A Novel Approach for Multichannel Epileptic Seizure Classification Based on Internet of Things Framework Using Critical Spectral Verge Feature Derived from Flower Pollination Algorithm
Abstract
1. Introduction
2. Literature
2.1. Epileptic Seizure Classification Techniques
2.2. Sensor Technology in the Seizure Classification
3. Methodology
3.1. Segmentation of EEG Signal
3.2. MRAF-Based Pre-Processing
- (i)
- To begin with, the EEG signal that is input is decomposed into multiple frequency levels making use of DWT;
- (ii)
- Next, soft thresholding is brought into use with the DWT coefficients to generate a signal with minimized abrupt changes;
- (iii)
- Finally, MRAF is undertaken to produce an EEG signal that contains minimal physiological EEG artifacts.
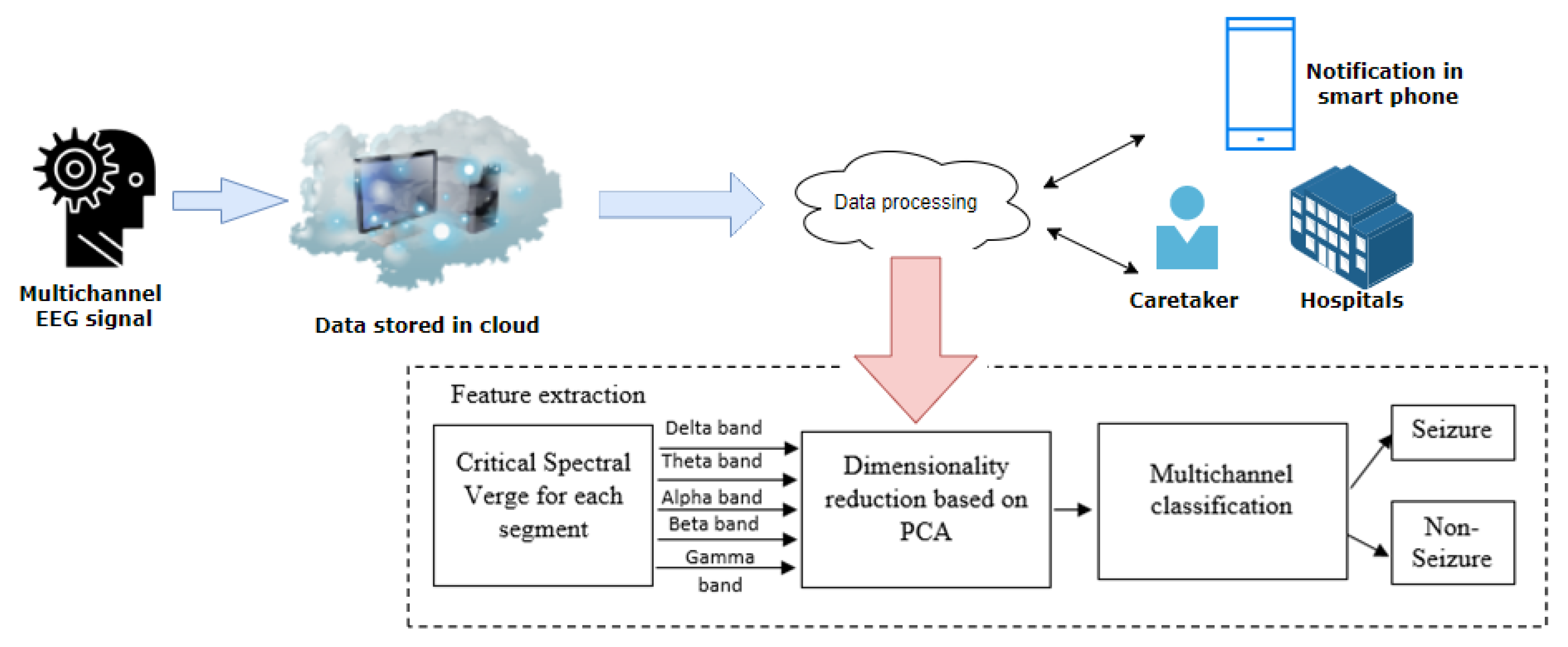
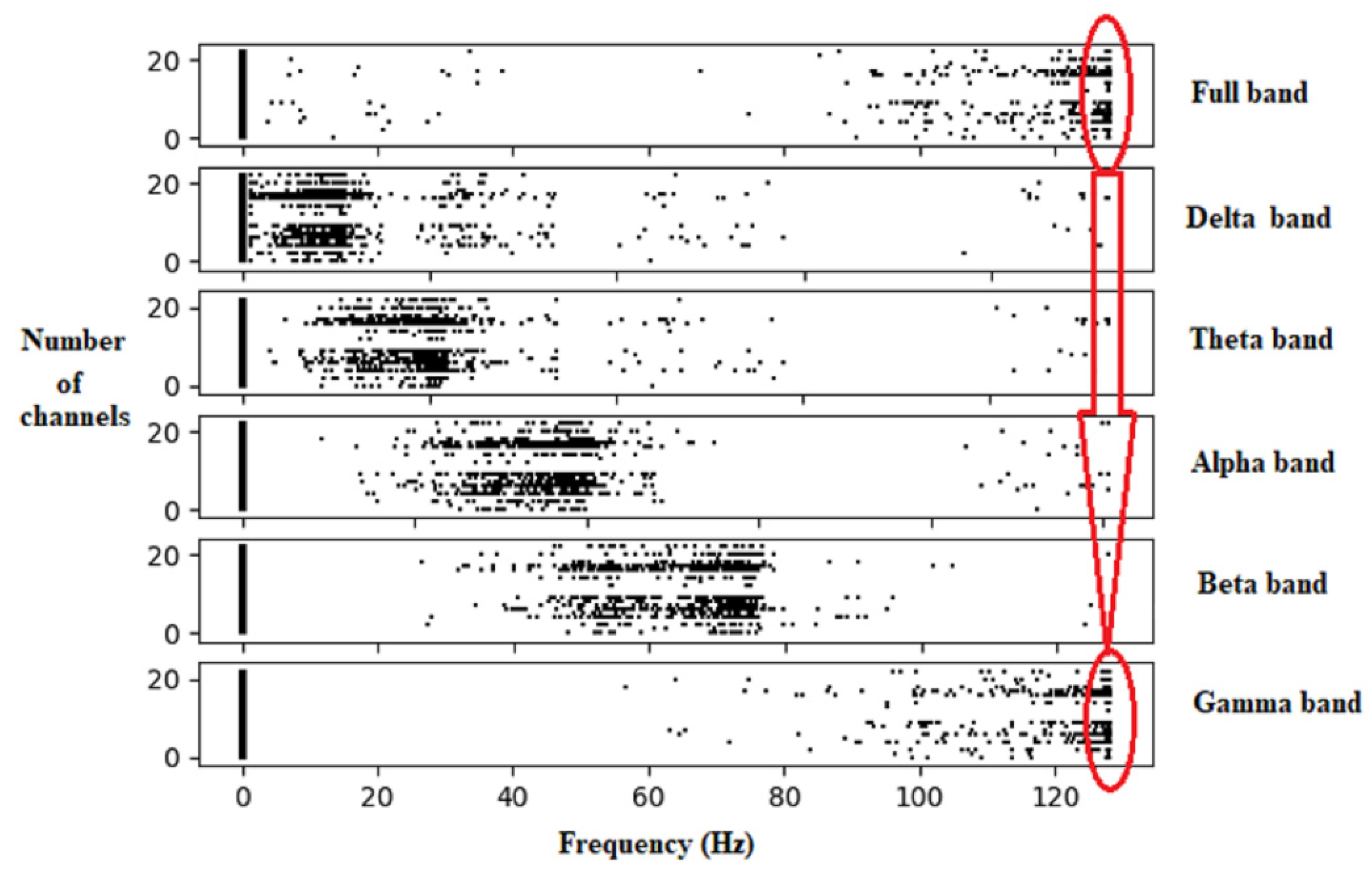
3.3. Proposed Feature Extraction Technique
- Step 1:
- Process segment k of an EEG signal
- Step 2:
- Process band b of an EEG signal
- Step 3:
- Calculate power spectral values of all segments and all bands.
- Step 4:
- Take the average of the calculated power spectral values.
- Step 5:
- Apply the FPA to optimize and calculate the CSV.
- Step 6:
- Add values of each calculated CSV to the feature map.
