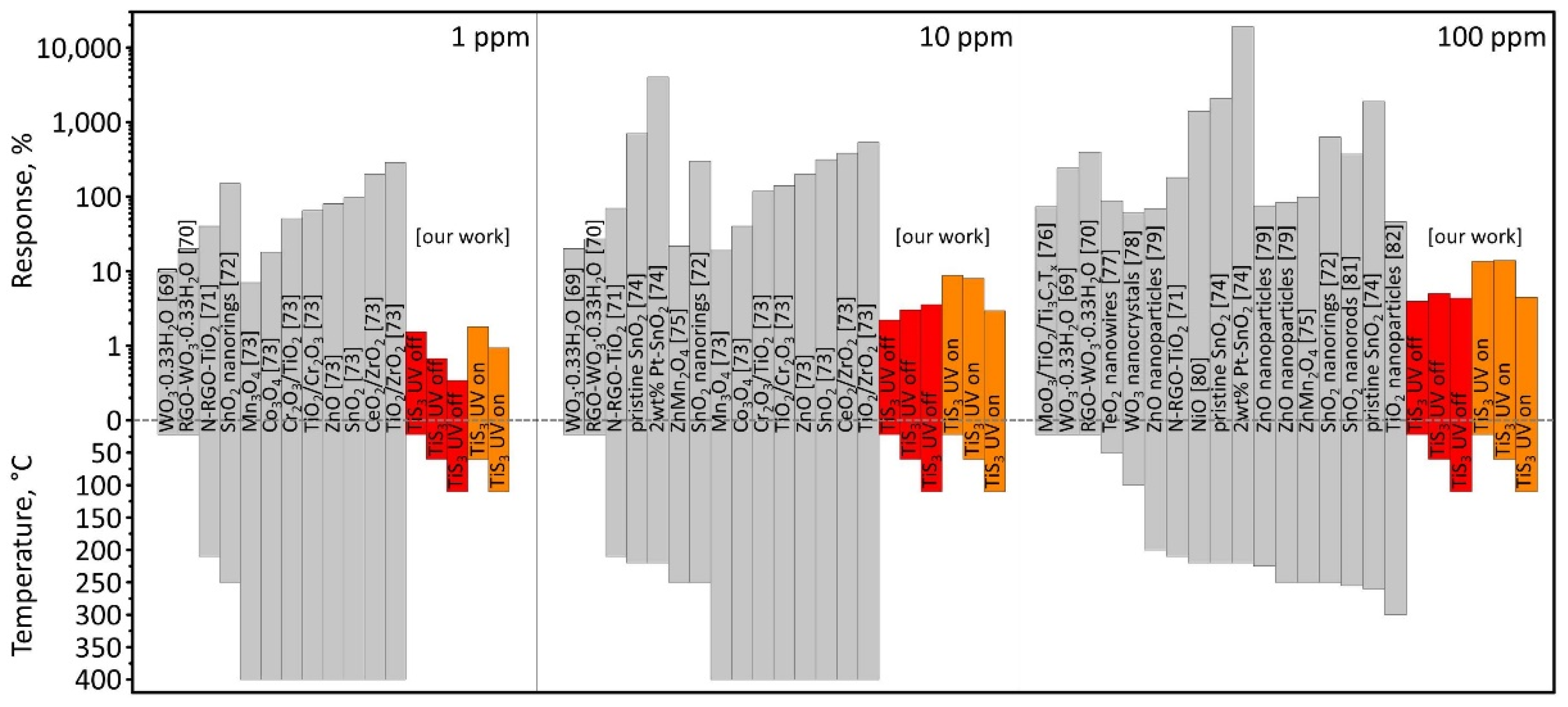
3.2. Gas-Sensing Performance of TiS3 NWs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.C.; Ng, K.; Zach, M.P.; Penner, R.M.; Favier, F. Electronic devices from electrodeposited metal nanowires. Microelectron. Eng. 2002, 61–62, 555–561. [Google Scholar] [CrossRef]
- Comini, E.; Faglia, G.; Sberveglieri, G.; Pan, Z.; Wang, Z.L. Stable and highly sensitive gas sensors based on semiconducting oxide nanobelts. Appl. Phys. Lett. 2002, 81, 1869–1871. [Google Scholar] [CrossRef]
- Comini, E. Metal oxides nanowires chemical/gas sensors: Recent advances. Mater. Today Adv. 2020, 7, 100099. [Google Scholar] [CrossRef]
- Ziegler, J.M.; Andoni, I.; Choi, E.J.; Fang, L.; Flores-Zuleta, H.; Humphrey, N.J.; Kim, D.-H.; Shin, J.; Youn, H.; Penner, R.M. Sensors based upon nanowires, nanotubes, and nanoribbons: 2016–2020. Anal. Chem. 2021, 93, 124–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, L.; Sun, S.; Wang, J.; Yan, W. One-dimensional nanomaterials in resistive gas sensor: From material design to application. Chemosensors 2021, 9, 198. [Google Scholar] [CrossRef]
- Tonezzer, M.; Thi Thanh Le, D.; Van Duy, L.; Hoa, N.D.; Gasperi, F.; Van Duy, N.; Biasioli, F. Electronic noses based on metal oxide nanowires: A review. Nanotechnol. Rev. 2022, 11, 897–925. [Google Scholar] [CrossRef]
- Sysoev, V.V.; Strelcov, E.; Kolmakov, A. Multisensor micro-arrays based on metal oxide nanowires for electronic nose applications. In Metal Oxide Nanomaterials for Chemical Sensors: Integrated Analytical Systems; Carpenter, M., Mathur, S., Kolmakov, A., Eds.; Springer: New York, NY, USA, 2013; pp. 465–502. [Google Scholar] [CrossRef]
- Barth, S.; Hernandez-Ramirez, F.; Holmes, J.D.; Romano-Rodriguez, A. Synthesis and applications of one-dimensional semiconductors. Prog. Mater. Sci. 2010, 55, 563–627. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, L.; Deng, Z.; Liao, J. Electrically transduced gas sensors based on semiconducting metal oxide nanowires. Sensors 2020, 20, 6781. [Google Scholar] [CrossRef]
- Chen, X.; Wong, C.K.Y.; Yuan, C.A.; Zhang, G. Nanowire-based gas sensors. Sens. Actuators B 2013, 177, 178–195. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Nat. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [PubMed]
- Zobel, A.; Boson, A.; Wilson, P.M.; Muratov, D.S.; Kuznetsov, D.V.; Sinitskii, A. Chemical vapour deposition and characterization of uniform bilayer and trilayer MoS2 crystals. J. Mater. Chem. C 2016, 4, 11081–11087. [Google Scholar] [CrossRef]
- Lipatov, A.; Varezhnikov, A.; Augustin, M.; Bruns, M.; Sommer, M.; Sysoev, V.; Kolmakov, A.; Sinitskii, A. Intrinsic device-to-device variation in graphene field-effect transistors on a Si/SiO2 substrate as a platform for discriminative gas sensing. Appl. Phys. Lett. 2014, 104, 013114. [Google Scholar] [CrossRef]
