Acetone Detection and Classification as Biomarker of Diabetes Mellitus Using a Quartz Crystal Microbalance Gas Sensor Array
Abstract
:1. Introduction
2. Theory
Sensing Film Thickness Estimation Using the Sauerbrey Equation
3. Materials and Methods
3.1. Materials
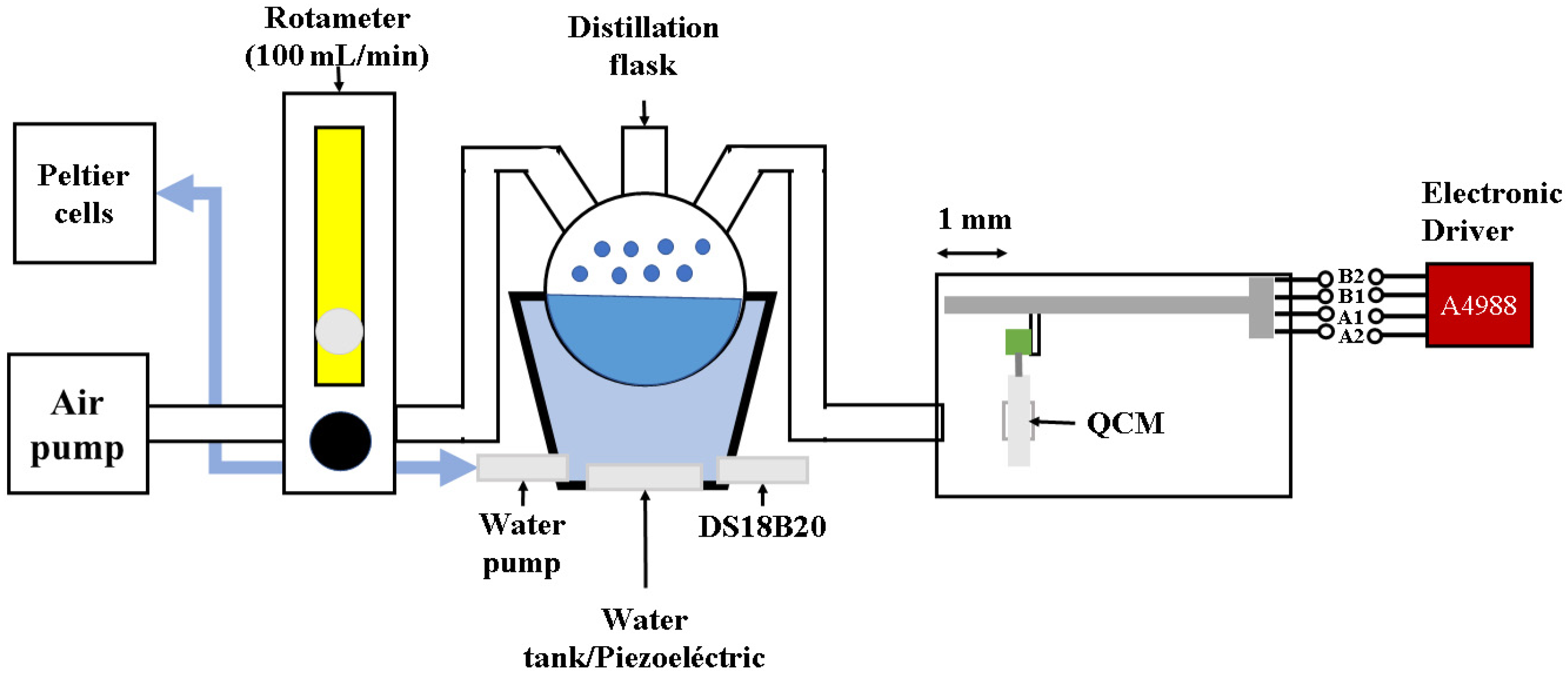
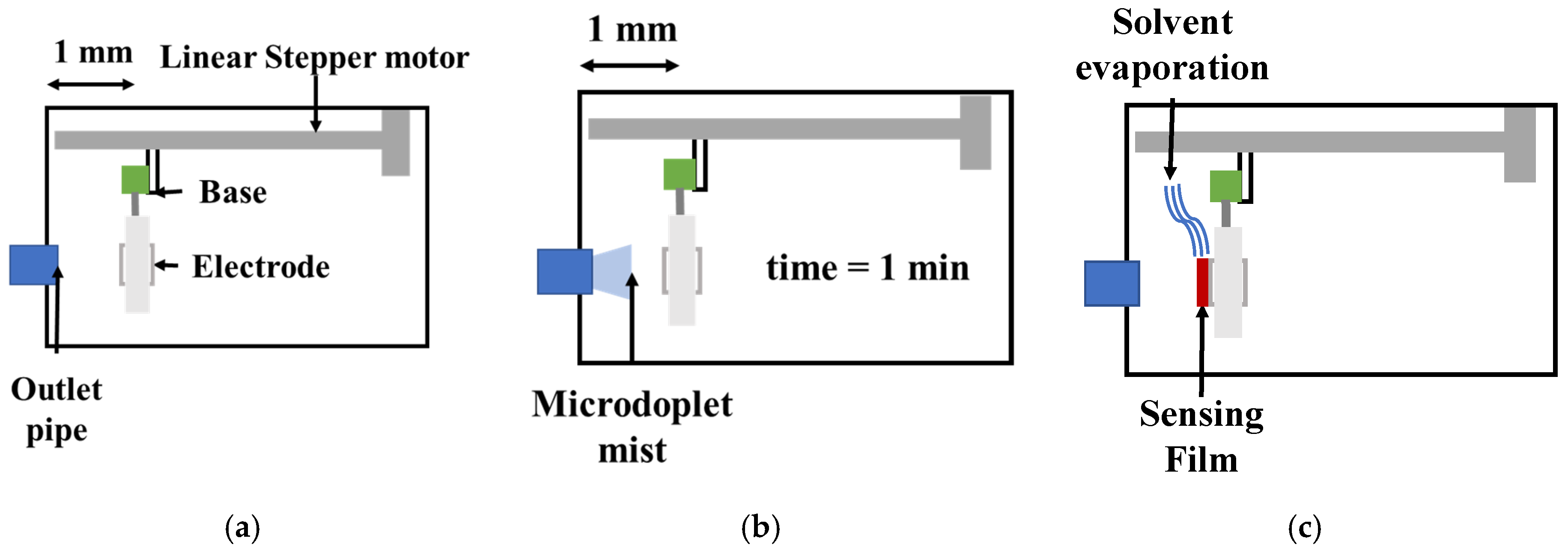
3.2. QCM Sensors’ Construction
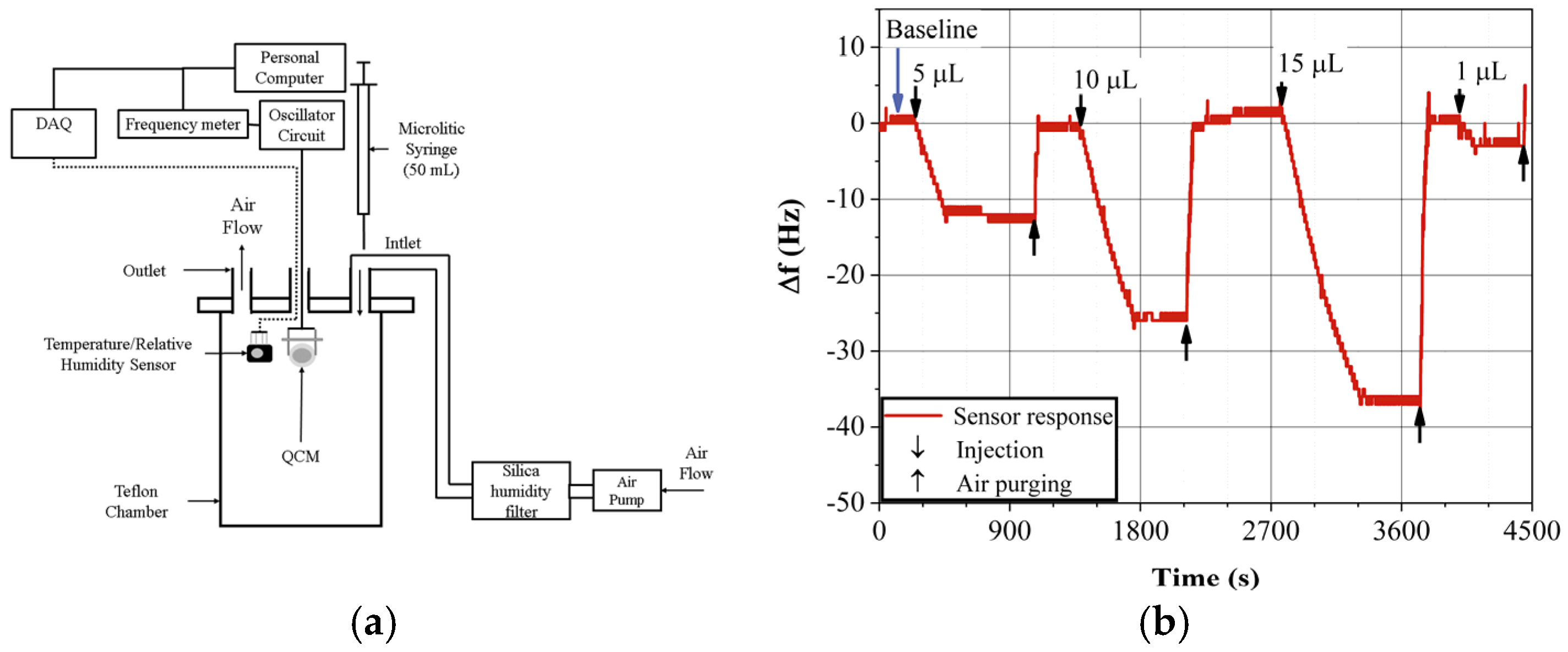
3.3. Gas Sensor Response Measurement
