Digital Biomarkers of Gait and Balance in Diabetic Foot, Measurable by Wearable Inertial Measurement Units: A Mini Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Search Results
3.2. Study Characteristics
3.3. Study Design and Participant Characteristics
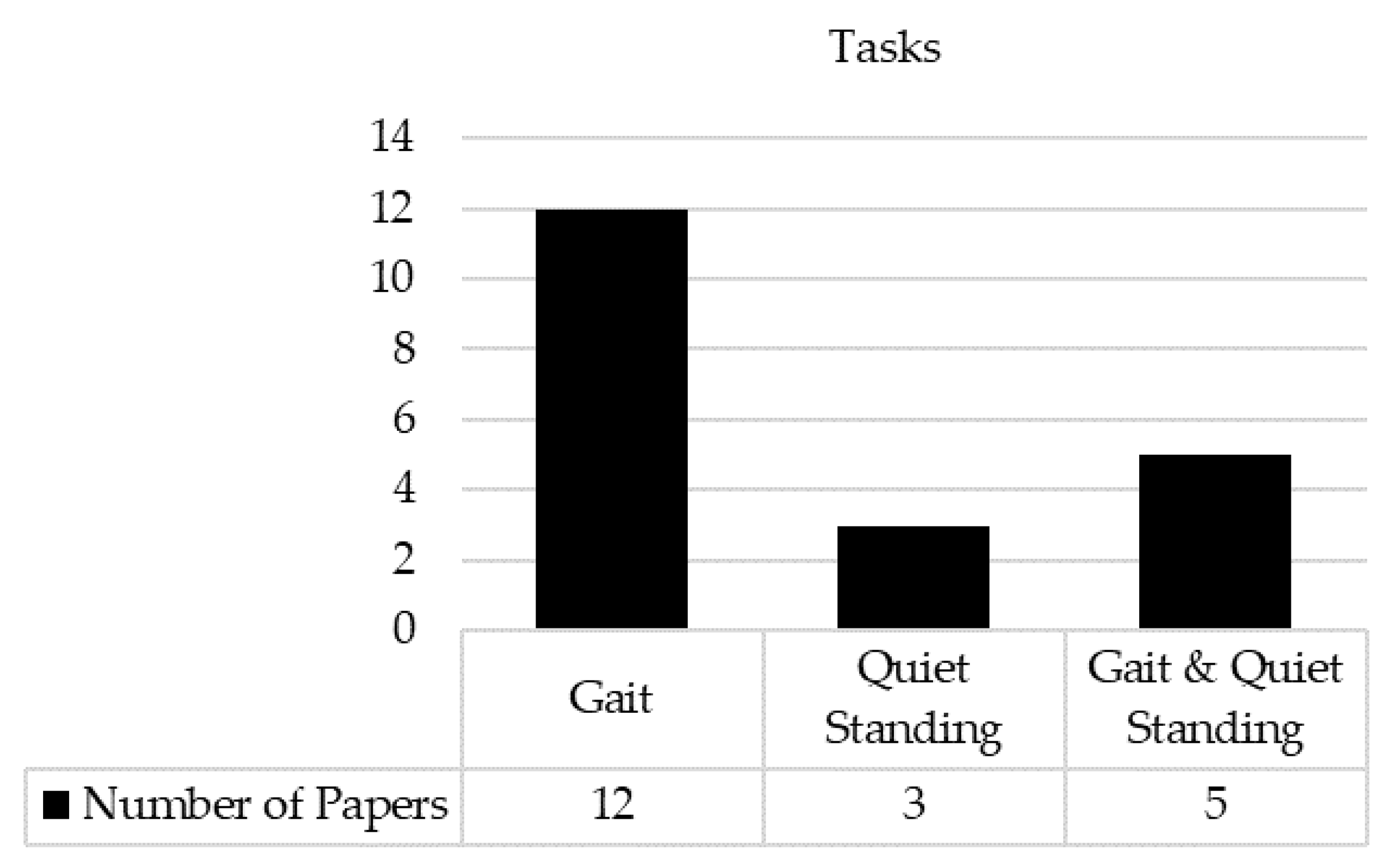
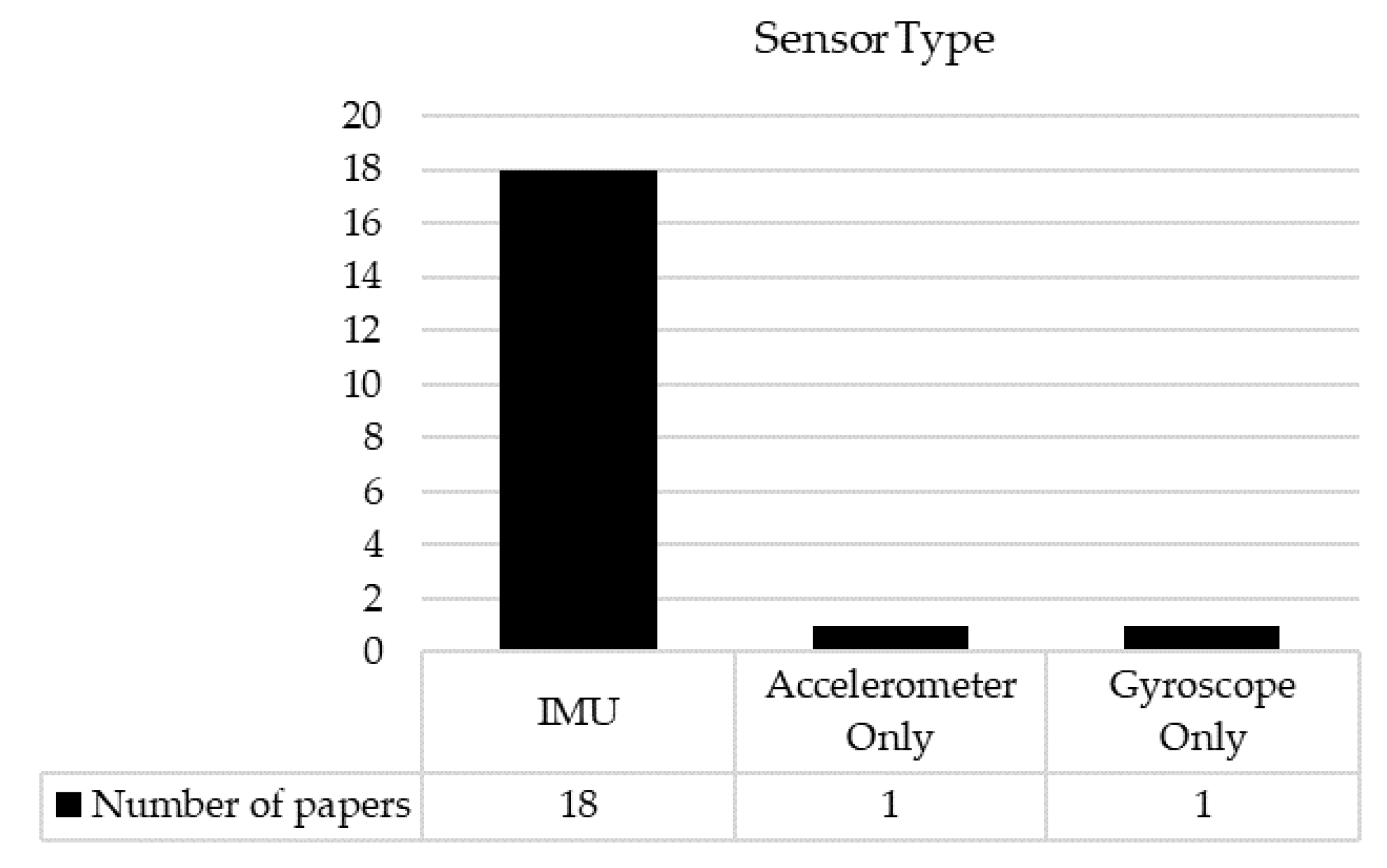
3.4. Tasks and IMUs
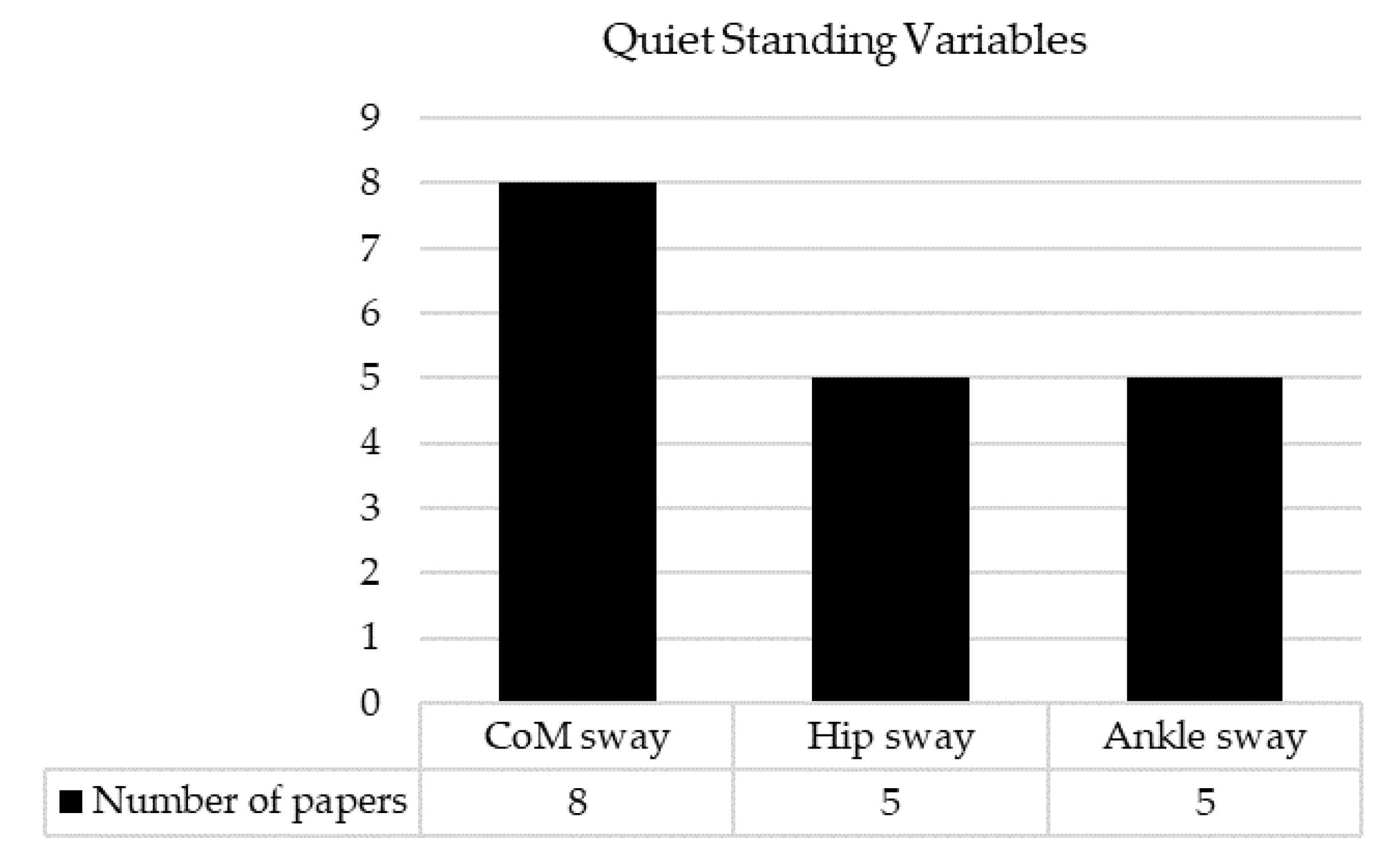
3.5. Measures and Key Findings
4. Discussion
4.1. Summary
4.2. Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herman, W.H. The global burden of diabetes: An overview. In Diabetes Mellitus in Developing Countries and Underserved Communities; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–5. [Google Scholar]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. Economic costs of diabetes in the US in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- van Netten, J.J.; Bus, S.A.; Apelqvist, J.; Lipsky, B.A.; Hinchliffe, R.J.; Game, F.; Rayman, G.; Lazzarini, P.A.; Forsythe, R.O.; Peters, E.J. Definitions and criteria for diabetic foot disease. Diabetes/Metab. Res. Rev. 2020, 36, e3268. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Boulton, A.J.; Bus, S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.; Armstrong, D.G.; Kirsner, R.S.; Attinger, C.E.; Lavery, L.A.; Lipsky, B.A.; Mills, J.L.; Steinberg, J.S. Diagnosis and management of diabetic foot complications. Compendia 2018, 2018. [Google Scholar] [CrossRef]
- Bakker, K.; Schaper, N.C.; on behalf of the International Working Group on the Diabetic Foot Editorial Board. The development of global consensus guidelines on the management and prevention of the diabetic foot 2011. Diabetes/Metab. Res. Rev. 2012, 28, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Geiss, L.S.; Li, Y.; Hora, I.; Albright, A.; Rolka, D.; Gregg, E.W. Resurgence of diabetes-related nontraumatic lower-extremity amputation in the young and middle-aged adult US population. Diabetes Care 2019, 42, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Wukich, D.K.; Raspovic, K.M.; Suder, N.C. Patients with diabetic foot disease fear major lower-extremity amputation more than death. Foot Ankle Spec. 2018, 11, 17–21. [Google Scholar] [CrossRef]