- Lipatov, A.; Varezhnikov, A.; Wilson, P.; Sysoev, V.; Kolmakov, A.; Sinitskii, A. Highly selective gas sensor arrays based on thermally reduced graphene oxide. Nanoscale 2013, 5, 5426–5434. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zeng, Z.; Yin, Z.; Li, H.; Wu, S.; Huang, X.; Zhang, H. Fabrication of flexible MoS2 thin-film transistor arrays for practical gas-sensing applications. Small 2012, 8, 2994–2999. [Google Scholar] [CrossRef]
- Lee, E.; Vahidmohammadi, A.; Prorok, B.C.; Yoon, Y.S.; Beidaghi, M.; Kim, D.-J. Room temperature gas sensing of two-dimensional titanium carbide (MXene). ACS Appl. Mater. Int. 2017, 9, 37184–37190. [Google Scholar] [CrossRef]
- Pazniak, H.; Plugin, I.A.; Loes, M.J.; Inerbaev, T.M.; Burmistrov, I.N.; Gorshenkov, M.; Polcak, J.; Varezhnikov, A.S.; Sommer, M.; Kuznetsov, D.V.; et al. Partially oxidized Ti3C2Tx MXenes for fast and selective detection of organic vapors at part-per-million concentrations. ACS Appl. Nano Mater. 2020, 3, 3195–3204. [Google Scholar] [CrossRef]
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically-transduced chemical sensors based on two-dimensional nanomaterials. Chem. Rev. 2019, 119, 478–598. [Google Scholar] [CrossRef]
- Kiani, M.; Rehman, M.U.; Tian, X.; Yakobson, B. Two-dimensional nanomaterials for the development of efficient gas sensors: Recent advances, challenges, and future perspectives. Adv. Mater. Technol. 2022, 7, 2101252. [Google Scholar] [CrossRef]
- Island, J.O.; Molina-Mendoza, A.J.; Barawi, M.; Biele, R.; Flores, E.; Clamagirand, J.M.; Ares, J.R.; Sánchez, C.; Van der Zant, H.S.J.; D’Agosta, R.; et al. Electronics and optoelectronics of quasi-1D layered transition metal trichalcogenides. 2D Mater. 2017, 4, 022003. [Google Scholar] [CrossRef]
- Yi, H.; Komesu, T.; Gilbert, S.; Hao, G.; Yost, A.J.; Lipatov, A.; Sinitskii, A.; Avila, J.; Chen, C.; Asensio, M.C.; et al. The band structure of the quasi-one-dimensional layered semiconductor TiS3(001). Appl. Phys. Lett. 2018, 112, 052102. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, X.; Xie, J.; Lu, G.; Zhang, J. Emerging van der Waals junctions based on TMDs materials for advanced gas sensors. Coordinat. Chem. Rev. 2021, 447, 214151. [Google Scholar] [CrossRef]
- Tang, H.; Sacco, L.N.; Vollebregt, S.; Ye, H.; Fan, X.; Zhang, G. Recent advances in 2D/nanostructured metal sulfide-based gas sensors: Mechanisms, applications, and perspectives. J. Mater. Chem. A 2020, 8, 24943–24976. [Google Scholar] [CrossRef]