4. Results and Discussion
4.1. Impedance Curves
4.2. QCM Sensing Film Surface Roughness Analysis
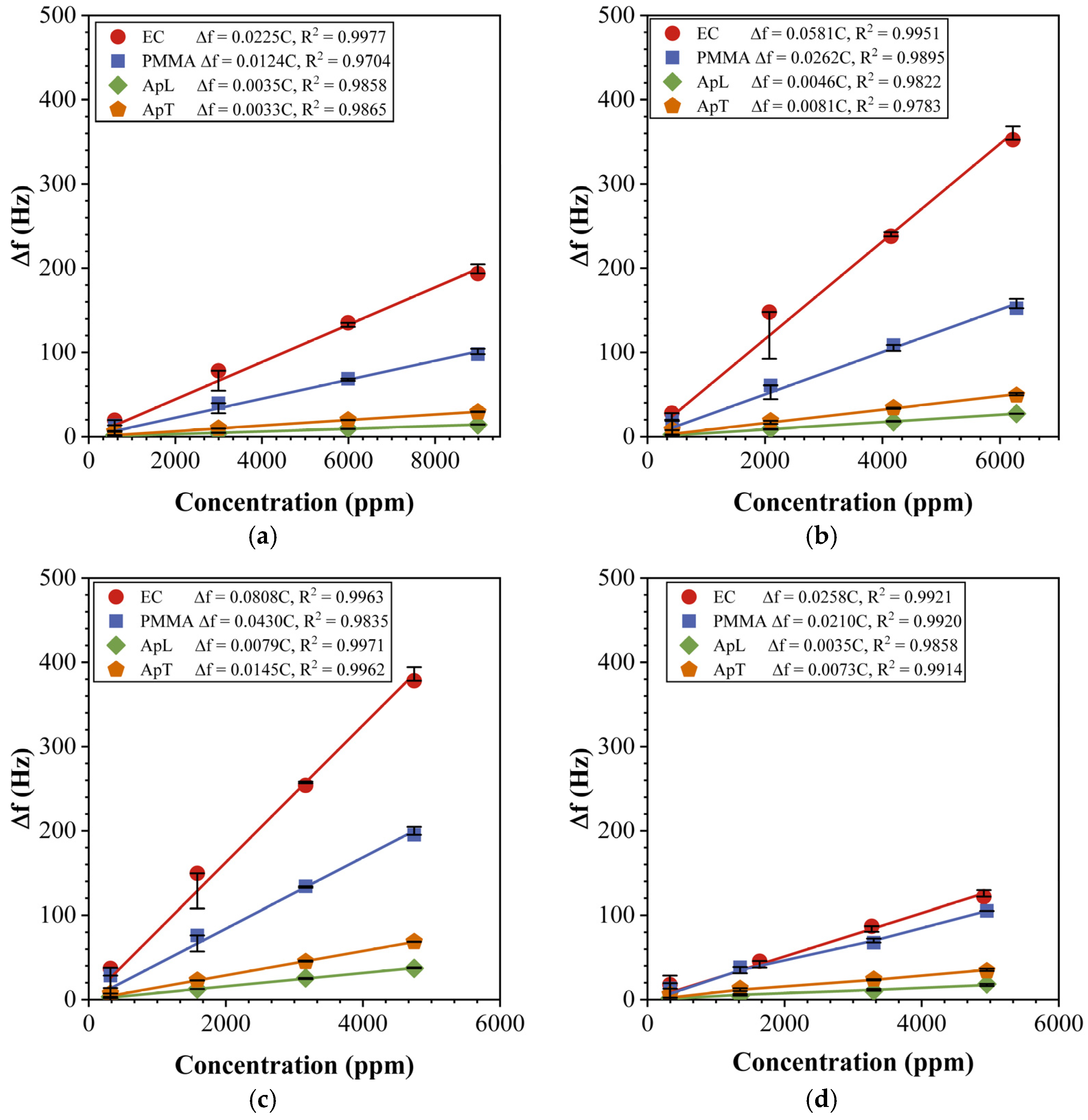
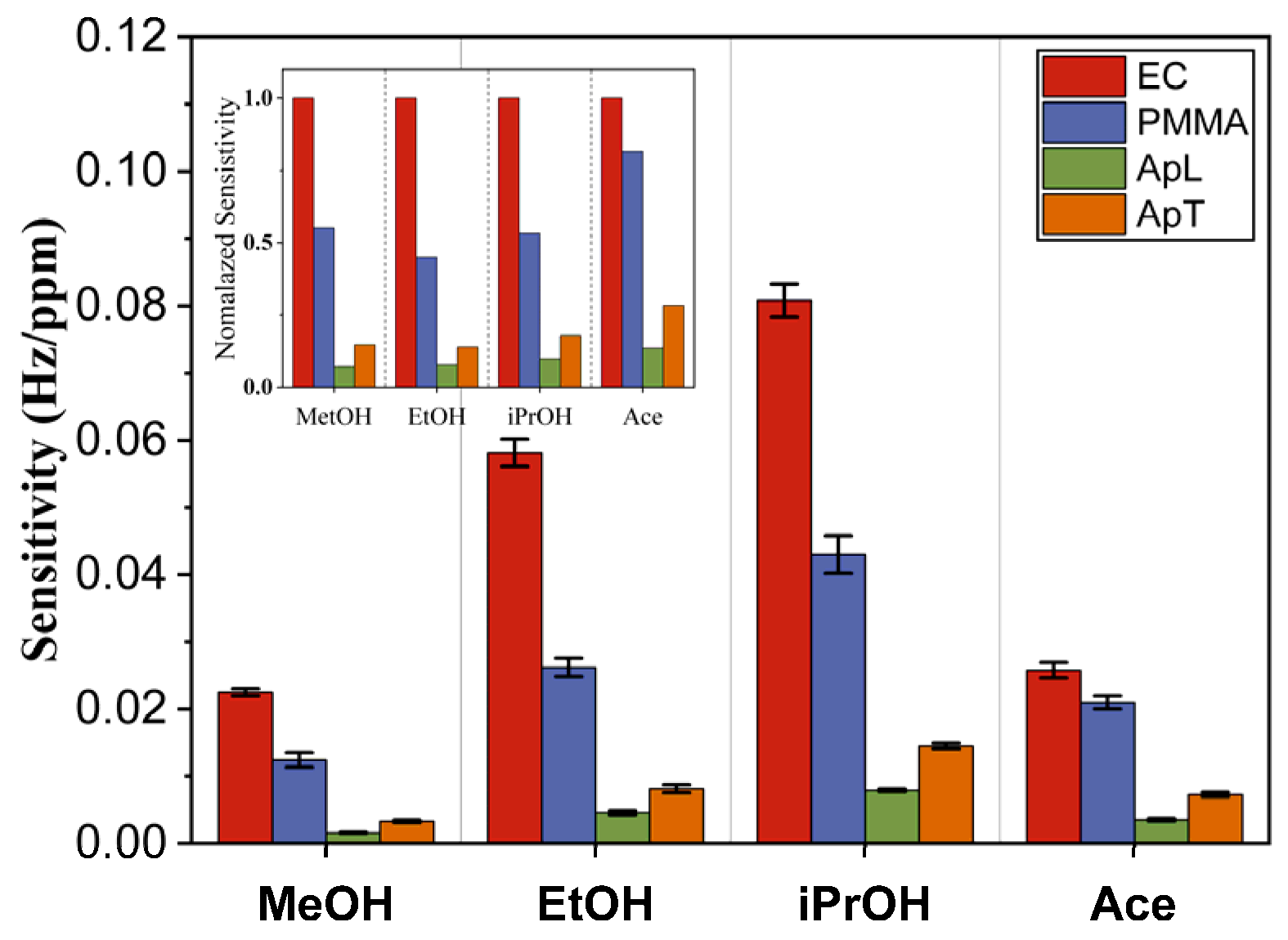
4.3. Sensor Array Characterization
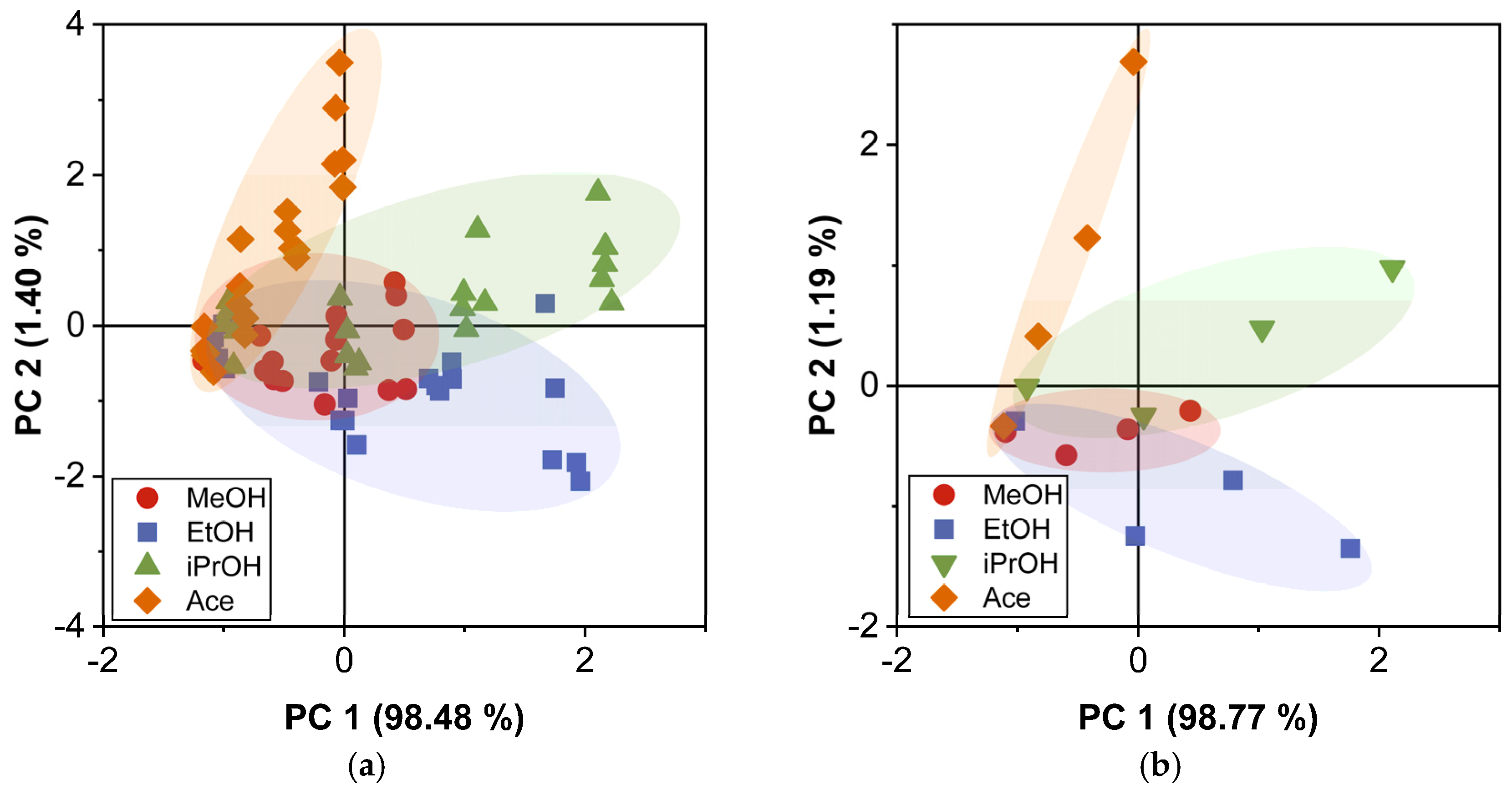
4.4. PCA and DA Application for Acetone Classification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solis-Herrera, C.; Triplitt, C.; Reasner, C.; DeFronzo, R.A.; Cersosimo, E.H.R.; Depkes, R.I.; Soelistijo, S.A.; Novida, H.; Rudijanto, A. Classification of Diabetes Mellitus. Dep. Manag. NCD Disabil. Violence Inj. Prev. 2018, 138, 271–281. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279119/ (accessed on 30 October 2023).
