Ambulation Mode Classification of Individuals with Transfemoral Amputation through A-Mode Sonomyography and Convolutional Neural Networks
Abstract
1. Introduction
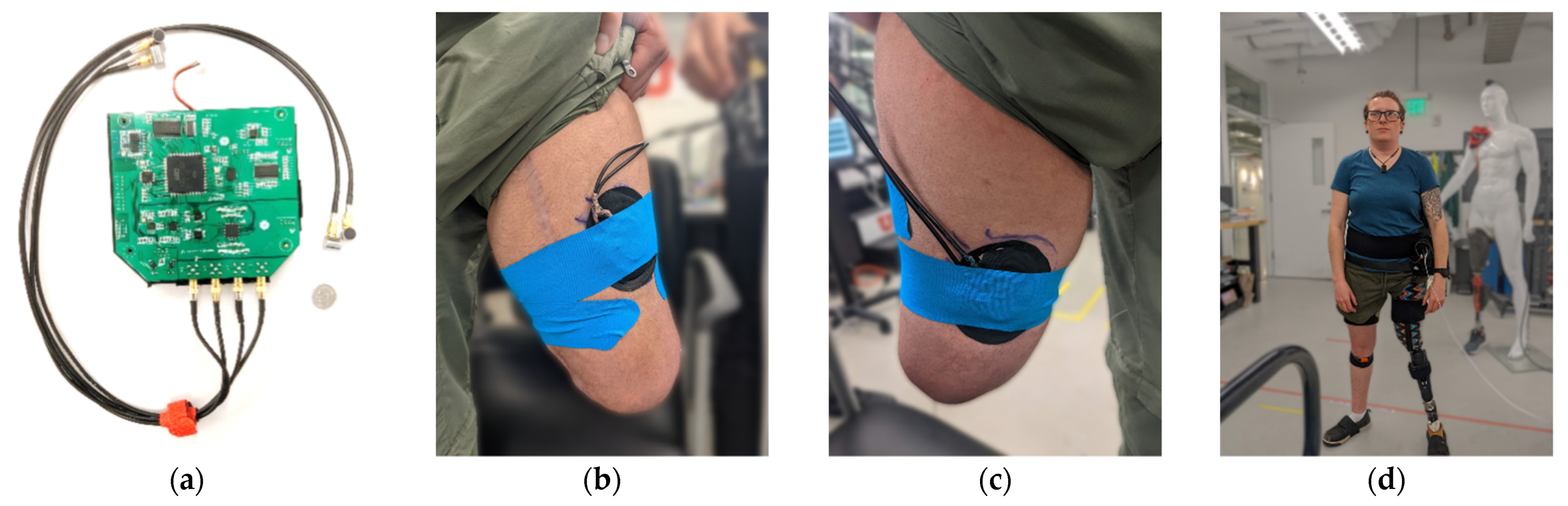
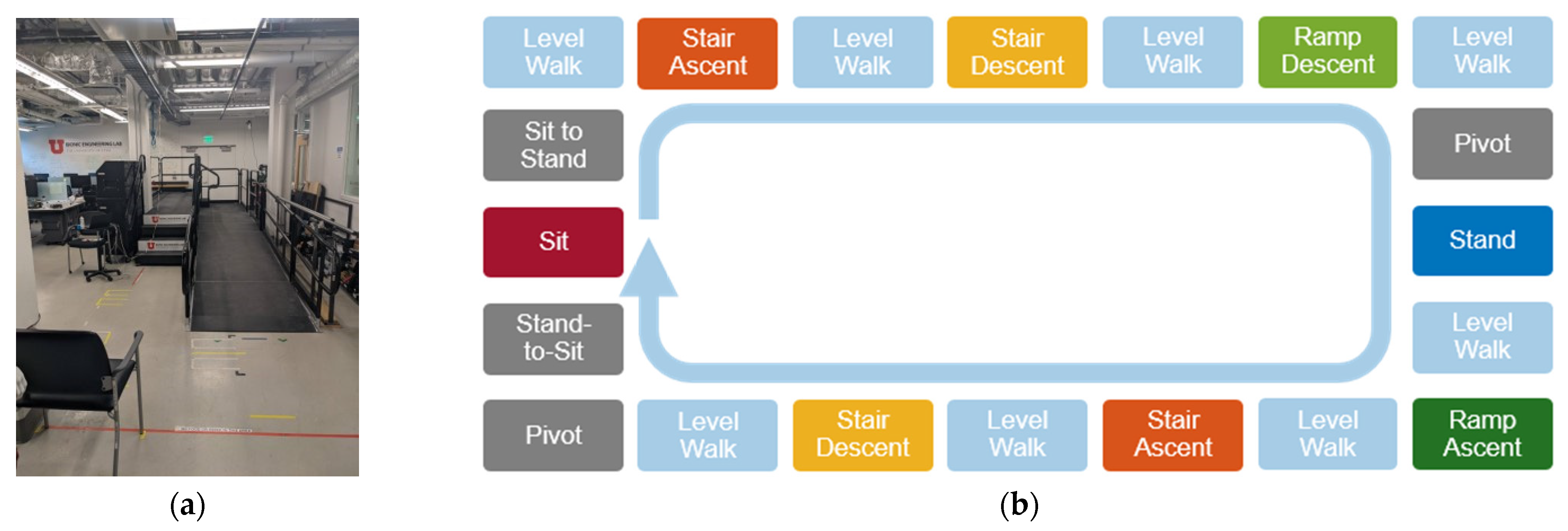
2. Materials and Methods
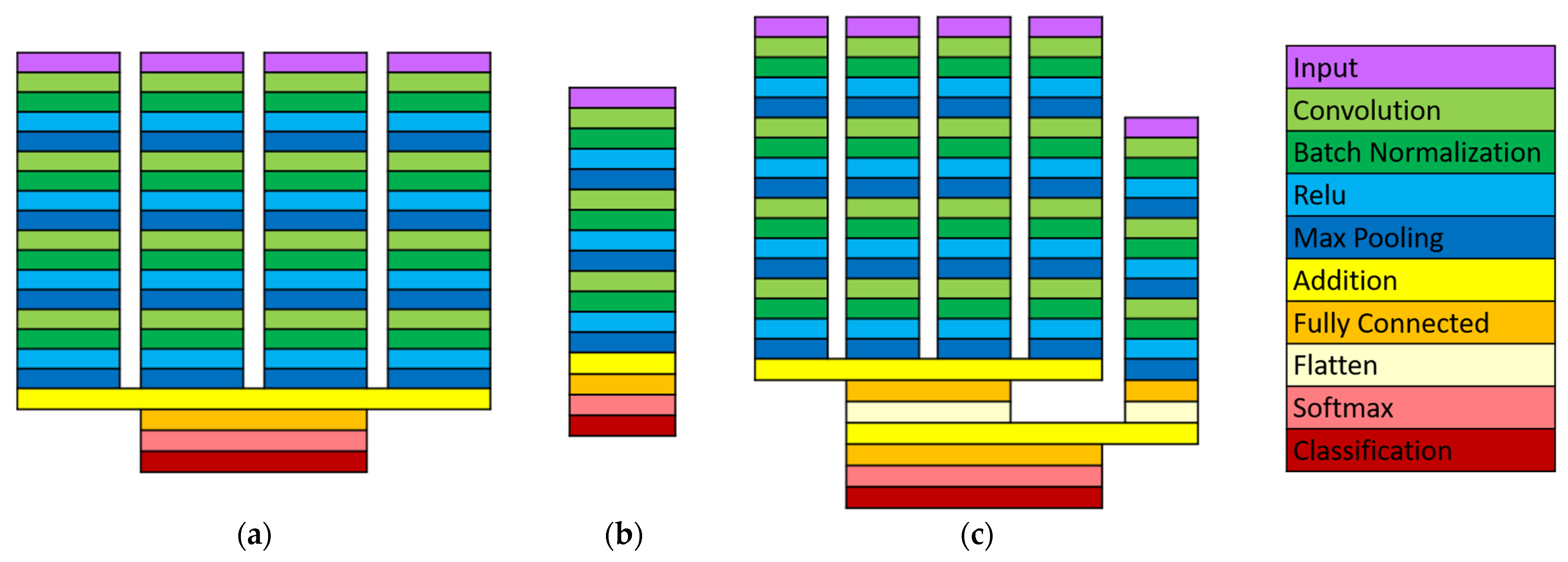
2.1. Data Processing
2.2. Outcome Measures
3. Results
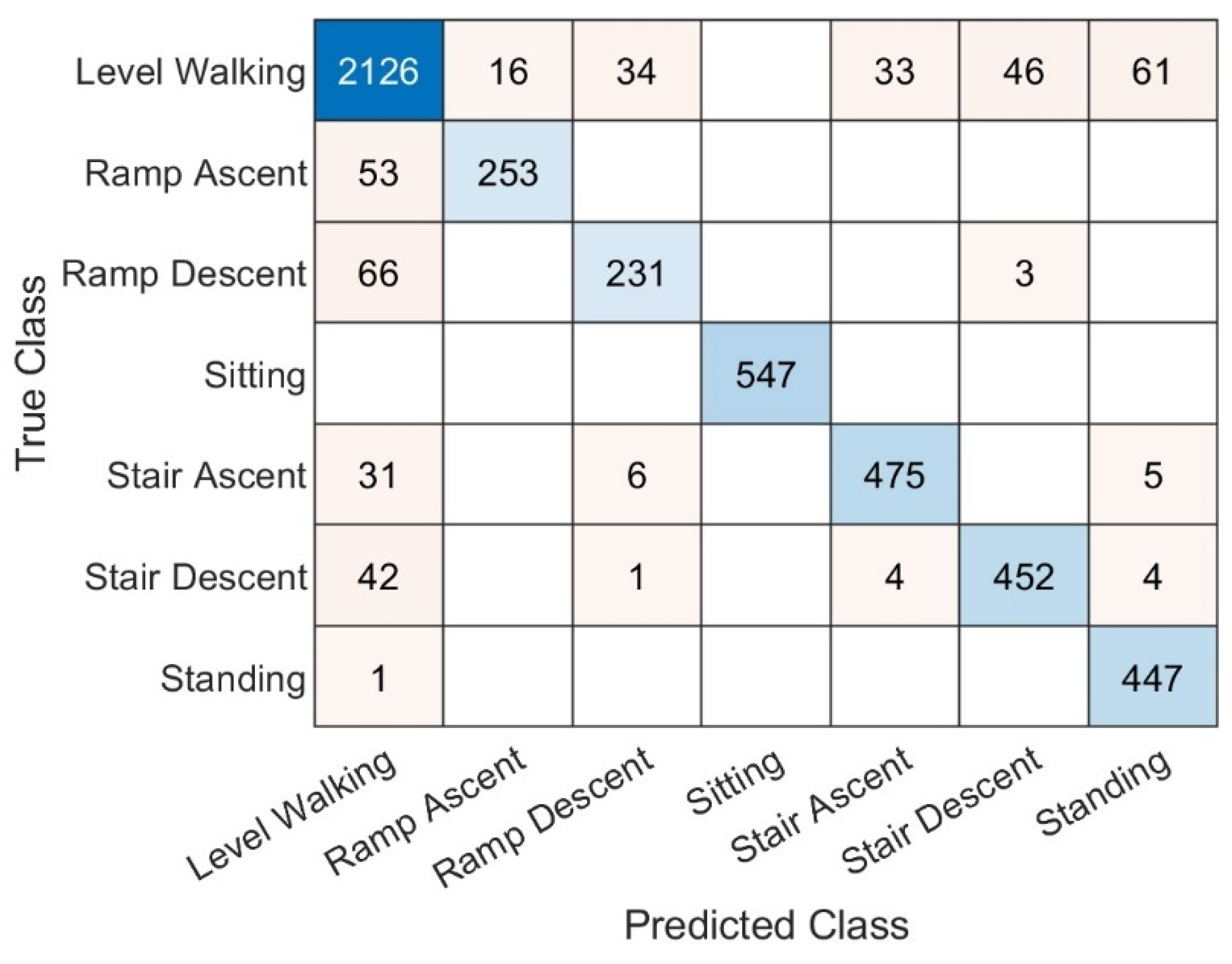
3.1. Overall Performance
3.2. Performance by Ambulation Mode
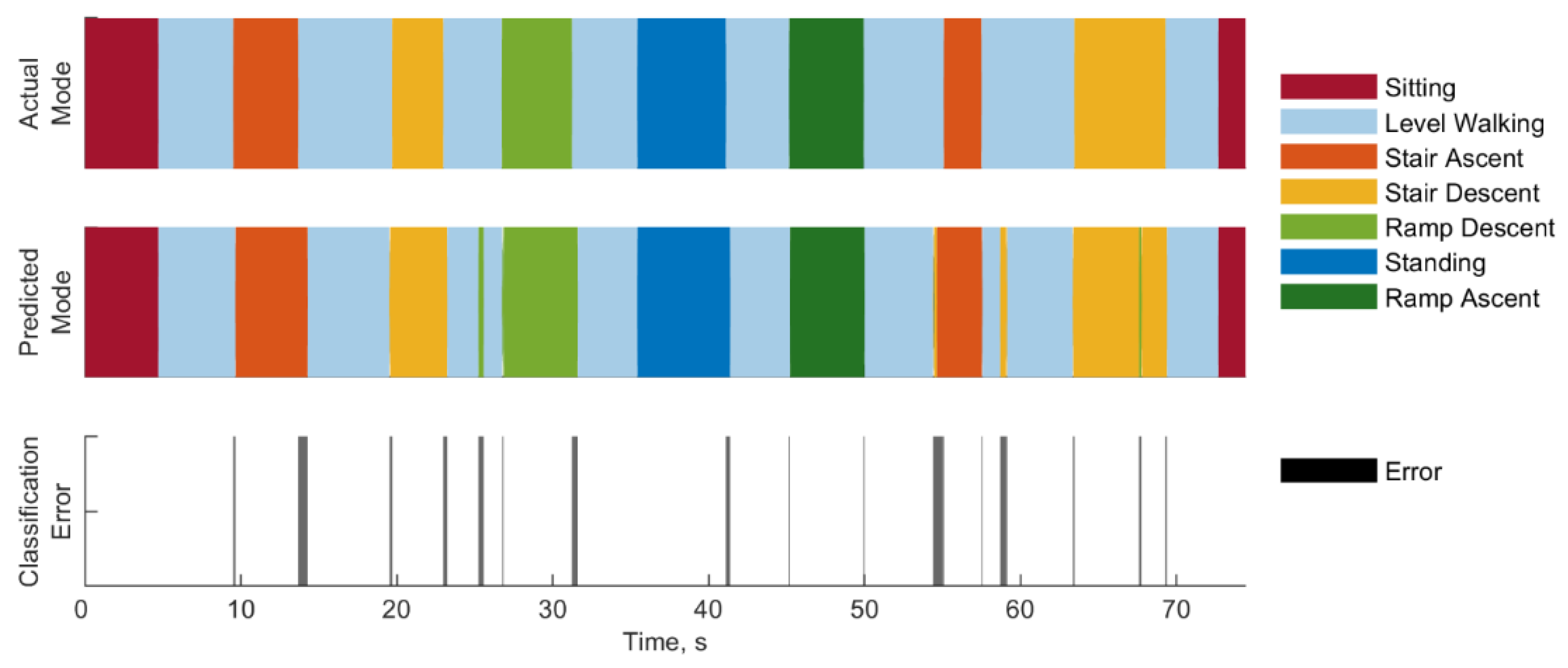
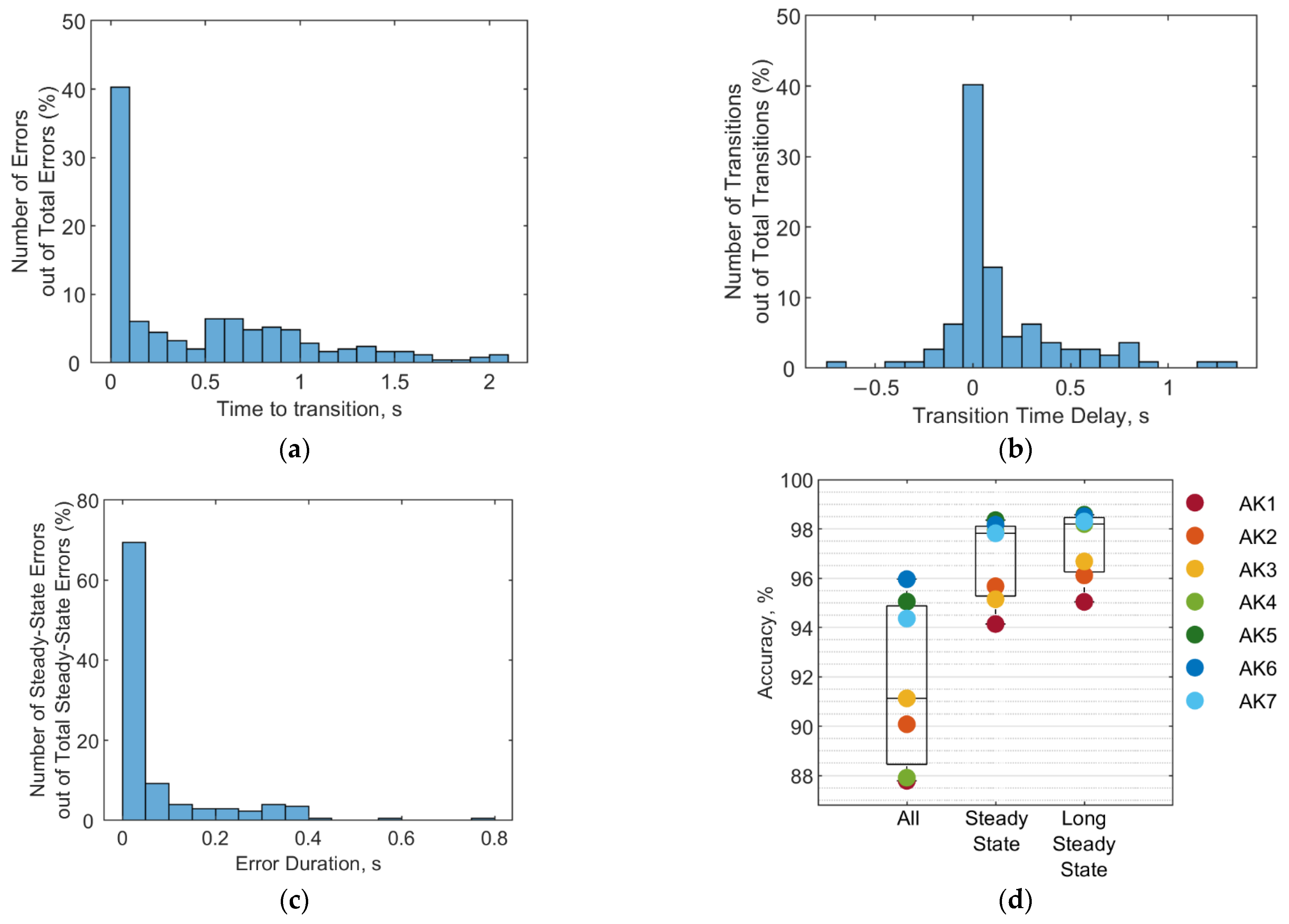
3.3. Error Types and Duration
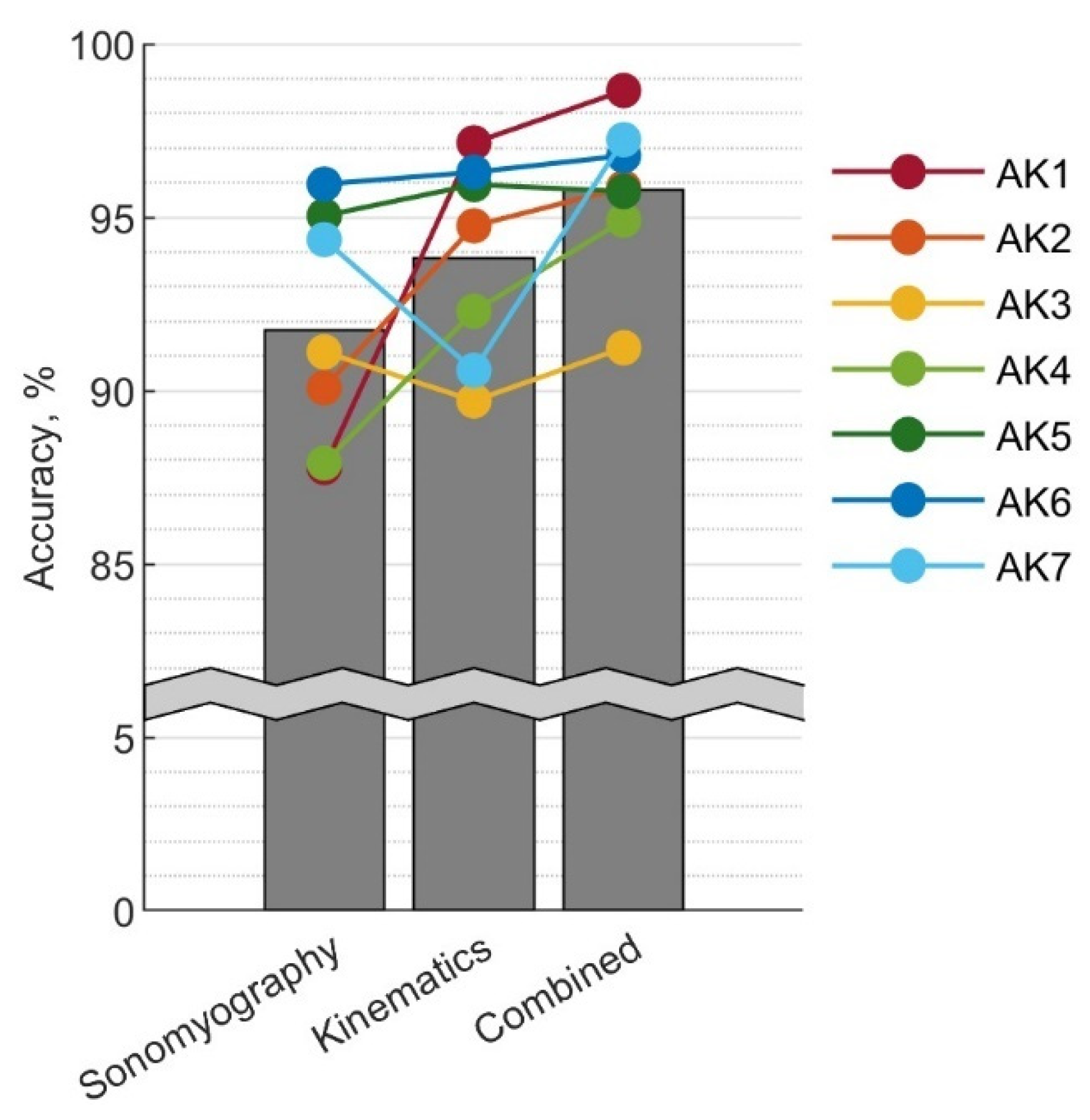
3.4. Performance Comparison
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziegler-Graham, K.; MacKenzie, E.J.; Ephraim, P.L.; Travison, T.G.; Brookmeyer, R. Estimating the Prevalence of Limb Loss in the United States: 2005 to 2050. Arch. Phys. Med. Rehabil. 2008, 89, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Dillingham, T.R.; Pezzin, L.E.; MacKenzie, E.J. Limb Amputation and Limb Deficiency: Epidemiology and Recent Trends in the United States. South Med. J. 2002, 95, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, M.; Lawson, B.E.; Shultz, A.H. Realizing the Promise of Robotic Leg Prostheses. Sci. Transl. Med. 2013, 5, 210ps15. [Google Scholar] [CrossRef]
- Tucker, M.R.; Olivier, J.; Pagel, A.; Bleuler, H.; Bouri, M.; Lambercy, O.; del Millán, J.R.; Riener, R.; Vallery, H.; Gassert, R. Control Strategies for Active Lower Extremity Prosthetics and Orthotics: A Review. J. Neuroeng. Rehabil. 2015, 12, 1. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, M.; Huang, H. Effects of Locomotion Mode Recognition Errors on Volitional Control of Powered Above-Knee Prostheses. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 64–72. [Google Scholar] [CrossRef]