- Randle, M.; Lipatov, A.; Kumar, A.; Kwan, C.-P.; Nathawat, J.; Barut, B.; Yin, S.; He, K.; Arabchigavkani, N.; Dixit, R.; et al. Gate-controlled metal-insulator transition in TiS3 nanowire field-effect transistors. ACS Nano 2019, 13, 803–811. [Google Scholar] [CrossRef]
- Lipatov, A.; Loes, M.J.; Lu, H.; Dai, J.; Patoka, P.; Vorobeva, N.S.; Muratov, D.S.; Ulrich, G.; Kästner, B.; Hoehl, A.; et al. Quasi-1D TiS3 nanoribbons: Mechanical exfoliation and thickness-dependent Raman spectroscopy. ACS Nano 2018, 12, 12713–12720. [Google Scholar] [CrossRef]
- Island, J.O.; Buscema, M.; Barawi, M.; Clamagirand, J.M.; Ares, J.R.; Sánchez, C.; Ferrer, I.J.; Steele, G.A.; van der Zant, H.S.J.; Castellanos-Gomez, A. Ultrahigh photoresponse of few-layer TiS3 nanoribbon transistors. Adv. Opt. Mater. 2014, 2, 641–645. [Google Scholar] [CrossRef]
- Lipatov, A.; Wilson, P.M.; Shekhirev, M.; Teeter, J.D.; Netusil, R.; Sinitskii, A. Few-layered titanium trisulfide (TiS3) field-effect transistors. Nanoscale 2016, 7, 12291–12296. [Google Scholar] [CrossRef]
- Dai, J.; Zeng, X.C. Titanium trisulfide monolayer: Theoretical prediction of a new direct-gap semiconductor with high and anisotropic carrier mobility. Angew. Chem. Int. Ed. 2015, 54, 7572–7576. [Google Scholar] [CrossRef]
- Cui, Q.; Lipatov, A.; Wilt, J.S.; Bellus, M.Z.; Zeng, X.C.; Wu, J.; Sinitskii, A.; Zhao, H. Time-resolved measurements of photocarrier dynamics in TiS3 nanoribbons. ACS Appl. Mater. Interfaces 2016, 8, 18334–18338. [Google Scholar] [CrossRef]
- Island, J.O.; Barawi, M.; Biele, R.; Almazán, A.; Clamagirand, J.M.; Ares, J.R.; Sánchez, C.; van der Zant, H.S.J.; Álvarez, J.V.; D’Agosta, R.; et al. TiS3 transistors with tailored morphology and electrical properties. Adv. Mater. 2015, 27, 2595–2601. [Google Scholar] [CrossRef]
- Gilbert, S.J.; Yi, H.; Chen, J.-S.; Yost, A.J.; Dhingra, A.; Abourahma, J.; Lipatov, A.; Avila, J.; Komesu, T.; Sinitskii, A.; et al. Effect of band symmetry on photocurrent production in quasi-one-dimensional transition-metal trichalcogenides. ACS Appl. Mater. Interfaces 2020, 12, 40525–40531. [Google Scholar] [CrossRef]
- Gilbert, S.J.; Lipatov, A.; Yost, A.J.; Loes, M.J.; Sinitskii, A.; Dowben, P.A. The electronic properties of Au and Pt metal contacts on quasi-one-dimensional layered TiS3(001). Appl. Phys. Lett. 2019, 114, 101604. [Google Scholar] [CrossRef]
- Randle, M.D.; Lipatov, A.; Mansaray, I.; Han, J.E.; Sinitskii, A.; Bird, J.P. Collective states and charge density waves in the group IV transition metal trichalcogenides. Appl. Phys. Lett. 2021, 118, 210502. [Google Scholar] [CrossRef]
- Chen, P.-C.; Shen, G.; Zhou, C. Chemical sensors and electronic noses based on 1-D metal oxide nanostructures. IEEE Trans. Nanotechnol. 2008, 7, 668–682. [Google Scholar] [CrossRef]