- Lavery, L.A.; Grigoropoulos, K.; Killeen, A.L.; La Fontaine, J. Elective Surgery in the Diabetic Foot to Heal Foot Ulcerations and Prevent Re-ulceration. In Diabetic Foot Reconstruction; Springer: Berlin/Heidelberg, Germany, 2022; pp. 53–76. [Google Scholar]
- Wrobel, J.S.; Najafi, B. Diabetic foot biomechanics and gait dysfunction. J. Diabetes Sci. Technol. 2010, 4, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Alam, U.; Riley, D.R.; Jugdey, R.S.; Azmi, S.; Rajbhandari, S.; D’Août, K.; Malik, R.A. Diabetic neuropathy and gait: A review. Diabetes Ther. 2017, 8, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, P.; Derr, J.; Ulbrecht, J.; Maser, R.; Orchard, T. Problems with gait and posture in neuropathic patients with insulin—Dependent diabetes mellitus. Diabet. Med. 1992, 9, 469–474. [Google Scholar] [CrossRef]
- Allet, L.; Armand, S.; De Bie, R.; Golay, A.; Pataky, Z.; Aminian, K.; De Bruin, E.D. Clinical factors associated with gait alterations in diabetic patients. Diabet. Med. 2009, 26, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.E.; Najafi, B. Sensor-based daily physical activity: Towards prediction of the level of concern about falling in peripheral neuropathy. Sensors 2020, 20, 505. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.E.; Murphy, T.K.; Kunik, M.E.; Badr, H.J.; Workeneh, B.T.; Yellapragada, S.V.; Sada, Y.H.; Najafi, B. The detrimental association between fear of falling and motor performance in older cancer patients with chemotherapy-induced peripheral neuropathy. Gait Posture 2021, 88, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sawacha, Z.; Guarneri, G.; Cristoferi, G.; Guiotto, A.; Avogaro, A.; Cobelli, C. Integrated kinematics-kinetics-plantar pressure data analysis: A useful tool for characterizing diabetic foot biomechanics. Gait Posture 2012, 36, 20–26. [Google Scholar] [CrossRef]
