Lateral Flow Test System to Control Total Content of Muscle Tissues in Raw Meat Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Biotinylation of Antibodies
2.3. Sandwich ELISA
2.4. Preparation of Gold Nanoparticles and Their Characterization
2.5. Immobilization of the Antibodies on the GNPs
2.6. Production of Test Strips
2.7. Sample Preparation
2.8. Performance of the ICA
2.9. Data Processing
3. Results and Discussion
3.1. Obtaining and Characterization of Reagents for ICA
3.2. Development of Individual ICAs
3.3. Development of Combined ICA
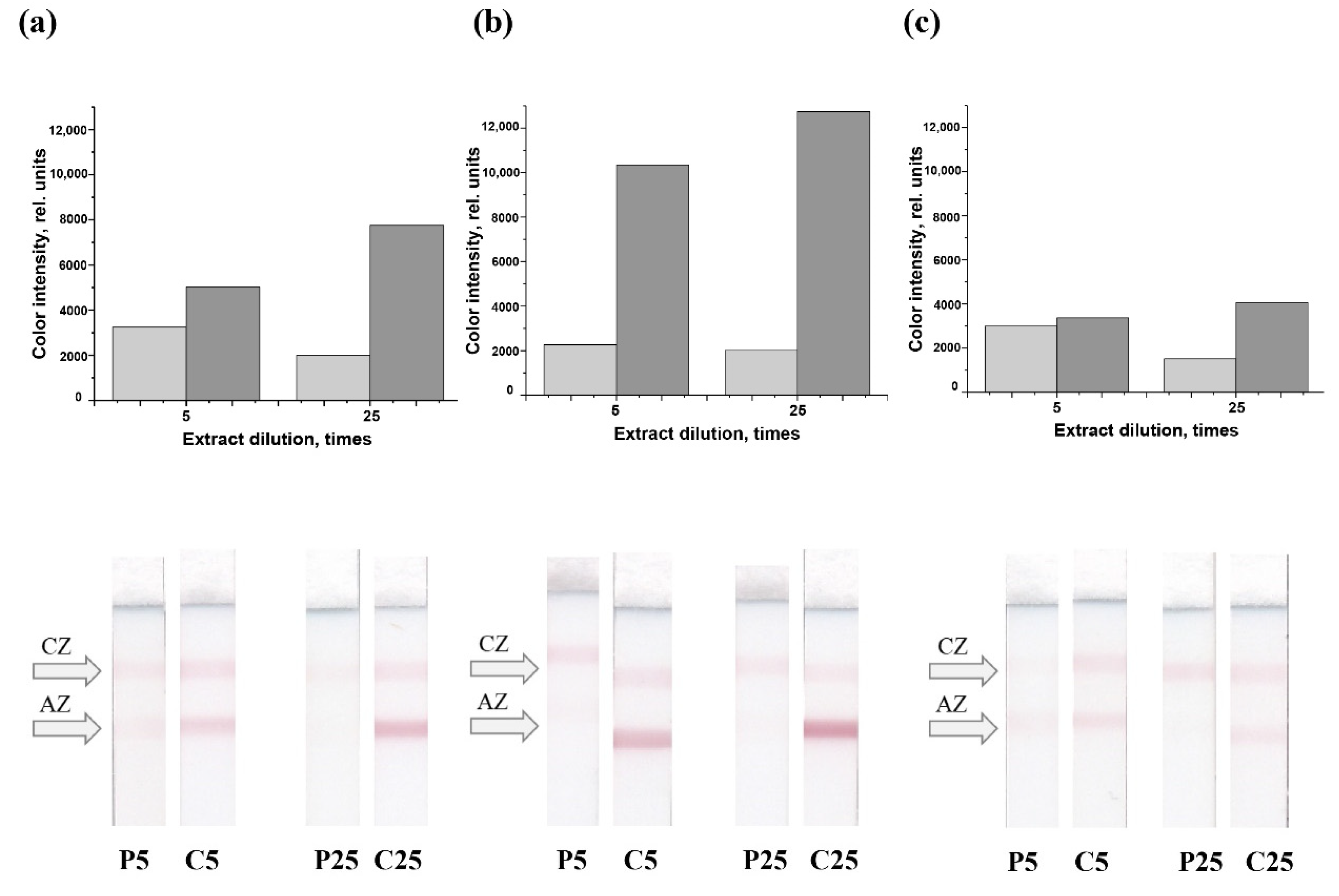
3.4. Choosing Assay Parameters for Total Meat Content Estimation
3.5. Analysis of Meat Extracts of Different Compositions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, P.M.; Vicente, A.F. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ma, Y.; Cai, L.; Jiang, S.; Li, C. Reconsidering meat intake and human health: A review of current research. Mol. Nutr. Food Res. 2022, 66, 2101066. [Google Scholar] [CrossRef]
- Kulczyński, B.; Sidor, A.; Gramza-Michałowska, A. Characteristics of selected antioxidative and bioactive compounds in meat and animal origin products. Antioxidants 2019, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Stangierski, J.; Lesnierowski, G. Nutritional and health-promoting aspects of poultry meat and its processed products. World’s Poult. Sci. J. 2015, 71, 71–82. [Google Scholar] [CrossRef]
- Yousefi, M.; Khorshidian, N.; Hosseini, H. An overview of the functionality of inulin in meat and poultry products. Nutr. Food Sci. 2018, 48, 819–835. [Google Scholar] [CrossRef]
- Li, Y.C.; Liu, S.Y.; Meng, F.B.; Liu, D.Y.; Zhang, Y.; Wang, W.; Zhang, J.M. Comparative review and the recent progress in detection technologies of meat product adulteration. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2256–2296. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Marquès, M.; Nadal, M.; Domingo, J.L. Meat consumption: Which are the current global risks? A review of recent (2010–2020) evidences. Food Res. Int. 2020, 137, 109341. [Google Scholar] [CrossRef] [PubMed]
- Jurica, K.; Karaconji, I.B.; Lasić, D.; Kovacević, D.B.; Putnik, P. Unauthorized food manipulation as a criminal offense: Food authenticity, legal frameworks, analytical tools and cases. Foods 2021, 10, 2570. [Google Scholar] [CrossRef]
- Canan, C.; Adamante, D.; Kalschne, D.; Corso, M.; Zanatta, E.R. Soy protein: A food allergen frequently used in the preparation of meat products. Rev. Chil. Nutr. 2020, 47, 463–469. [Google Scholar] [CrossRef]
- Alikord, M.; Momtaz, H.; Keramat, J.; Kadivar, M.; Rad, A.H. Species identification and animal authentication in meatproducts: A review. Food Measur. 2018, 12, 145–155. [Google Scholar] [CrossRef]
- Geiker, N.R.W.; Bertram, H.C.; Mejborn, H.; Dragsted, L.O.; Kristensen, L.; Carrascal, J.R.; Bügel, S.; Astrup, A. Meat and human health—Current knowledge and research gaps. Foods 2021, 10, 1556. [Google Scholar] [CrossRef]