- Kolmakov, A. Some recent trends in the fabrication, functionalisation and characterisation of metal oxide nanowire gas sensors. Int. J. Nanotechnol. 2008, 5, 450–474. [Google Scholar] [CrossRef]
- Brattas, L.; Kjekshus, A. On the properties of compounds with the ZrSe3 type structure. Acta Chem. Scand. 1972, 26, 3441–3449. [Google Scholar] [CrossRef]
- Finkman, E.; Fisher, B. Electrical transport measurements in TiS3. Solid State Commun. 1984, 50, 25–28. [Google Scholar] [CrossRef]
- Kikkawa, S.; Koizumi, M.; Yamanaka, S.; Onuki, Y.; Tanuma, S. Electrical conductivity of TiS3. Phys. Status Solidi 1980, 61, K55–K57. [Google Scholar] [CrossRef]
- Lévy, F.; Berger, H. Single crystals of transition metal trichalcogenides. J. Cryst. Growth 1983, 61, 61–68. [Google Scholar] [CrossRef]
- Slaughter, R.J.; Mason, R.W.; Beasley, D.M.G.; Vale, J.A.; Schep, L.J. Isopropanol poisoning. Clin. Toxicol. 2014, 52, 470–478. [Google Scholar] [CrossRef]
- García-Gavín, J.; Lissens, R.; Timmermans, A.; Goossens, A. Allergic contact dermatitis caused by isopropyl alcohol: A missed allergen? Contact Dermat. 2011, 65, 101–106. [Google Scholar] [CrossRef]
- Adib, M.; Eckstein, R.; Hernandez-Sosa, G.; Sommer, M.; Lemmer, U. SnO2 nanowire-based aerosol jet printed electronic nose as fire detector. IEEE Sens. J. 2018, 18, 494–500. [Google Scholar] [CrossRef]
- Goschnick, J. An electronic nose for intelligent consumer products based on a gas analytical gradient microarray. Microelectron. Eng. 2001, 57–58, 693–704. [Google Scholar] [CrossRef]
- Lipatov, A.; Guinel, M.J.-F.; Muratov, D.S.; Vanyushin, V.O.; Wilson, P.M.; Kolmakov, A.; Sinitskii, A. Low-temperature thermal reduction of graphene oxide: In situ correlative structural, thermal desorption, and electrical transport measurements. Appl. Phys. Lett. 2018, 112, 053103. [Google Scholar] [CrossRef]
- Mehdi Pour, M.; Lashkov, A.; Radocea, A.; Liu, X.; Sun, T.; Lipatov, A.; Korlacki, R.A.; Shekhirev, M.; Aluru, N.R.; Lyding, J.W.; et al. Laterally extended atomically precise graphene nanoribbons with improved electrical conductivity for efficient gas sensing. Nat. Commun. 2017, 8, 820. [Google Scholar] [CrossRef]
- Shekhirev, M.; Lipatov, A.; Torres, A.; Vorobeva, N.S.; Harkleroad, A.; Lashkov, A.; Sysoev, V.; Sinitskii, A. Highly selective gas sensors based on graphene nanoribbons grown by chemical vapor deposition. ACS Appl. Mater. Interfaces 2020, 12, 7392–7402. [Google Scholar] [CrossRef]
- Parry, K.L.; Shard, A.G.; Short, R.D.; White, R.G.; Whittle, J.D.; Wright, A. ARXPS characterization of plasma polymerized surface chemical gradients. Surf. Interface Anal. 2006, 38, 1497–1504. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths. V. Data for 14 organic compounds over the 50–2000 eV range. Surf. Interface Anal. 1994, 21, 165–176. [Google Scholar] [CrossRef]