- Woodward, R.B.; Spanias, J.A.; Hargrove, L.J. User Intent Prediction with a Scaled Conjugate Gradient Trained Artificial Neural Network for Lower Limb Amputees Using a Powered Prosthesis. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 6405–6408. [Google Scholar]
- Young, A.J.; Simon, A.; Hargrove, L.J. An Intent Recognition Strategy for Transfemoral Amputee Ambulation across Different Locomotion Modes. In Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Osaka, Japan, 3–7 July 2013; pp. 1587–1590. [Google Scholar]
- Liu, Z.; Lin, W.; Geng, Y.; Yang, P. Intent Pattern Recognition of Lower-Limb Motion Based on Mechanical Sensors. IEEE/CAA J. Autom. Sin. 2017, 4, 651–660. [Google Scholar] [CrossRef]
- Simon, A.M.; Ingraham, K.A.; Spanias, J.A.; Young, A.J.; Finucane, S.B.; Halsne, E.G.; Hargrove, L.J. Delaying Ambulation Mode Transition Decisions Improves Accuracy of a Flexible Control System for Powered Knee-Ankle Prosthesis. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1164–1171. [Google Scholar] [CrossRef]
- Stolyarov, R.; Burnett, G.; Herr, H. Translational Motion Tracking of Leg Joints for Enhanced Prediction of Walking Tasks. IEEE Trans. Biomed. Eng. 2018, 65, 763–769. [Google Scholar] [CrossRef]
- Su, B.Y.; Wang, J.; Liu, S.Q.; Sheng, M.; Jiang, J.; Xiang, K. A Cnn-Based Method for Intent Recognition Using Inertial Measurement Units and Intelligent Lower Limb Prosthesis. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1032–1042. [Google Scholar] [CrossRef]
- Young, A.J.; Simon, A.M.; Hargrove, L.J. A Training Method for Locomotion Mode Prediction Using Powered Lower Limb Prostheses. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 671–677. [Google Scholar] [CrossRef]
- Spanias, J.A.; Simon, A.M.; Perreault, E.J.; Hargrove, L.J. Preliminary Results for an Adaptive Pattern Recognition System for Novel Users Using a Powered Lower Limb Prosthesis. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Orlando, FL, USA, 16–20 August 2016. [Google Scholar]