- Haddad, M. The impact of CB1 receptor on nuclear receptors in skeletal muscle cells. Pathophysiology 2021, 28, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M. Impact of adenosine A2 receptor ligands on BCL2 expression in skeletal muscle cells. Appl. Sci. 2021, 11, 2272. [Google Scholar] [CrossRef]
- Chernukha, I.M.; Vostrikova, N.L.; Khvostov, D.V.; Zvereva, E.A.; Taranova, N.A.; Zherdev, A.V. Methods of identification of muscle tissue in meat products. Prerequisites for creating a multi–level control system. Theory Pract. Meat Proces. 2019, 4, 32–40. [Google Scholar] [CrossRef]
- Stachniuk, A.; Sumara, A.; Montowska, M.; Fornal, E. Liquid chromatography–mass spectrometry bottom-up proteomic methods in animal species analysis of processed meat for food authentication and the detection of adulterations. Mass Spectrom. Rev. 2021, 40, 3–30. [Google Scholar] [CrossRef]
- Kumar, Y.; Narsaiah, K. Rapid point-of-care testing methods/devices for meat species identification: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 900–923. [Google Scholar] [CrossRef] [PubMed]
- Harlina, P.W.; Maritha, V.; Musfiroh, I.; Huda, S.; Sukri, N.; Muchtaridi, M. Possibilities of liquid chromatography mass spectrometry (LC-MS)-based metabolomics and lipidomics in the authentication of meat products: A mini review. Food Sci. Anim. Resour. 2022, 42, 744–761. [Google Scholar] [CrossRef]
- Dzantiev, B.B.; Byzova, N.A.; Urusov, A.E.; Zherdev, A.V. Immunochromatographic methods in food analysis. Trend Anal. Chem. 2014, 55, 81–93. [Google Scholar] [CrossRef]
- Chen, F.C.; Hsieh, Y.P. Porcine troponin I: A thermostable species marker protein. Meat Sci. 2002, 61, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Masiri, J.; Benoit, L.; Barrios-Lopez, B.; Thienes, C.; Meshgi, M.; Agapov, A.; Dobritsa, A.; Nadala, C.; Samadpour, M. Development and validation of a rapid test system for detection of pork meat and collagen residues. Meat Sci. 2016, 121, 397–402. [Google Scholar] [CrossRef]
- Zvereva, E.A.; Kovalev, L.I.; Ivanov, A.V.; Kovaleva, M.A.; Zherdev, A.V.; Shishkin, S.S.; Lisitsyn, A.B.; Chernukha, I.M.; Dzantiev, B.B. Enzyme immunoassay and proteomic characterization of troponin I as a marker of mammalian muscle compounds in raw meat and some meat products. Meat Sci. 2015, 105, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Kotoura, S.; Murakami-Yamaguchi, Y.; Kizu, K.; Nakamura, M.; Fuchu, H.; Kiyotaka Miake, K.; Sugiyama, M.; Narita, H. Establishment of a sandwich ELISA for the determination of beef content in processed foods by using monoclonal antibodies to myoglobin. Food Agric. Immunol. 2012, 23, 289–301. [Google Scholar] [CrossRef]
- Jiang, X.; Rao, Q.; Mittl, K.; Hsieh, Y.P. Monoclonal antibody-based sandwich ELISA for the detection of mammalian meats. Food Control 2020, 110, 107045. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Zvereva, E.A.; Vostrikova, N.L.; Chernukha, I.M.; Dzantiev, B.B.; Zherdev, A.V. Lateral flow immunoassay for sensitive detection of undeclared chicken meat in meat products. Food Chem. 2021, 344, 128598. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Gani, A.A.; Ahmad, M. Immuno strip test for detection of pork adulteration in cooked meatballs. Food Biosci. 2017, 19, 1–6. [Google Scholar] [CrossRef]