3.4. Mathematical Model
3.5. Feature Dimensionality Reduction
3.6. Classifier
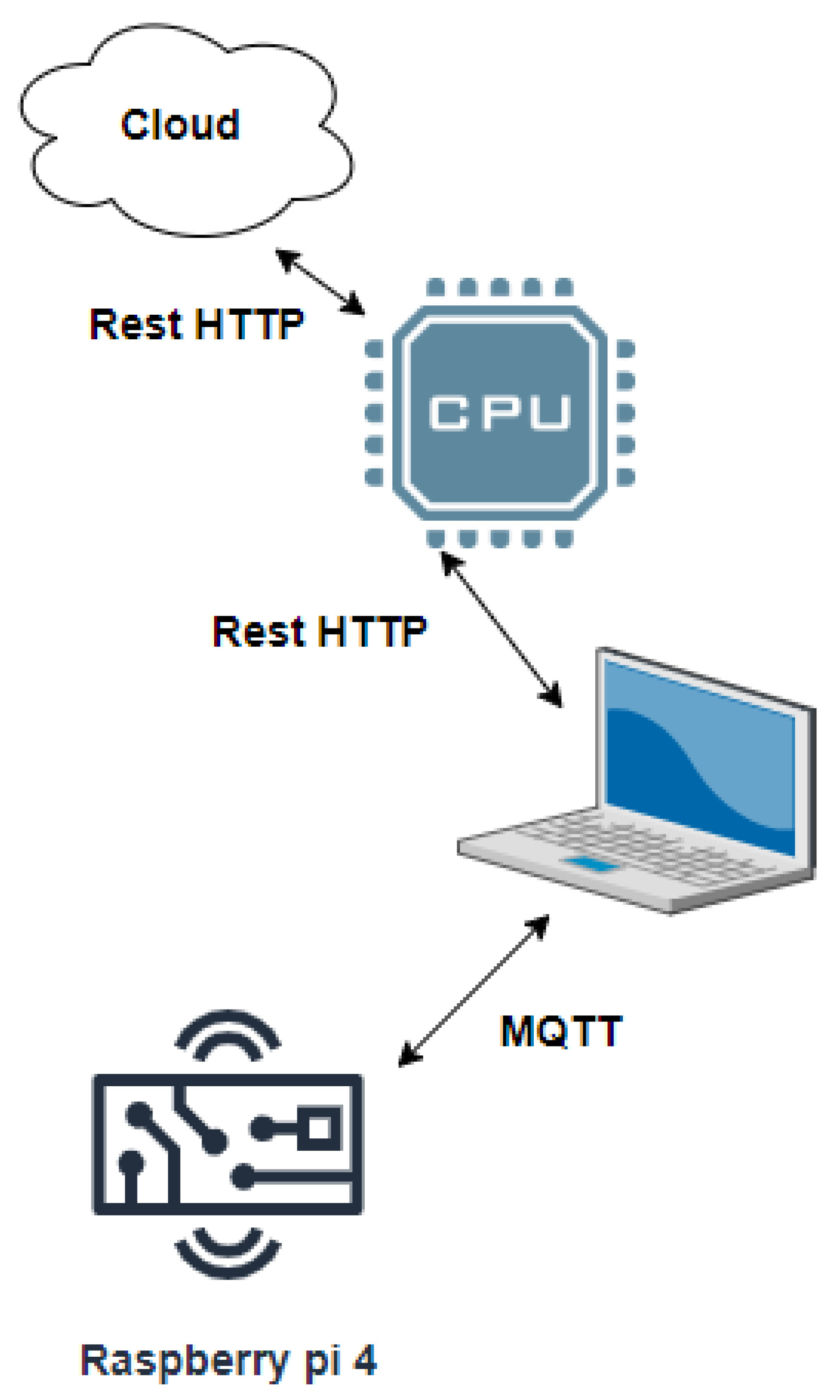
3.7. Data Processing Using IoT- Cloud
4. Results
4.1. Dataset Description
4.1.1. Dataset 1
4.1.2. Dataset 2
4.2. Evaluation Parameters
4.3. Selection of Segment Size
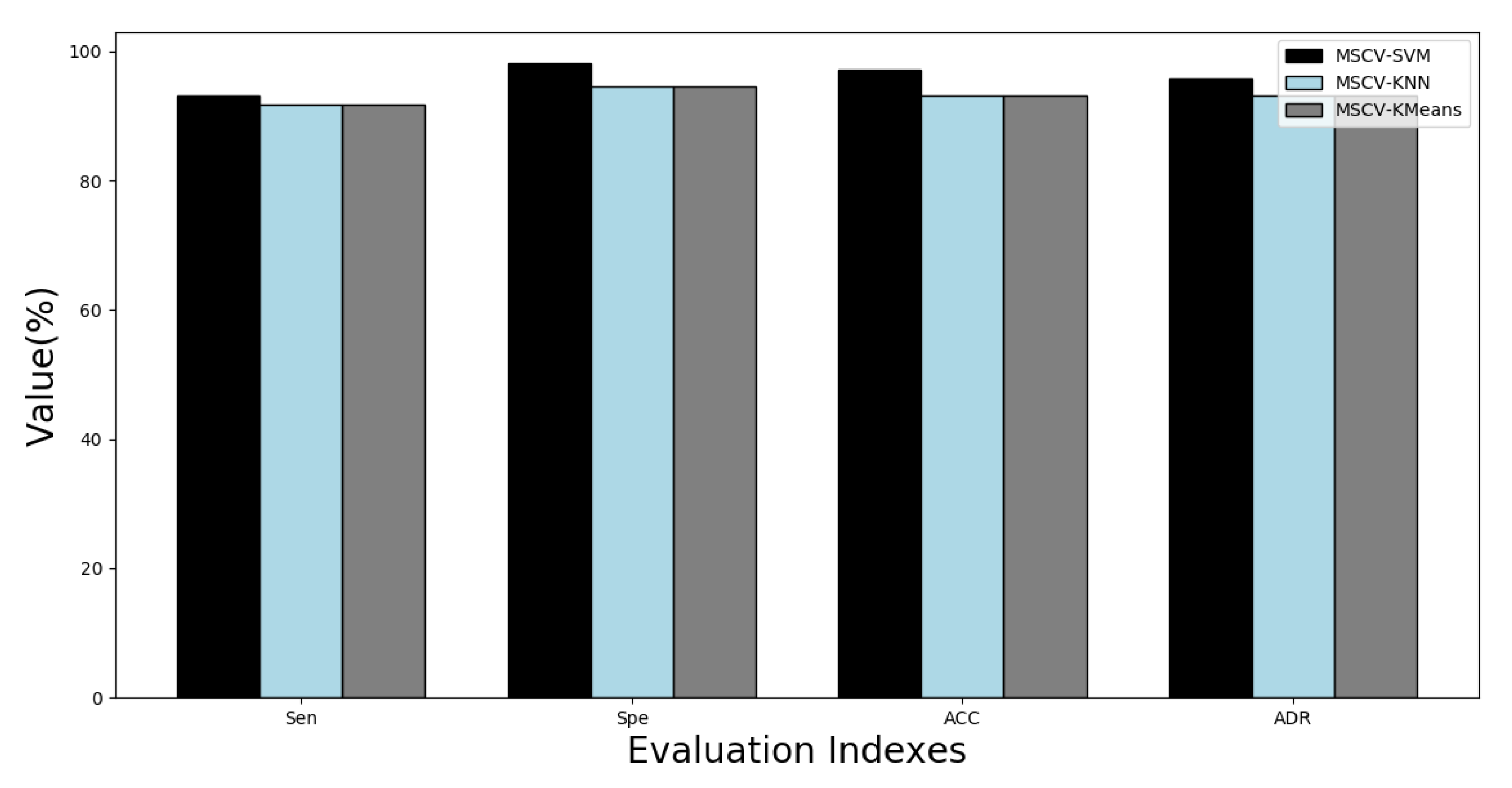
4.4. Seizure Detection Using the Proposed MCSV Approach
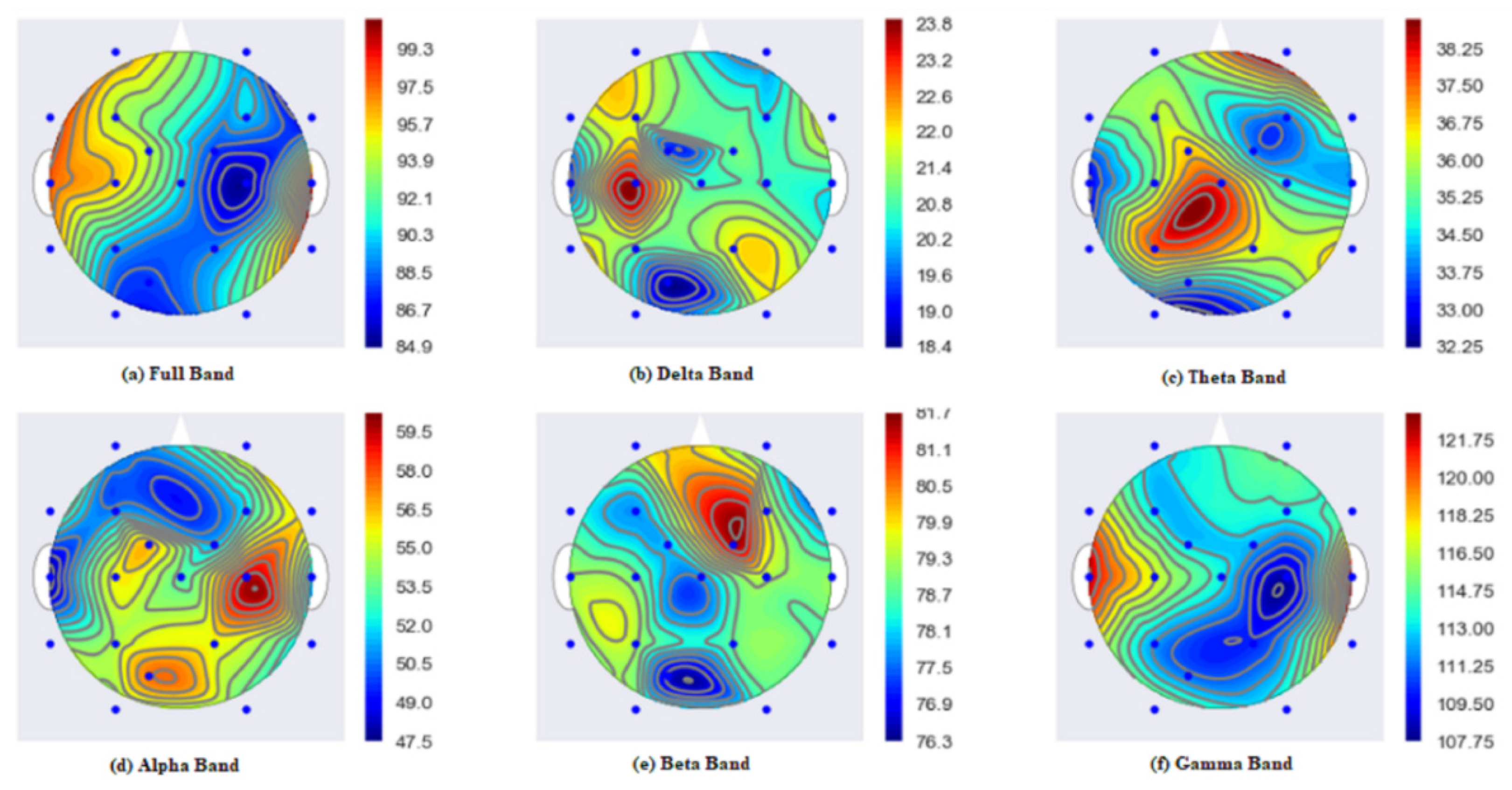
4.5. Localization of the Brain Focal Region of the Seizure Activity
5. Discussion
6. Comparative Performance Analysis of the Proposed MCSV Approach
6.1. Single Channel vs. Multichannel
6.2. Comparison of the Proposed MCSV Approach with Existing Approach
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Epilepsy. Available online: http://www.who.int/mental_health/neurology/epilepsy/en/index.html (accessed on 28 September 2022).
- Fisher, R.S.; EmdeBoas, W.V.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J. Epileptic seizures and epilepsy: Definitions proposed by the international league against epilepsy (ILAE) and the international bureau for epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, H.; Ersoy, C. Wireless sensor networks for healthcare: A survey. Comput. Netw. 2014, 54, 2688–2710. [Google Scholar] [CrossRef]
- Osorio, I.; Frei, M.G. Real-time detection, quantification, warning, and control of epileptic seizures: The foundations for a scientific epileptology. Epilepsy Behav. 2009, 16, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Andrzejak, R.G.; Lehnertz, K.; Mormann, F.; Rieke, C.; David, P.; Elger, C.E. Indications of nonlinear deterministic and finite-dimensional structures in time series of brain electrical activity: Dependence on recording region and brain state. Phys. Rev. E 2001, 64, 061907. [Google Scholar] [CrossRef] [PubMed]