- Watts, J.; Wolstenholme, J. An Introduction to Surface Analysis by XPS and AES, 2nd ed.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 74–77. ISBN 9781119417583. [Google Scholar] [CrossRef]
- Fedorov, F.S.; Varezhnikov, A.S.; Kiselev, I.; Kolesnichenko, V.V.; Burmistrov, I.N.; Sommer, M.; Fuchs, D.; Kübel, C.; Gorokhovsky, A.V.; Sysoev, V.V. Potassium polytitanate gas-sensor study by impedance spectroscopy. Anal. Chim. Acta 2015, 897, 81–86. [Google Scholar] [CrossRef]
- Augustin, M.; Sommer, M.; Sysoev, V.V. UV-VIS sensor system based on SnO2 nanowires. Sens. Actuators A 2014, 210, 205–208. [Google Scholar] [CrossRef]
- Henrion, R.; Henrion, G. Multivariate Datenanalyse; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Yi, H.; Gilbert, S.J.; Lipatov, A.; Sinitskii, A.; Avila, J.; Abourahma, J.; Komesu, T.; Asensio, M.C.; Dowben, P.A. The electronic band structure of quasi-one-dimensional van der Waals semiconductors: The effective hole mass of ZrS3 compared to TiS3. J. Phys. Condens. Matter. 2020, 32, 29LT01. [Google Scholar] [CrossRef]
- Fleet, M.E.; Harmer, S.L.; Liu, X.; Nesbitt, H.W. Polarized X-ray absorption spectroscopy and XPS of TiS3: S K- and Ti L-edge XANES and S and Ti 2p XPS. Surf. Sci. 2005, 584, 133–145. [Google Scholar] [CrossRef]
- Tunc, I.; Bruns, M.; Gliemann, H.; Grunze, M.; Koelsch, P. Bandgap determination and charge separation in Ag@TiO2 core shell nanoparticle films. Surf. Interface Anal. 2010, 42, 835–841. [Google Scholar] [CrossRef]
- Fedorov, F.; Vasilkov, M.; Lashkov, A.; Varezhnikov, A.; Fuchs, D.; Kübel, C.; Bruns, M.; Sommer, M.; Sysoev, V. Toward new gas-analytical multisensor chips based on titanium oxide nanotube array. Sci. Rep. 2017, 7, 9732. [Google Scholar] [CrossRef]
- Fuchs, M.; Breitenstein, D.; Fartmann, M.; Grehl, T.; Kayser, S.; Koester, R.; Ochs, R.; Schlabach, S.; Szabó, D.V.; Bruns, M. Characterization of core/shell nanoparticle thin films for gas analytical applications. Surf. Interface Anal. 2010, 42, 1131–1134. [Google Scholar] [CrossRef]
- Sakhuja, N.; Jha, R.K.; Chaurasiya, R.; Dixit, A.; Bhat, N. 1T-phase titanium disulfide nanosheets for sensing H2S and O2. ACS Appl. Nano Mater. 2020, 3, 3382–3394. [Google Scholar] [CrossRef]
- Ruminski, A.M.; King, B.H.; Salonen, J.; Snyder, J.L.; Sailor, M.J. Porous silicon-based optical microsensors for volatile organic analytes: Effect of surface chemistry on stability and specificity. Adv. Funct. Mater. 2010, 20, 2874–2883. [Google Scholar] [CrossRef]
- Gurlo, A. Interplay between O2 and SnO2: Oxygen ionosorption and spectroscopic evidence for adsorbed oxygen. ChemPhysChem 2006, 7, 2041–2052. [Google Scholar] [CrossRef]