- Lazzarini, P.A.; Crews, R.T.; van Netten, J.J.; Bus, S.A.; Fernando, M.E.; Chadwick, P.J.; Najafi, B. Measuring plantar tissue stress in people with diabetic peripheral neuropathy: A critical concept in diabetic foot management. J. Diabetes Sci. Technol. 2019, 13, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Shull, P.B.; Jirattigalachote, W.; Hunt, M.A.; Cutkosky, M.R.; Delp, S.L. Quantified self and human movement: A review on the clinical impact of wearable sensing and feedback for gait analysis and intervention. Gait Posture 2014, 40, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Myers, M. Qualitative research and the generalizability question: Standing firm with Proteus. Qual. Rep. 2000, 4, 9. [Google Scholar] [CrossRef]
- Gordt, K.; Gerhardy, T.; Najafi, B.; Schwenk, M. Effects of wearable sensor-based balance and gait training on balance, gait, and functional performance in healthy and patient populations: A systematic review and meta-analysis of randomized controlled trials. Gerontology 2018, 64, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Hubble, R.P.; Naughton, G.A.; Silburn, P.A.; Cole, M.H. Wearable sensor use for assessing standing balance and walking stability in people with Parkinson’s disease: A systematic review. PLoS ONE 2015, 10, e0123705. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, M.; Mohler, J.; Wendel, C.; Fain, M.; Taylor-Piliae, R.; Najafi, B. Wearable sensor-based in-home assessment of gait, balance, and physical activity for discrimination of frailty status: Baseline results of the Arizona frailty cohort study. Gerontology 2015, 61, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Najafi, B.; Reeves, N.D.; Armstrong, D.G. Leveraging smart technologies to improve the management of diabetic foot ulcers and extend ulcer-free days in remission. Diabetes/Metab. Res. Rev. 2020, 36, e3239. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.E.; Patriquin, M.A.; Nguyen, H.; Oh, H.; Rufino, K.A.; Storch, E.A.; Schanzer, B.; Mathew, S.J.; Salas, R.; Najafi, B. Objective measurement of sleep, heart rate, heart rate variability, and physical activity in suicidality: A systematic review. J. Affect. Disord. 2020, 273, 318–327. [Google Scholar] [CrossRef]