- Adeli, H.; Zhou, Z.; Dadmehr, N. Analysis of EEG records in an epileptic patient using wavelet transform. J. Neurosci. Methods 2003, 123, 69–87. [Google Scholar] [CrossRef]
- Musselman, M.; Djurdjanovic, D. Time-frequency distributions in the classification of epilepsy from EEG signals. Expert Syst. Appl. 2012, 39, 11413–11422. [Google Scholar] [CrossRef]
- Hassanpour, H.; Mesbah, M.; Boashash, B. Time-frequency based newborn EEG seizure detection using low and high frequency signatures. Physiol. Meas. 2004, 25, 935–944. [Google Scholar] [CrossRef]
- Jones, D.L.; Baraniuk, R.G. An adaptive optimal-kernel time-frequency representation. IEEE Trans. Signal Process. 1995, 43, 2361–2371. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhou, W.; Liu, Y.; Wang, J. Epileptic seizure detection with linear and nonlinear features. Epilepsy Behav. 2012, 24, 415–421. [Google Scholar] [CrossRef]
- Adeli, H.; Ghosh-Dastidar, S.; Dadmehr, N. A wavelet-chaos methodology for analysis of EEGs and EEG subbands to detect seizure and epilepsy. IEEE Trans. Biomed. Eng. 2007, 54, 205–211. [Google Scholar] [CrossRef]
- Subasi, A.; Gursoy, I. EEG signal classification using PCA, ICA, LDA and support vector machines. Expert Syst. Appl. 2010, 37, 8659–8666. [Google Scholar] [CrossRef]
- Sharma, R.; Pachori, R.B. Classification of epileptic seizures in EEG signals based on phase space representation of intrinsic mode functions. Expert Syst. Appl. 2015, 42, 1106–1117. [Google Scholar] [CrossRef]
- Doyle, W.D. Magnetization reversal in films with biaxial anisotropy. IEEE Trans. Magn. 1966, 2, 68–73. [Google Scholar] [CrossRef]
- Dastidar, S.G.; Adeli, H.; Dadmehr, N. Principle component analysis-enhanced cosine radial basis function neural network for robust epilepsy and seizure detection. IEEE Trans. Biomed. Eng. 2008, 55, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Bower, M.R.; Stead, M.; Meyer, F.B.; Marsh, W.R.; Worrell, G.A. Spatiotemporal neuronal correlates of seizure generation in focal epilepsy. Epilepsia 2012, 53, 807–816. [Google Scholar] [CrossRef]