- Randle, M.D.; Lipatov, A.; Datta, A.; Kumar, A.; Mansaray, I.; Sinitskii, A.; Singisetti, U.; Han, J.E.; Bird, J.P. High-electric-field behavior of the metal-insulator transition in TiS3 nanowire transistors. Appl. Phys. Lett. 2022, 120, 073102. [Google Scholar] [CrossRef]
- Pazniak, H.; Varezhnikov, A.S.; Kolosov, D.A.; Plugin, I.A.; Vito, A.D.; Glukhova, O.E.; Sheverdyaeva, P.M.; Spasova, M.; Kaikov, I.; Kolesnikov, E.A.; et al. 2D molybdenum carbide MXenes for enhanced selective detection of humidity in air. Adv. Mater. 2021, 33, 2104878. [Google Scholar] [CrossRef]
- Dhingra, A.; Lipatov, A.; Loes, M.J.; Abourahma, J.; Pink, M.; Sinitskii, A.; Dowben, P.A. Effect of Au/HfS3 interfacial interactions on properties of HfS3-based devices. Phys. Chem. Chem. Phys. 2022, 24, 14016–14021. [Google Scholar] [CrossRef]
- Gurlo, A.; Bârsan, N.; Oprea, A.; Sahm, M.; Sahm, T.; Weimar, U. An n- to p-type conductivity transition induced by oxygen adsorption on α-Fe2O3. Appl. Phys. Lett. 2004, 85, 2280–2282. [Google Scholar] [CrossRef]
- Dai, Z.; Lee, C.-S.; Tian, Y.; Kim, I.-D.; Lee, J.-H. Highly reversible switching from P- to N-type NO2 sensing in a monolayer Fe2O3 inverse opal film and the associated P-N transition phase diagram. J. Mater. Chem. A 2015, 3, 3372–3381. [Google Scholar] [CrossRef]
- Zhou, J.; Xue, K.; Liu, Y.; Liang, T.; Zhang, P.; Zhang, X.; Zhang, W.; Dai, Z. Highly sensitive NO2 response and abnormal P-N sensing transition with ultrathin Mo-doped SnS2 nanosheets. Chem. Eng. J. 2021, 420, 127572. [Google Scholar] [CrossRef]
- Perfecto, T.M.; Zito, C.A.; Volanti, D.P. Design of nanostructured WO3·0.33H2O via combination of ultrasonic spray nozzle and microwave-assisted hydrothermal methods for enhancing isopropanol gas sensing at room temperature. CrystEngComm 2017, 19, 2733–2738. [Google Scholar] [CrossRef]
- Perfecto, T.M.; Zito, C.A.; Mazon, T.; Volanti, D.P. Flexible room-temperature volatile organic compound sensors based on reduced graphene oxide–WO3·0.33H2O nano-needles. J. Mater. Chem. C 2018, 6, 2822–2829. [Google Scholar] [CrossRef]
- Yan, W.Y.; Zhou, Q.; Chen, X.; Huang, X.J.; Wu, Y.C. C-doped and N-doped reduced graphene oxide/TiO2 composites with exposed (0 0 1) and (1 0 1) facets controllably synthesized by a hydrothermal route and their gas sensing characteristics. Sens. Actuators B 2016, 230, 761–772. [Google Scholar] [CrossRef]
- Li, S.H.; Chu, Z.; Meng, F.F.; Luo, T.; Hu, X.Y.; Huang, S.Z.; Jin, Z. Highly sensitive gas sensor based on SnO2 nanorings for detection of isopropanol. J. Alloys Compd. 2016, 688, 712–717. [Google Scholar] [CrossRef]