- Kang, G.E.; Frederick, R.; Nunley, B.; Lavery, L.; Dhaher, Y.; Najafi, B.; Cogan, S. The effect of implanted functional electrical stimulation on gait performance in stroke survivors: A systematic review. Sensors 2021, 21, 8323. [Google Scholar] [CrossRef]
- Menz, H.B.; Lord, S.R.; St George, R.; Fitzpatrick, R.C. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch. Phys. Med. Rehabil. 2004, 85, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Allet, L.; Armand, S.; de Bie, R.A.; Pataky, Z.; Aminian, K.; Herrmann, F.R.; de Bruin, E.D. Gait alterations of diabetic patients while walking on different surfaces. Gait Posture 2009, 29, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Allet, L.; Armand, S.; Aminian, K.; Pataky, Z.; Golay, A.; De Bie, R.; de Bruin, E.D. An exercise intervention to improve diabetic patients’ gait in a real-life environment. Gait Posture 2010, 32, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Crews, R.T.; Sayeed, F.; Najafi, B. Impact of strut height on offloading capacity of removable cast walkers. Clin. Biomech. 2012, 27, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Najafi, B.; Khan, T.; Fleischer, A.; Wrobel, J. The impact of footwear and walking distance on gait stability in diabetic patients with peripheral neuropathy. J. Am. Podiatr. Med. Assoc. 2013, 103, 165–173. [Google Scholar] [PubMed]
- Najafi, B.; Crews, R.T.; Wrobel, J.S. A novel plantar stimulation technology for improving protective sensation and postural control in patients with diabetic peripheral neuropathy: A double-blinded, randomized study. Gerontology 2013, 59, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Grewal, G.S.; Bharara, M.; Menzies, R.; Talal, T.K.; Armstrong, D.; Najafi, B. Diabetic peripheral neuropathy and gait: Does footwear modify this association? J. Diabetes Sci. Technol. 2013, 7, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; Fleischer, A.; Yalla, S.; Grewal, G.S.; Albright, R.; Berns, D.; Crews, R.; Najafi, B. Fear of falling is prevalent in older adults with diabetes mellitus but is unrelated to level of neuropathy. J. Am. Podiatr. Med. Assoc. 2013, 103, 480. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, J.S.; Ammanath, P.; Le, T.; Luring, C.; Wensman, J.; Grewal, G.S.; Najafi, B.; Pop-Busui, R. A novel shear reduction insole effect on the thermal response to walking stress, balance, and gait. J. Diabetes Sci. Technol. 2014, 8, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Grewal, G.S.; Schwenk, M.; Lee-Eng, J.; Parvaneh, S.; Bharara, M.; Menzies, R.A.; Talal, T.K.; Armstrong, D.G.; Najafi, B. Sensor-based interactive balance training with visual joint movement feedback for improving postural stability in diabetics with peripheral neuropathy: A randomized controlled trial. Gerontology 2015, 61, 567–574. [Google Scholar] [CrossRef]
- Toosizadeh, N.; Mohler, J.; Armstrong, D.G.; Talal, T.K.; Najafi, B. The influence of diabetic peripheral neuropathy on local postural muscle and central sensory feedback balance control. PLoS ONE 2015, 10, e0135255. [Google Scholar] [CrossRef] [PubMed]
- Toosizadeh, N.; Stocker, H.; Thiede, R.; Mohler, J.; Mills, J.L.; Najafi, B. Alterations in gait parameters with peripheral artery disease: The importance of pre-frailty as a confounding variable. Vasc. Med. 2016, 21, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Thiede, R.; Toosizadeh, N.; Mills, J.L.; Zaky, M.; Mohler, J.; Najafi, B. Gait and balance assessments as early indicators of frailty in patients with known peripheral artery disease. Clin. Biomech. 2016, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Najafi, B.; Talal, T.K.; Grewal, G.S.; Menzies, R.; Armstrong, D.G.; Lavery, L.A. Using plantar electrical stimulation to improve postural balance and plantar sensation among patients with diabetic peripheral neuropathy: A randomized double blinded study. J. Diabetes Sci. Technol. 2017, 11, 693–701. [Google Scholar] [CrossRef]
- Esser, P.; Collett, J.; Maynard, K.; Steins, D.; Hillier, A.; Buckingham, J.; Tan, G.D.; King, L.; Dawes, H. Single sensor gait analysis to detect diabetic peripheral neuropathy: A proof of principle study. Diabetes Metab. J. 2018, 42, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.E.; Zahiri, M.; Lepow, B.; Saleem, N.; Najafi, B. The effect of daily use of plantar mechanical stimulation through micro-mobile foot compression device installed in shoe insoles on vibration perception, gait, and balance in people with diabetic peripheral neuropathy. J. Diabetes Sci. Technol. 2019, 13, 847–856. [Google Scholar] [CrossRef]