- Zvereva, E.A.; Popravko, D.S.; Hendrickson, O.D.; Vostrikova, N.L.; Chernukha, I.M.; Dzantiev, B.B.; Zherdev, A.V. Lateral flow immunoassay to detect the addition of beef, pork, lamb, and horse muscles in raw meat mixtures and finished meat products. Foods 2020, 9, 1662. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.M.; Uddin, S.M.K.; Sultana, S.; Wahab, Y.A.; Sagadevan, S.; Johan, M.R.; Eaqub, A. Authentication of Halal and Kosher meat and meat products: Analytical approaches, current progresses and future prospects. Crit. Rev. Food Sci. Nutr. 2022, 62, 285–310. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Hussain, M.; Shahid, F.; Siddeeg, A.; Al-Farga, A. Proteomics as a promising biomarker in food authentication, quality and safety: A review. Food Sci. Nutr. 2022, 10, 2333–2346. [Google Scholar] [CrossRef]
- Bayer, E.A.; Wilchek, M. Protein biotinylation. Meth. Enzymol. 1990, 184, 138–160. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Wild, D.G. Immunoassay Handbook: Theory and Applications of Ligand Binding, ELISA and Related Techniques, 4th ed.; Elsevier Science: Amsterdam, The Netherlands, 2013; 1036p. [Google Scholar]





| Chicken Meat/Porcine Meat Ratio, % | ICA Responses (n = 3) |
|---|---|
| 25/75 | 3181 ± 183 |
| 50/50 | 2961 ± 156 |
| 75/25 | 3172 ± 64 |
| 90/10 | 3144 ± 177 |
| 95/5 | 3112 ± 182 |
| Chicken Meat/Mammalian Meat Ratio, % | ICA Response (n = 3) | ||
|---|---|---|---|
| Chicken/Porcine | Chicken/Beef | Chicken/Rabbit | |
| 25/75 | 4677 ± 309 | 4205 ± 239 | 4261 ± 243 |
| 50/50 | 4673 ± 271 | 4342 ± 129 | 4252 ± 225 |
| 75/25 | 4621 ± 259 | 4348 ± 289 | 4760 ± 309 |
| 90/10 | 4145 ± 235 | 4332 ± 115 | 4515 ± 452 |
| 95/5 | 4484 ± 430 | 4317 ± 208 | 4298 ± 222 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zvereva, E.A.; Hendrickson, O.D.; Dzantiev, B.B.; Zherdev, A.V. Lateral Flow Test System to Control Total Content of Muscle Tissues in Raw Meat Products. Sensors 2022, 22, 9724. https://doi.org/10.3390/s22249724
Zvereva EA, Hendrickson OD, Dzantiev BB, Zherdev AV. Lateral Flow Test System to Control Total Content of Muscle Tissues in Raw Meat Products. Sensors. 2022; 22(24):9724. https://doi.org/10.3390/s22249724
Chicago/Turabian StyleZvereva, Elena A., Olga D. Hendrickson, Boris B. Dzantiev, and Anatoly V. Zherdev. 2022. "Lateral Flow Test System to Control Total Content of Muscle Tissues in Raw Meat Products" Sensors 22, no. 24: 9724. https://doi.org/10.3390/s22249724
APA StyleZvereva, E. A., Hendrickson, O. D., Dzantiev, B. B., & Zherdev, A. V. (2022). Lateral Flow Test System to Control Total Content of Muscle Tissues in Raw Meat Products. Sensors, 22(24), 9724. https://doi.org/10.3390/s22249724