- Spanias, J.A.; Simon, A.M.; Hargrove, L.J. Across-User Adaptation for a Powered Lower Limb Prosthesis. In Proceedings of the IEEE International Conference on Rehabilitation Robotics, London, UK, 17–20 July 2017. [Google Scholar]
- Young, A.J.; Simon, A.M.; Fey, N.P.; Hargrove, L.J. Intent Recognition in a Powered Lower Limb Prosthesis Using Time History Information. Ann. Biomed. Eng. 2014, 42, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J.; Hargrove, L.J. A Classification Method for User-Independent Intent Recognition for Transfemoral Amputees Using Powered Lower Limb Prostheses. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 217–225. [Google Scholar] [CrossRef]
- Labarrière, F.; Thomas, E.; Calistri, L.; Optasanu, V.; Gueugnon, M.; Ornetti, P.; Laroche, D. Machine Learning Approaches for Activity Recognition and/or Activity Prediction in Locomotion Assistive Devices—A Systematic Review. Sensors 2020, 20, 6345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, D.; Yang, Q.; Huang, H. An Automatic and User-Driven Training Method for Locomotion Mode Recognition for Artificial Leg Control. In Proceedings of the 34th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, San Diego, CA, USA, 28 August–1 September 2012. [Google Scholar]
- Liu, M.; Wang, D.; Helen Huang, H. Development of an Environment-Aware Locomotion Mode Recognition System for Powered Lower Limb Prostheses. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Laschowski, B.; McNally, W.; Wong, A.; McPhee, J. ExoNet Database: Wearable Camera Images of Human Locomotion Environments. Front. Robot. AI 2020, 7, 188. [Google Scholar] [CrossRef]