- Kang, G.E.; Zhou, H.; Varghese, V.; Najafi, B. Characteristics of the gait initiation phase in older adults with diabetic peripheral neuropathy compared to control older adults. Clin. Biomech. 2020, 72, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Ling, E.; Lepow, B.; Zhou, H.; Enriquez, A.; Mullen, A.; Najafi, B. The impact of diabetic foot ulcers and unilateral offloading footwear on gait in people with diabetes. Clin. Biomech. 2020, 73, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Wang, H.; Chen, H.; Fan, X.; Liu, D.; Du, D.; Wu, M.; Wang, G.; Boey, J.; Armstrong, D.G. The feasibility and effectiveness of wearable sensor technology in the management of elderly diabetics with foot ulcer remission: A proof-of-concept pilot study with six cases. Gerontology 2021, 67, 493–502. [Google Scholar] [CrossRef]
- Lanzi, S.; Boichat, J.; Calanca, L.; Mazzolai, L.; Malatesta, D. Supervised Exercise Training Improves 6 min Walking Distance and Modifies Gait Pattern during Pain-Free Walking Condition in Patients with Symptomatic Lower Extremity Peripheral Artery Disease. Sensors 2021, 21, 7989. [Google Scholar] [CrossRef]
- Kirby, K. Biomechanics of the normal and abnormal foot. J. Am. Podiatr. Med. Assoc. 2000, 90, 30–34. [Google Scholar] [CrossRef]
- Van Schie, C.M. A review of the biomechanics of the diabetic foot. Int. J. Low. Extrem. Wounds 2005, 4, 160–170. [Google Scholar] [CrossRef]
- Khan, K.S.; Andersen, H. The impact of diabetic neuropathy on activities of daily living, postural balance and risk of falls—A systematic review. J. Diabetes Sci. Technol. 2022, 16, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Yau, R.K.; Strotmeyer, E.S.; Resnick, H.E.; Sellmeyer, D.E.; Feingold, K.R.; Cauley, J.A.; Vittinghoff, E.; De Rekeneire, N.; Harris, T.B.; Nevitt, M.C. Diabetes and risk of hospitalized fall injury among older adults. Diabetes Care 2013, 36, 3985–3991. [Google Scholar] [CrossRef]