- Fedorov, F.S.; Simonenko, N.P.; Trouillet, V.; Volkov, I.A.; Plugin, I.A.; Rupasov, D.P.; Mokrushin, A.S.; Nagornov, I.A.; Simonenko, T.L.; Vlasov, I.S.; et al. Microplotter-printed on-chip combinatorial library of ink-derived multiple metal oxides as an “electronic olfaction” unit. ACS Appl. Mater. Interfaces 2020, 12, 56135–56150. [Google Scholar] [CrossRef]
- Dong, C.; Liu, X.; Xiao, X.; Chen, G.; Wang, Y.; Djerdj, I. Combustion synthesis of porous Pt-functionalized SnO2 sheets for isopropanol gas detection with a significant enhancement in response. J. Mater. Chem. A 2014, 2, 20089–20095. [Google Scholar] [CrossRef]
- Guillien-Lopez, E.S.; Lopez-Urias, F.; Munoz-Sandoval, E.; Courel-Piedrahita, M.; Sanchez-Tizapa, M.; Guillen-Bonilla, H.; Rodríguez-Betancourtt, V.M.; Blanco-Alonso, O.; Guillen-Bonilla, A.; Moran-Lazaro, J.P. High performance isopropanol sensor based on spinel ZnMn2O4 nanoparticles. Mater. Today Commun. 2021, 26, 102138. [Google Scholar] [CrossRef]
- Yao, Y.; Han, Y.; Zhou, M.; Xie, L.; Zhao, X.; Wang, Z.; Barsan, N.; Zhu, Z. MoO3/TiO2/Ti3C2Tx nanocomposite based gas sensors for highly sensitive and selective isopropanol detection at room temperature. J. Mater. Chem. A 2022, 10, 8283–8292. [Google Scholar] [CrossRef]
- Shen, Y.; Fan, A.; Wei, D.; Gao, S.; Liu, W.; Han, C.; Cui, B. A low-temperature n-propanol gas sensor based on TeO2 nanowires as the sensing layer. RSC Adv. 2015, 5, 29126–29130. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, M.; Wang, Y.; Liu, X.; Qin, Y. Effect of solvothermal reaction temperature on the morphology of WO3 nanocrystals and their low-temperature NO2-sensing properties. J. Alloys Compd. 2016, 665, 173–179. [Google Scholar] [CrossRef]
- Prajapati, C.S.; Sahay, P.P. Alcohol-sensing characteristics of spray deposited ZnO nano-particle thin films. Sens. Actuators B 2011, 160, 1043–1049. [Google Scholar] [CrossRef]
- Li, Q.; Chen, N.; Xing, X.; Xiao, X.; Wang, Y.; Djerdj, I. NiO nanosheets assembled into hollow microspheres for highly sensitive and fast-responding VOC sensors. RSC Adv. 2015, 5, 80786–80792. [Google Scholar] [CrossRef]
- Hu, D.; Han, B.; Deng, S.; Feng, Z.; Wang, Y.; Popovic, J.; Nuskol, M.; Wang, Y.; Djerdj, I. Novel mixed phase SnO2 nanorods assembled with SnO2 nanocrystals for enhancing gas-sensing performance toward isopropanol gas. J. Phys. Chem. C 2014, 118, 9832–9840. [Google Scholar] [CrossRef]
- Das, P.; Mondal, B.; Mukherjee, K. Chemi-resistive response of rutile titania nano-particles towards isopropanol and formaldehyde: A correlation with the volatility and chemical reactivity of vapors. Mater. Res. Express 2017, 4, 015503. [Google Scholar] [CrossRef]