- Acharya, U.R.; Sree, S.V.; Swapna, G.; Martis, R.J.; Suri, J.S. Automated EEG analysis of epilepsy: A review. Knowl. Based Syst. 2013, 45, 147–165. [Google Scholar] [CrossRef]
- Aldana, Y.R.; Hunyadi, B.; Reyes, E.J.; Rodriguez, V.R.; Van, H.S. Nonconvulsive epileptic seizure detection in scalp EEG using multiway data analysis. IEEE J. Biomed. Health Inform. 2018, 23, 660–671. [Google Scholar] [CrossRef]
- Tanaka, T.; Saito, Y. Rhythmic component extraction for multichannel EEG data analysis. In Proceedings of the IEEE International Conference on Acoustics, Speech and Signal Processing, Las Vegas, NV, USA, 31 March–4 April 2008; pp. 425–428. [Google Scholar]
- Peters, B.; Pfurtscheller, G.; Flyvbjerg, H. Automatic differentiation of multichannel EEG signals. IEEE Trans. Biomed. Eng. 2001, 48, 111–116. [Google Scholar] [CrossRef]
- Kiranyazet, S.; Ince, T.; Zabihi, M.; Ince, D. Automated patient-specific classification of long-term Electroencephalography. J. Biomed. Inform. 2014, 49, 16–31. [Google Scholar] [CrossRef]
- Dong, W.; Ren, D.; Li, K.; Feng, Y.; Ma, D.; Yan, X.; Wang, G. Epileptic seizure detection in long-term EEG recordings by using wavelet-based directed transfer function. IEEE Trans. Biomed. Eng. 2018, 65, 2591–2599. [Google Scholar]
- Roy, S.; Kiral-Kornek, I.; Mirmomeni, M.; Mummert, T.; Braz, A.; Tsai, J.; Harrer, S. Evaluation of combined artificial intelligence and neurologist assessment to annotate scalp electroencephalography data. EBioMedicine 2021, 66, 103275. [Google Scholar] [CrossRef]
- Alyasseri, Z.A.; Khader, A.T.; Al-Betar, M.A.; Papa, J.P.; Alomari, O.A. EEG Feature Extraction for Person Identification Using Wavelet Decomposition and Multi-Objective Flower Pollination Algorithm. IEEE Access Spec. Sect. New Trends Brain Signal Process. Anal. 2018, 6, 76007–76024. [Google Scholar] [CrossRef]