- Minh, T.D.C.; Blake, D.R.; Galassetti, P.R. The clinical potential of exhaled breath analysis for diabetes mellitus. Diabetes Res. Clin. Pract. 2012, 97, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Blackman, S.M.; Cooke, D.W. Diabetes. Encycl. Biol. Chem. Second Ed. 2013, 1, 649–658. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C. Is breath acetone a biomarker of diabetes? A historical review on breath acetone measurements. J. Breath Res. 2013, 7, 037109. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, R.; Varadwaj, P. Smelling the Disease: Diagnostic Potential of Breath Analysis. Mol. Diagn. Ther. 2023, 27, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.; Wang, S.; Duan, Z.; Jiang, Y. Evolution of breath analysis based on humidity and gas sensors: Potential and challenges. Sens. Actuators B Chem. 2020, 318, 128104. [Google Scholar] [CrossRef]
- Sánchez, C.; Santos, J.P.; Lozano, J. Use of electronic noses for diagnosis of digestive and respiratory diseases through the breath. Biosensors 2019, 9, 35. [Google Scholar] [CrossRef]
- Wilson, A.D. Advances in Electronic-Nose Technologies for the Detection of Volatile Biomarker Metabolites in the Human Breath. Metabolites 2015, 5, 140–163. [Google Scholar] [CrossRef]
- Smith, D.; Turner, C.; Spanel, P. Volatile metabolites in the exhaled breath of healthy volunteers: Their levels and distributions. J. Breath Res. 2007, 1, 014004. [Google Scholar] [CrossRef]
- Montuschi, P.; Mores, N.; Trové, A.; Mondino, C.; Barnes, P.J. The Electronic Nose in Respiratory Medicine. Respiration 2012, 85, 72–84. [Google Scholar] [CrossRef]
- Ali, A.; Mansol, A.S.; Khan, A.A.; Muthoosamy, K.; Siddiqui, Y. Electronic nose as a tool for early detection of diseases and quality monitoring in fresh postharvest produce: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2408–2432. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, X.; Zhou, Y.; Wang, J.; You, R. Research progress of electronic nose technology in exhaled breath disease analysis. Microsyst. Nanoeng. 2023, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, D.S.; Abramov, A.A.; Gaidarzhi, V.P.; Kaplun, D.S.; Agina, E.V.; Ponomarenko, S.A. Food Freshness Measurements and Product Distinguishing by a Portable Electronic Nose Based on Organic Field-Effect Transistors. ACS Omega 2023, 8, 4649–4654. [Google Scholar] [CrossRef] [PubMed]
- Rath, R.J.; Farajikhah, S.; Oveissi, F.; Dehghani, F.; Naficy, S. Chemiresistive Sensor Arrays for Gas/Volatile Organic Compounds Monitoring: A Review. Adv. Eng. Mater. 2023, 25, 2200830. [Google Scholar] [CrossRef]
- Zhang, S.; Vinod Ram, K.; Chua, R.Z.T.; Foo, J.C.Y.; Perumal, J.; Dinish, U.S.; Olivo, M. Biophotonics technologies for the detection of VOCs in healthcare applications: Are we there yet? Appl. Phys. Rev. 2023, 10, 31304. [Google Scholar] [CrossRef]
- Gan, Z.; Zhou, Q.; Zheng, C.; Wang, J. Challenges and applications of volatile organic compounds monitoring technology in plant disease diagnosis. Biosens. Bioelectron. 2023, 237, 115540. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Aguirre, S.; Yoshino, A.; Nakamoto, T.; Moriizumi, T. Odor approximation of fruit flavors using a QCM odor sensing system. Sens. Actuators B Chem. 2007, 123, 1101–1106. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, C. Volatile organic compounds gas sensor based on quartz crystal microbalance for fruit freshness detection: A review. Food Chem. 2021, 334, 127615. [Google Scholar] [CrossRef]
- Pérez, R.L.; Ayala, C.E.; Park, J.Y.; Choi, J.W.; Warner, I.M. Coating-Based Quartz Crystal Microbalance Detection Methods of Environmentally Relevant Volatile Organic Compounds. Chemosensors 2021, 9, 153. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Joseph, A.; Emadi, A. A High Frequency Dual Inverted Mesa QCM Sensor Array with Concentric Electrodes. IEEE Access 2020, 8, 92669–92676. [Google Scholar] [CrossRef]