- Laschowski, B.; McNally, W.; Wong, A.; McPhee, J. Environment Classification for Robotic Leg Prostheses and Exoskeletons Using Deep Convolutional Neural Networks. Front. Neurorobot. 2022, 15, 191. [Google Scholar] [CrossRef]
- Zhang, K.; Xiong, C.; Zhang, W.; Liu, H.; Lai, D.; Rong, Y.; Fu, C. Environmental Features Recognition for Lower Limb Prostheses Toward Predictive Walking. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 465–476. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, W.; Xiao, W.; Liu, H.; de Silva, C.W.; Fu, C. Sequential Decision Fusion for Environmental Classification in Assistive Walking. IEEE Trans. Neural. Syst. Rehabil. Eng. 2019, 27, 1780–1790. [Google Scholar] [CrossRef]
- Godiyal, A.K.; Mondal, M.; Joshi, S.D.; Joshi, D. Force Myography Based Novel Strategy for Locomotion Classification. IEEE Trans. Hum. Mach. Syst. 2018, 48, 648–657. [Google Scholar] [CrossRef]
- Miller, J.D.; Beazer, S.; Hahn, M.E. Myoelectric Walking Mode Classification for Transtibial Amputees. IEEE Trans. Biomed. Eng. 2013, 60, 2745–2750. [Google Scholar] [CrossRef]
- Hargrove, L.J.; Englehart, K.; Hudgins, B. A Comparison of Surface and Intramuscular Myoelectric Signal Classification. IEEE Trans. Biomed. Eng. 2007, 54, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Parker, P.; Englehart, K.; Hudgins, B. Myoelectric Signal Processing for Control of Powered Limb Prostheses. J. Electromyogr. Kinesiol. 2006, 16, 541–548. [Google Scholar] [CrossRef]
- Rabe, K.G.; Jahanandish, M.H.; Boehm, J.R.; Majewicz Fey, A.; Hoyt, K.; Fey, N.P. Ultrasound Sensing Can Improve Continuous Classification of Discrete Ambulation Modes Compared to Surface Electromyography. IEEE Trans. Biomed. Eng. 2021, 68, 1379–1388. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, F.; Hargrove, L.J.; Dou, Z.; Rogers, D.R.; Englehart, K.B. Continuous Locomotion-Mode Identification for Prosthetic Legs Based on Neuromuscular—Mechanical Fusion. IEEE Trans. Biomed. Eng. 2011, 58, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J.; Kuiken, T.A.; Hargrove, L.J. Analysis of Using EMG and Mechanical Sensors to Enhance Intent Recognition in Powered Lower Limb Prostheses. J. Neural. Eng. 2014, 11, 056021. [Google Scholar] [CrossRef] [PubMed]
- Spanias, J.A.; Simon, A.M.; Ingraham, K.A.; Hargrove, L.J. Effect of Additional Mechanical Sensor Data on an EMG-Based Pattern Recognition System for a Powered Leg Prosthesis. In Proceedings of the 7th International IEEE/EMBS Conference on Neural Engineering, NER, Montpellier, France, 22–24 April 2015. [Google Scholar]
- Huang, H.; Kuiken, T.A.; Lipschutz, R.D. A Strategy for Identifying Locomotion Modes Using Surface Electromyography. IEEE Trans. Biomed. Eng. 2009, 56, 65–73. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, F.; Sun, Y.L.; He, H. Design of a Robust EMG Sensing Interface for Pattern Classification. J. Neural. Eng. 2010, 7, 056005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Disanto, W.; Ren, J.; Dou, Z.; Yang, Q.; Huang, H. A Novel CPS System for Evaluating a Neural-Machine Interface for Artificial Legs. In Proceedings of the 2011 IEEE/ACM 2nd International Conference on Cyber-Physical Systems, ICCPS 2011, Chicago, IL, USA, 12–14 April 2011. [Google Scholar]
- Hernandez, R.; Zhang, F.; Zhang, X.; Huang, H.; Yang, Q. Promise of a Low Power Mobile CPU Based Embedded System in Artificial Leg Control. In Proceedings of the 34th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, San Diego, CA, USA, 28 August–1 September 2012. [Google Scholar]
- Du, L.; Zhang, F.; Liu, M.; Huang, H. Toward Design of an Environment-Aware Adaptive Locomotion-Mode-Recognition System. IEEE Trans. Biomed. Eng. 2012, 59, 2716–2725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Huang, H. Source Selection for Real-Time User Intent Recognition toward Volitional Control of Artificial Legs. IEEE J. Biomed. Health Inform. 2013, 17, 907–914. [Google Scholar] [CrossRef]