- Forkan, A.; Khalil, I.; Tari, Z. CoCaMAAL: A cloud-oriented context-aware middleware in ambient assisted living. Future Gener. Comput. Syst. 2014, 35, 114–127. [Google Scholar] [CrossRef]
- Fortino, G.; Parisi, D.; Pirrone, V.; Fatta, D.G. BodyCloud: A SaaS approach for community body sensor networks. Future Gener. Comput. Syst. 2014, 35, 62–79. [Google Scholar] [CrossRef]
- Quwaider, M.; Jararweh, Y. Cloudlet-based efficient data collection in wireless body area networks. Simul. Model. Pract. Theory 2015, 50, 57–71. [Google Scholar] [CrossRef]
- Lounis, A.; Hadjidj, A.; Bouabdallah, A.; Challal, Y. Healing on the cloud: Secure cloud architecture for medical wireless sensor networks. Future Gener. Comput. Syst. 2016, 55, 266–277. [Google Scholar] [CrossRef]
- Yedurkar, D.P.; Metkar, S.P. Multiresolution approach for artifacts removal and localization of seizure onset zone in epileptic EEG signal. Biomed. Signal Process. Control. 2020, 57, 101794. [Google Scholar] [CrossRef]
- Schalk, G.; McFarland, D.J.; Hinterberger, T.; Birbaumer, N.; Wolpaw, J.R. BCI2000: A general-purpose brain-computer interface (BCI) system. IEEE Trans. Biomed. Eng. 2004, 51, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Supriya, S.; Siuly, S.; Wang, H.; Cao, J.; Zhang, Y. Weighted visibility graph with complex network features in the detection of epilepsy. IEEE Access 2016, 4, 6554–6566. [Google Scholar] [CrossRef]
- Harati, A.; Choi, S.; Tabrizi, M.; Obeid, I.; Picone, J.; Jacobson, M.P. The Temple University Hospital EEG Corpus. In Proceedings of the IEEE Global Conference on Signal and Information Processing, Austin, TX, USA, 3–5 December 2013; Available online: www.nedcdata.org (accessed on 28 September 2022).