- Alanazi, N.; Almutairi, M.; Alodhayb, A.N. A Review of Quartz Crystal Microbalance for Chemical and Biological Sensing Applications. Sens. Imaging 2023, 24, 10. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.K.; Swargiary, K.; Kongsawang, N.; Jitpratak, P.; Ajchareeyasoontorn, N.; Udomkittivorakul, J.; Viphavakit, C. Recent Advances in Sensing Materials Targeting Clinical Volatile Organic Compound (VOC) Biomarkers: A Review. Biosensors 2023, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Khatib, M.; Haick, H. Sensors for Volatile Organic Compounds. ACS Nano 2021, 16, 7080–7115. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Torres, M.; Altuzar, V.; Mendoza-Barrera, C.; Beltrán-Pérez, G.; Castillo-Mixcóatl, J.; Muñoz-Aguirre, S. Discrimination improvement of a gas sensors’ array using high-frequency quartz crystal microbalance coated with polymeric films. Sensors 2020, 20, 6972. [Google Scholar] [CrossRef] [PubMed]
- Cova, C.M.; Rincón, E.; Espinosa, E.; Serrano, L.; Zuliani, A. Paving the Way for a Green Transition in the Design of Sensors and Biosensors for the Detection of Volatile Organic Compounds (VOCs). Biosensors 2022, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Liu, Y.; Cheng, S.; Duan, Y. Exhaled isopropanol: New potential biomarker in diabetic breathomics and its metabolic correlations with acetone. RSC Adv. 2017, 7, 17480–17488. [Google Scholar] [CrossRef]
- Török, Z.M.; Blaser, A.F.; Kavianynejad, K.; de Torrella, C.G.M.G.; Nsubuga, L.; Mishra, Y.K.; Rubahn, H.G.; de Oliveira Hansen, R. Breath Biomarkers as Disease Indicators: Sensing Techniques Approach for Detecting Breath Gas and COVID-19. Chemosensors 2022, 10, 167. [Google Scholar] [CrossRef]
- Kabir, K.M.M.; Baker, M.J.; Donald, W.A. Micro- and nanoscale sensing of volatile organic compounds for early-stage cancer diagnosis. TrAC Trends Anal. Chem. 2022, 153, 116655. [Google Scholar] [CrossRef]
- Behera, B.; Joshi, R.; Anil Vishnu, G.K.; Bhalerao, S.; Pandya, H.J. Electronic nose: A non-invasive technology for breath analysis of diabetes and lung cancer patients. J. Breath Res. 2019, 13, 024001. [Google Scholar] [CrossRef]
- Sarno, R.; Sabilla, S.I.; Rahmanwijaya, D. Electronic Nose for Detecting Multilevel Diabetes Using Optimized Deep Neural Network. Eng. Lett. 2020, 28, 31–42. [Google Scholar]
- Saidi, T.; Zaim, O.; Moufid, M.; El Bari, N.; Ionescu, R.; Bouchikhi, B. Exhaled breath analysis using electronic nose and gas chromatography–mass spectrometry for non-invasive diagnosis of chronic kidney disease, diabetes mellitus and healthy subjects. Sens. Actuators B Chem. 2018, 257, 178–188. [Google Scholar] [CrossRef]
- Jahangiri-Manesh, A.; Mousazadeh, M.; Nikkhah, M. Fabrication of chemiresistive nanosensor using molecularly imprinted polymers for acetone detection in gaseous state. Iran. Polym. J. 2022, 31, 883–891. [Google Scholar] [CrossRef]
- Mitsubayashi, K.; Toma, K.; Iitani, K.; Arakawa, T. Gas-phase biosensors: A review. Sens. Actuators B Chem. 2022, 367, 132053. [Google Scholar] [CrossRef]
- Saraoğlu, H.M.; Koçan, M. Determination of Blood Glucose Level-Based Breath Analysis by a Quartz Crystal Microbalance Sensor Array. IEEE Sens. J. 2010, 10, 104–109. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9781420006834. [Google Scholar]
- Muñoz-Aguirre, S.; Nakamoto, T.; Moriizumi, T. Study of deposition of gas sensing films on quartz crystal microbalance using an ultrasonic atomizer. Sens. Actuators B Chem. 2005, 105, 144–149. [Google Scholar] [CrossRef]
- Kudo, T.; Sekiguchi, K.; Sankoda, K.; Namiki, N.; Nii, S. Effect of ultrasonic frequency on size distributions of nanosized mist generated by ultrasonic atomization. Ultrason. Sonochem. 2017, 37, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Lang, J. Ultrasonic Atomization of Liquids. Acustica 1962, 341, 6–8. [Google Scholar] [CrossRef]
- Muñoz-Aguirre, S.; Muñoz-Mata, J.L.; Castillo-Mixcóatl, J.; Beltrán-Pérez, G. Medidor de Frecuencia de Alto Rendimiento. Mexican Patent 359609, 27 August 2018. [Google Scholar]