- Du, L.; Zhang, F.; He, H.; Huang, H. Improving the Performance of a Neural-Machine Interface for Prosthetic Legs Using Adaptive Pattern Classifiers. In Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Osaka, Japan, 3–7 July 2013. [Google Scholar]
- Hernandez, R.; Yang, Q.; Huang, H.; Zhang, F.; Zhang, X. Design and Implementation of a Low Power Mobile CPU Based Embedded System for Artificial Leg Control. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 5769–5772. [Google Scholar]
- Young, A.J.; Simon, A.M.; Fey, N.P.; Hargrove, L.J. Classifying the Intent of Novel Users during Human Locomotion Using Powered Lower Limb Prostheses. In Proceedings of the International IEEE/EMBS Conference on Neural Engineering, NER, San Diego, CA, USA, 6–8 November 2013. [Google Scholar]
- Spanias, J.A.; Perreault, E.J.; Hargrove, L.J. A Strategy for Labeling Data for the Neural Adaptation of a Powered Lower Limb Prosthesis. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC, Chicago, IL, USA, 26–30 August 2014. [Google Scholar]
- Spanias, J.A.; Perreault, E.J.; Hargrove, L.J. Detection of and Compensation for EMG Disturbances for Powered Lower Limb Prosthesis Control. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 226–234. [Google Scholar] [CrossRef]
- Spanias, J.A.; Simon, A.M.; Finucane, S.B.; Perreault, E.J.; Hargrove, L.J. Online Adaptive Neural Control of a Robotic Lower Limb Prosthesis. J. Neural. Eng. 2018, 15, 016015. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, F.; Huang, H.H. An Adaptive Classification Strategy for Reliable Locomotion Mode Recognition. Sensors 2017, 17, 2020. [Google Scholar] [CrossRef] [PubMed]