- Ko, C.W.; Chung, H.W. Automatic spike detection via an artificial neural network using raw EEG data: Effects of data preparation and implications in the limitations of online recognition. Clin. Neurophysiol. 2000, 111, 477–481. [Google Scholar] [CrossRef]
- Miroslaw, L.; Was, Z.; Kozik, A.; West, B.J. Wavelet analysis of epileptic spikes. Phys. Rev. E 2003, 67, 052902. [Google Scholar]
- Sriraam, N.; Raghu, S.; Tamanna, K.; Narayan, L.; Khanum, M.; Hegde, A.; Kumar, A.B. Automated epileptic seizures detection using multi-features and multilayer perceptron neural network. Brain Inform. 2018, 5, 1–10. [Google Scholar] [CrossRef]
- Bashashati, A.; Fatourechi, M.; Ward, R.K.; Birch, G.E. A survey of signal processing algorithms in brain–computer interfaces based on electrical brain signals. J. Neural Eng. 2007, 4, R32–R57. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, P.; Karlekar, M. A Novel Signal Modeling Approach for Classification of Seizure and Seizure-free EEG Signals. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1–11. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Z.; Chen, Y.; Mahara, G.; Huang, J.; Lin, Z.; Zhang, J. Automatic epileptic seizure classification in multichannel EEG time series with linear discriminant analysis. Technol. Health Care 2020, 28, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Oh, S.L.; Hagiwara, Y.; Tan, J.H.; Adeli, H. Deep convolutional neural network for the automated detection and diagnosis of seizure using EEG signals. Comput. Biol. Med. 2018, 100, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Golmohammadi, M.; Obeid, I.; Picone, J. Deep residual learning for automatic seizure detection. Seizure Hrs. 2018, 21, 16. [Google Scholar]
- Wang, Z.; Wu, D.; Dong, F.; Cao, J.; Jiang, T.; Liu, J. A novel spike detection algorithm based on multi-channel of bect eeg signals. IEEE Trans. Circuits Syst. II Express Briefs 2020, 67, 3592–3596. [Google Scholar] [CrossRef]
- Cura, O.; Akan, A. Analysis of epileptic EEG signals by using dynamic mode decomposition and spectrum. Biocybern. Biomed. Eng. 2021, 41, 1–17. [Google Scholar] [CrossRef]
- Yao, X.; Li, X.; Ye, Q.; Huang, Y.; Cheng, Q.; Zhang, G.Q. A robust deep learning approach for automatic classification of seizures against non-seizures. Biomed. Signal Process. Control. 2021, 64, 2215. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.D.; Luo, M.L.; Li, K.; Yang, X.F.; Guo, Q. Epileptic seizure classification of EEGs using time–frequency analysis based multiscale radial basis functions. IEEE J. Biomed. Health Inform. 2017, 22, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Li, Y.; En, P.P. Epileptic seizure detection in EEGs signals using a fast weighted horizontal visibility algorithm. Comput. Methods Programs Biomed. 2014, 115, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.U.; Yusoff, M.Z.; Ahmad, R.F. A novel approach based on wavelet analysis and arithmetic coding for automated detection and diagnosis of epileptic seizure in EEG signals using machine learning techniques. Biomed. Signal Process. Control. 2020, 56, 101707. [Google Scholar] [CrossRef]
| Subject Index | Dataset | Total No. of Samples | Sampling Frequency (Hz) | No. of Seizures | Start of Seizure (s) | End of Seizure (s) |
|---|---|---|---|---|---|---|
| 1 | TUH | 79,200 | 400 | 2 | 5.15 | 37.207 |
| 2 | TUH | 135,600 | 400 | 2 | 10.27 | 142.98 |
| 3 | TUH | 357,000 | 250 | 5 | 83.94 | 1271.23 |