- Solomatin, M.A.; Glukhova, O.E.; Fedorov, F.S.; Sommer, M.; Shunaev, V.V.; Varezhnikov, A.S.; Nasibulin, A.G.; Ushakov, N.M.; Sysoev, V.V. The UV effect on the chemiresistive response of ZnO nanostructures to isopropanol and benzene at PPM concentrations in mixture with dry and wet air. Chemosensors 2021, 9, 181. [Google Scholar] [CrossRef]
- Aryanpour, M.; Rafiefard, N.; Hosseini-Shokouh, S.H.; Fardindoost, S.; Iraji Zad, A. Computational investigation of gas detection and selectivity on TiS3 nanoflakes supported by experimental evidence. Phys. Chem. Chem. Phys. 2018, 20, 25458–25466. [Google Scholar] [CrossRef]
- Sysoev, V.V.; Kiselev, I.; Trouillet, V.; Bruns, M. Enhancing the gas selectivity of single-crystal SnO2:Pt thin-film chemiresistor microarray by SiO2 membrane coating. Sens. Actuators B 2013, 185, 59–69. [Google Scholar] [CrossRef]
- Muratov, D.S.; Vanyushin, V.O.; Vorobeva, N.S.; Jukova, P.; Lipatov, A.; Kolesnikov, E.A.; Karpenkov, D.; Kuznetsov, D.V.; Sinitskii, A. Synthesis and exfoliation of quasi-1D (Zr,Ti)S3 solid solutions for device measurements. J. Alloys Compd. 2020, 815, 152316. [Google Scholar] [CrossRef]
- Vorobeva, N.S.; Lipatov, A.; Torres, A.; Dai, J.; Abourahma, J.; Le, D.; Dhingra, A.; Gilbert, S.J.; Galiy, P.V.; Nenchuk, T.M.; et al. Anisotropic properties of quasi-1D In4Se3: Mechanical exfoliation, electronic transport, and polarization-dependent photoresponse. Adv. Funct. Mater. 2021, 31, 2106459. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sysoev, V.V.; Lashkov, A.V.; Lipatov, A.; Plugin, I.A.; Bruns, M.; Fuchs, D.; Varezhnikov, A.S.; Adib, M.; Sommer, M.; Sinitskii, A. UV-Light-Tunable p-/n-Type Chemiresistive Gas Sensors Based on Quasi-1D TiS3 Nanoribbons: Detection of Isopropanol at ppm Concentrations. Sensors 2022, 22, 9815. https://doi.org/10.3390/s22249815
Sysoev VV, Lashkov AV, Lipatov A, Plugin IA, Bruns M, Fuchs D, Varezhnikov AS, Adib M, Sommer M, Sinitskii A. UV-Light-Tunable p-/n-Type Chemiresistive Gas Sensors Based on Quasi-1D TiS3 Nanoribbons: Detection of Isopropanol at ppm Concentrations. Sensors. 2022; 22(24):9815. https://doi.org/10.3390/s22249815
Chicago/Turabian StyleSysoev, Victor V., Andrey V. Lashkov, Alexey Lipatov, Ilya A. Plugin, Michael Bruns, Dirk Fuchs, Alexey S. Varezhnikov, Mustahsin Adib, Martin Sommer, and Alexander Sinitskii. 2022. "UV-Light-Tunable p-/n-Type Chemiresistive Gas Sensors Based on Quasi-1D TiS3 Nanoribbons: Detection of Isopropanol at ppm Concentrations" Sensors 22, no. 24: 9815. https://doi.org/10.3390/s22249815
APA StyleSysoev, V. V., Lashkov, A. V., Lipatov, A., Plugin, I. A., Bruns, M., Fuchs, D., Varezhnikov, A. S., Adib, M., Sommer, M., & Sinitskii, A. (2022). UV-Light-Tunable p-/n-Type Chemiresistive Gas Sensors Based on Quasi-1D TiS3 Nanoribbons: Detection of Isopropanol at ppm Concentrations. Sensors, 22(24), 9815. https://doi.org/10.3390/s22249815