- Cimolato, A.; Driessen, J.J.M.; Mattos, L.S.; de Momi, E.; Laffranchi, M.; de Michieli, L. EMG-Driven Control in Lower Limb Prostheses: A Topic-Based Systematic Review. J. Neuroeng. Rehabil. 2022, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Englehart, K.B.; Parker, P.A. Extracting Simultaneous and Proportional Neural Control Information for Multiple-Dof Prostheses from the Surface Electromyographic Signal. IEEE Trans. Biomed. Eng. 2009, 56, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lu, Z.; Yao, L.; Cai, S. A Gesture Recognition Strategy Based on A-Mode Ultrasound for Identifying Known and Unknown Gestures. IEEE Sens. J. 2022, 22, 10730–10739. [Google Scholar] [CrossRef]
- Zeng, J.; Zhou, Y.; Yang, Y.; Yan, J.; Liu, H. Fatigue-Sensitivity Comparison of SEMG and A-Mode Ultrasound Based Hand Gesture Recognition. IEEE J. Biomed. Health Inform. 2022, 26, 1718–1725. [Google Scholar] [CrossRef]
- Yang, X.; Yan, J.; Liu, H. Comparative Analysis of Wearable A-Mode Ultrasound and SEMG for Muscle-Computer Interface. IEEE Trans. Biomed. Eng. 2020, 67, 2434–2442. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Hettiarachchi, N.; Yan, J.; Liu, H. A Wearable Ultrasound System for Sensing Muscular Morphological Deformations. IEEE Trans. Syst. Man Cybern. Syst. 2021, 51, 3370–3379. [Google Scholar] [CrossRef]
- Zhang, J.T.; Novak, A.C.; Brouwer, B.; Li, Q. Concurrent Validation of Xsens MVN Measurement of Lower Limb Joint Angular Kinematics. Physiol. Meas. 2013, 34, N63–N69. [Google Scholar] [CrossRef]
- Yang, X.; Yan, J.; Chen, Z.; Ding, H.; Member, S.; Liu, H. A Proportional Pattern Recognition Control Scheme for Wearable A-Mode Ultrasound Sensing. IEEE Trans. Ind. Electron. 2020, 67, 800–808. [Google Scholar] [CrossRef]
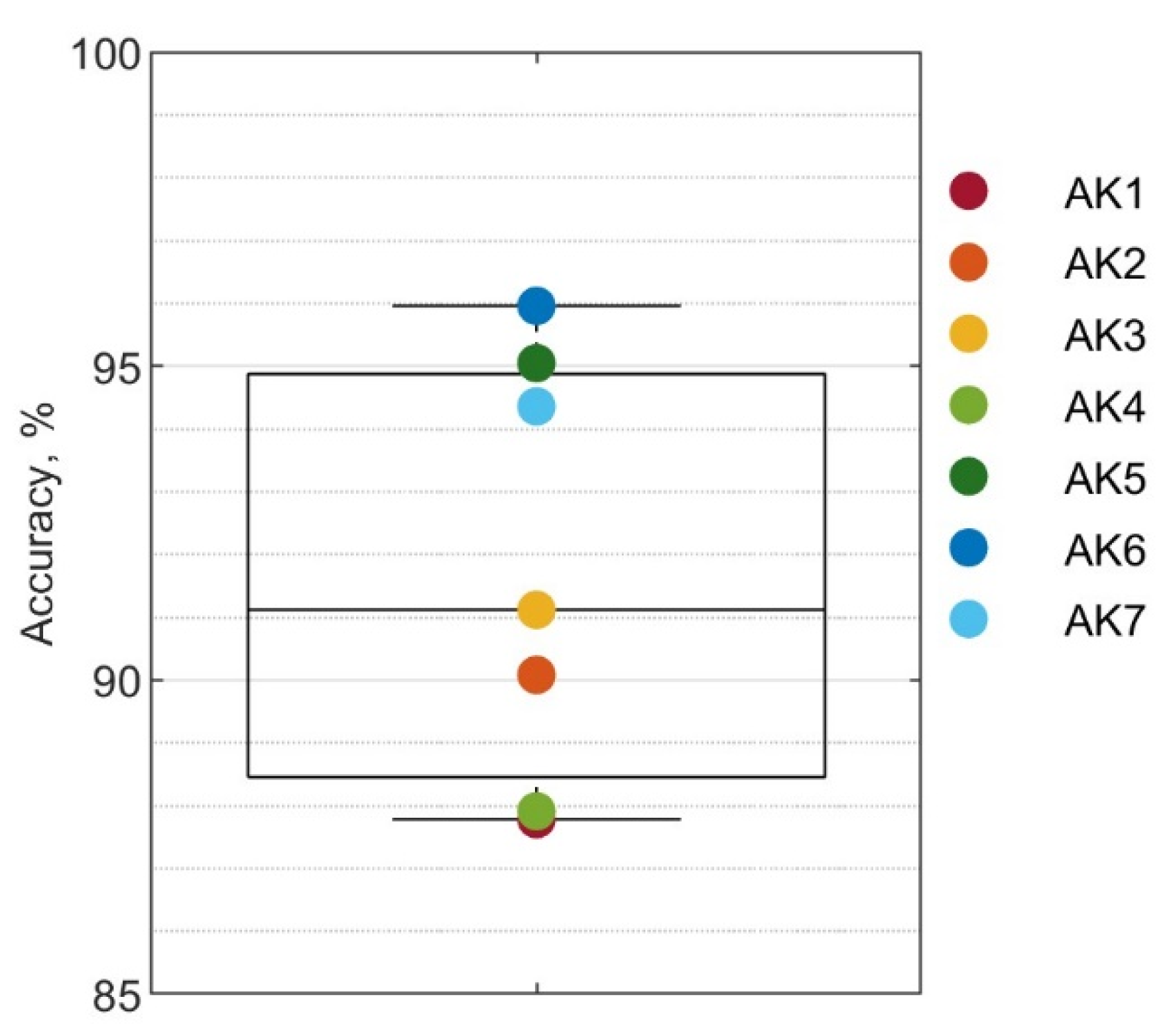
| Subject | Age | Years since Amputation | Socket Suspension | Sex | Weight (kg) | Height (m) |