| 4 | TUH | 400,250 | 250 | 10 | 20.63 | 1521.94 |
| 5 | TUH | 109,000 | 250 | 4 | 162.1 | 580.90 |
| 6 | TUH | 518,500 | 250 | 3 | 63.85 | 1355 |
| 7 | TUH | 373,500 | 250 | 17 | 57.86 | 1493 |
| 8 | TUH | 302,750 | 250 | 7 | 1 | 1112.276 |
| 9 | TUH | 96,250 | 250 | 4 | 52.54 | 374.48 |
| 10 | TUH | 269,250 | 250 | 16 | 1 | 678.93 |
| 11 | Local | 14,547,500 | 250 | 2 | 12.63 | 62.14 |
| 12 | Local | 10,412,470 | 250 | 4 | 46.8 | 192.12 |
| 13 | Local | 192,860 | 250 | 1 | 71.25 | 118.33 |
| Channel | MCSV-SVM Method | |||
|---|---|---|---|---|
| Average Values | ||||
| SEN (%) | SPE (%) | ACC (%) | ADR (%) | |
| Single | 92.8 | 98.13 | 96.53 | 95.46 |
| Multi | 93.83 | 97.94 | 97.38 | 95.89 |
| Authors with Reference Number | Channel/Segment (in Seconds, s) | Technique Used | Localization of Seizure Origination | SEN (%) | SPE (%) | ACC (%) | ADR (%) |
|---|---|---|---|---|---|---|---|
| Sriram et al. [36] | Multi/NS | Multifeatures and multi layer perceptron | NO | 97.1 | 97.8 | NS | NS |
| Anubha Gupta et al. [37] | Single/NS | Hurst component and autoregressive moving average based features are used | NO | NS | NS | 97 | NS |
| M.G.Moha mmadi et al. [26] | Multi/NS | CNN-MLP/LSTM/ ResNet | NO | 76.84/ 94.24 | NS | NS | NS |
| Acharya, U. R. et al. [38] | NS | 13 layer CNN | NO | 95 | 90 | 88.67 | NS |
| Gao et al. [39] | Multi/NS | Maximal overlap DWT | NO | NS | NS | 94.12 | NS |
| M.G.Moha mmadi et al. [40] | Multi/0.9 s | HMM-Deep learning | NO | Above 90 | 95 | NS | NS |
| Zimeng et al. [41] | Multi/NS | Multi-step spike detection algorithm | YES | 97.4 | 96.5 | 96.9 | NS |
| Cura et al. [42] | Multi/NS | DMD-spectral moments | NO | 92.5 | 98.6 | 96.5 | NS |
| Yao et al. [43] | Multi/NS | BiLSTM with CNN | NO | 87.3 | 88.3 | NS | NS |
| S.Roy et al. [27] | Multi/NS | Deep Learning | NO | 75 to 91.6 | NS | NS | NS |
| The proposed work | Multi/4 s | CSV based MCSV-SVM approach | YES | 93.83 | 97.94 | 97.38 | 95.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yedurkar, D.P.; Metkar, S.P.; Al-Turjman, F.; Stephan, T.; Kolhar, M.; Altrjman, C. A Novel Approach for Multichannel Epileptic Seizure Classification Based on Internet of Things Framework Using Critical Spectral Verge Feature Derived from Flower Pollination Algorithm. Sensors 2022, 22, 9302. https://doi.org/10.3390/s22239302
Yedurkar DP, Metkar SP, Al-Turjman F, Stephan T, Kolhar M, Altrjman C. A Novel Approach for Multichannel Epileptic Seizure Classification Based on Internet of Things Framework Using Critical Spectral Verge Feature Derived from Flower Pollination Algorithm. Sensors. 2022; 22(23):9302. https://doi.org/10.3390/s22239302
Chicago/Turabian StyleYedurkar, Dhanalekshmi Prasad, Shilpa P. Metkar, Fadi Al-Turjman, Thompson Stephan, Manjur Kolhar, and Chadi Altrjman. 2022. "A Novel Approach for Multichannel Epileptic Seizure Classification Based on Internet of Things Framework Using Critical Spectral Verge Feature Derived from Flower Pollination Algorithm" Sensors 22, no. 23: 9302. https://doi.org/10.3390/s22239302
APA StyleYedurkar, D. P., Metkar, S. P., Al-Turjman, F., Stephan, T., Kolhar, M., & Altrjman, C. (2022). A Novel Approach for Multichannel Epileptic Seizure Classification Based on Internet of Things Framework Using Critical Spectral Verge Feature Derived from Flower Pollination Algorithm. Sensors, 22(23), 9302. https://doi.org/10.3390/s22239302