- Muñoz-Mata, J.L.; Muñoz-Aguirre, S.; González-Santos, H.; Beltrán-Pérez, G.; Castillo-Mixcóatl, J. Development and implementation of a system to measure the response of quartz crystal resonator based gas sensors using a field-programmable gate array. Meas. Sci. Technol. 2012, 23, 055104. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, J.; Chen, S.; Zeng, L.; Huang, Y. A highly sensitive QCM sensor coated with Ag+-ZSM-5 film for medical diagnosis. Sens. Actuators B Chem. 2004, 101, 316–321. [Google Scholar] [CrossRef]
- Parmar, S.; Ray, B.; Vishwakarma, S.; Rath, S.; Datar, S. Polymer modified quartz tuning fork (QTF) sensor array for detection of breath as a biomarker for diabetes. Sens. Actuators B Chem. 2022, 358, 131524. [Google Scholar] [CrossRef]
- Naidu, H.; Kahraman, O.; Feng, H. Novel applications of ultrasonic atomization in the manufacturing of fine chemicals, pharmaceuticals, and medical devices. Ultrason. Sonochem. 2022, 86, 105984. [Google Scholar] [CrossRef]
- Hekiem, N.L.L.; Aini, A.; Ralib, M.; Akmal, M.; Ahmad, F.B.; Rahim, R.A.; Farahidah Za’bah, N.; Sugandi, G. Chitosan Coating on Quartz Crystal Microbalance Gas Sensor for Isopropyl Alcohol and Acetone Detection. Int. J. Integr. Eng. 2022, 14, 241–249. [Google Scholar] [CrossRef]
- Wang, L. Metal-organic frameworks for QCM-based gas sensors: A review. Sens. Actuators A Phys. 2020, 307, 111984. [Google Scholar] [CrossRef]
- Aliza Aini Ralib, M.; Bhattacharjee, S.; Ralib, A.A.; Vyakaranam, A.; Svpk, S.D.; Shameem, S.S.S.; Sulo, R.; Zainuddin, A.A. Study of Multichannel QCM Prospects in VOC Detection. J. Phys. Conf. Ser. 2021, 1900, 012020. [Google Scholar] [CrossRef]
- Wolf, W.R.; Sievers, R.E.; Brown, G.H. Vapor Pressure Measurements and Gas Chromatographic Studies of the Solution Thermodynamics of Metal β-Diketonates. Inorg. Chem. 1972, 11, 1995–2002. [Google Scholar] [CrossRef]
- Koch, W. Properties and Uses of Ethylcellulose. Ind. Eng. Chem. 1937, 29, 687–690. [Google Scholar] [CrossRef]
- Bellenger, V.; Kaltenecker-Commerçon, J.; Verdu, J.; Tordjeman, P. Interactions of solvents with poly(methyl methacrylate). Polymer 1997, 38, 4175–4184. [Google Scholar] [CrossRef]



| Volume | ||||
|---|---|---|---|---|
| µL | (ppm) | (ppm) | (ppm) | (ppm) |
| 1 | 598 | 414 | 317 | 327 |
| 5 | 2992 | 2072 | 1583 | 1636 |
| 10 | 5983 | 4144 | 3165 | 3272 |
| 15 | 8975 | 6216 | 4748 | 4908 |
| Sensing Film | (g/cm3) | (kHz) | (nm) | Thickness Increase Rate (nm/min) | ||||
|---|---|---|---|---|---|---|---|---|
(kHz) | (nm) | (kHz) | (nm) | |||||
| EC | 1.14 | 28.1 | 99 | 28.9 | 101 | 57.0 | 200 | 20 |
| PMMA | 1.18 | 23.5 | 99 | 23.8 | 100 | 47.3 | 198 | 2 |
| ApL | 0.896 | 19.1 | 105 | 17.5 | 96 | 36.6 | 201 | 5 |
| ApT | 0.912 | 21.0 | 113 | 6.6 | 36 | 27.6 | 149 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Torres, M.; Altuzar, V.; Mendoza-Barrera, C.; Beltrán-Pérez, G.; Castillo-Mixcóatl, J.; Muñoz-Aguirre, S. Acetone Detection and Classification as Biomarker of Diabetes Mellitus Using a Quartz Crystal Microbalance Gas Sensor Array. Sensors 2023, 23, 9823. https://doi.org/10.3390/s23249823
Rodríguez-Torres M, Altuzar V, Mendoza-Barrera C, Beltrán-Pérez G, Castillo-Mixcóatl J, Muñoz-Aguirre S. Acetone Detection and Classification as Biomarker of Diabetes Mellitus Using a Quartz Crystal Microbalance Gas Sensor Array. Sensors. 2023; 23(24):9823. https://doi.org/10.3390/s23249823
Chicago/Turabian StyleRodríguez-Torres, Marcos, Víctor Altuzar, Claudia Mendoza-Barrera, Georgina Beltrán-Pérez, Juan Castillo-Mixcóatl, and Severino Muñoz-Aguirre. 2023. "Acetone Detection and Classification as Biomarker of Diabetes Mellitus Using a Quartz Crystal Microbalance Gas Sensor Array" Sensors 23, no. 24: 9823. https://doi.org/10.3390/s23249823
APA StyleRodríguez-Torres, M., Altuzar, V., Mendoza-Barrera, C., Beltrán-Pérez, G., Castillo-Mixcóatl, J., & Muñoz-Aguirre, S. (2023). Acetone Detection and Classification as Biomarker of Diabetes Mellitus Using a Quartz Crystal Microbalance Gas Sensor Array. Sensors, 23(24), 9823. https://doi.org/10.3390/s23249823