|---|---|---|---|---|---|---|
| AK1 | 29 | 8 | Suction | Male | 65 | 1.78 |
| AK2 | 68 | 5 | Suction | Male | 70 | 1.70 |
| AK3 | 32 | 13 | Lanyard | Female | 59 | 1.60 |
| AK4 | 32 | 4 | Suction | Male | 77 | 1.80 |
| AK5 | 53 | 22 | Suction | Male | 100 | 1.93 |
| AK6 | 54 | 2 | Suction | Male | 78 | 1.73 |
| AK7 | 31 | 1 | Lanyard | Female | 59 | 1.68 |
| Precision | Recall | F1 | |
|---|---|---|---|
| Level Walking | 91.9 ± 5.9% | 91.9 ± 1.7% | 91.8 ± 3.2% |
| Ramp Ascent | 93.9 ± 3.8% | 82.4 ± 14.5% | 87.2 ± 8.6% |
| Ramp Descent | 86.0 ± 11.7% | 76.4 ± 21.5% | 79.4 ± 15.5% |
| Sitting | |||
| Stair Ascent | |||
| Stair Descent | |||
| Standing |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murray, R.; Mendez, J.; Gabert, L.; Fey, N.P.; Liu, H.; Lenzi, T. Ambulation Mode Classification of Individuals with Transfemoral Amputation through A-Mode Sonomyography and Convolutional Neural Networks. Sensors 2022, 22, 9350. https://doi.org/10.3390/s22239350
Murray R, Mendez J, Gabert L, Fey NP, Liu H, Lenzi T. Ambulation Mode Classification of Individuals with Transfemoral Amputation through A-Mode Sonomyography and Convolutional Neural Networks. Sensors. 2022; 22(23):9350. https://doi.org/10.3390/s22239350
Chicago/Turabian StyleMurray, Rosemarie, Joel Mendez, Lukas Gabert, Nicholas P. Fey, Honghai Liu, and Tommaso Lenzi. 2022. "Ambulation Mode Classification of Individuals with Transfemoral Amputation through A-Mode Sonomyography and Convolutional Neural Networks" Sensors 22, no. 23: 9350. https://doi.org/10.3390/s22239350
APA StyleMurray, R., Mendez, J., Gabert, L., Fey, N. P., Liu, H., & Lenzi, T. (2022). Ambulation Mode Classification of Individuals with Transfemoral Amputation through A-Mode Sonomyography and Convolutional Neural Networks. Sensors, 22(23), 9350. https://doi.org/10.3390/s22239350