- Lavery, L.A.; Armstrong, D.G.; Wunderlich, R.P.; Tredwell, J.; Boulton, A.J. Predictive value of foot pressure assessment as part of a population-based diabetes disease management program. Diabetes Care 2003, 26, 1069–1073. [Google Scholar] [CrossRef]
- Black, F.O.; Wall, C., III; Rockette, H.E., Jr.; Kitch, R. Normal subject postural sway during the Romberg test. Am. J. Otolaryngol. 1982, 3, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Washabaugh, E.P.; Kalyanaraman, T.; Adamczyk, P.G.; Claflin, E.S.; Krishnan, C. Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 2017, 55, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Felius, R.; Geerars, M.; Bruijn, S.; Wouda, N.; Van Diee, J.; Punt, M. Reliability of IMU-Based Balance Assessment in Clinical Stroke Rehabilitation. Gait Posture 2022, 98, 62–68. [Google Scholar] [CrossRef]
- Lavery, L.A.; Armstrong, D.G.; Wunderlich, R.P.; Mohler, M.J.; Wendel, C.S.; Lipsky, B.A. Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006, 29, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- La Fontaine, J.; Lavery, L.; Jude, E. Current concepts of Charcot foot in diabetic patients. Foot 2016, 26, 7–14. [Google Scholar] [CrossRef]
- Petersen, B.J.; Bus, S.A.; Rothenberg, G.M.; Linders, D.R.; Lavery, L.A.; Armstrong, D.G. Recurrence rates suggest delayed identification of plantar ulceration for patients in diabetic foot remission. BMJ Open Diabetes Res. Care 2020, 8, e001697. [Google Scholar] [CrossRef]
- Bus, S.A.; van Netten, J.J.; Monteiro-Soares, M.; Lipsky, B.A.; Schaper, N.C. Diabetic foot disease:“The Times They are A Changin’”. Diabetes/Metab. Res. Rev. 2020, 36, e3249. [Google Scholar] [CrossRef]
- Jayaram, P.; Kang, G.E.; Heldt, B.L.; Sokunbi, O.; Song, B.; Yeh, P.C.; Epstein, M.; Shybut, T.B.; Lee, B.H.; Najafi, B. Novel assessment of leukocyte-rich platelet-rich plasma on functional and patient-reported outcomes in knee osteoarthritis: A pilot study. Regen. Med. 2021, 16, 823–832. [Google Scholar] [CrossRef]
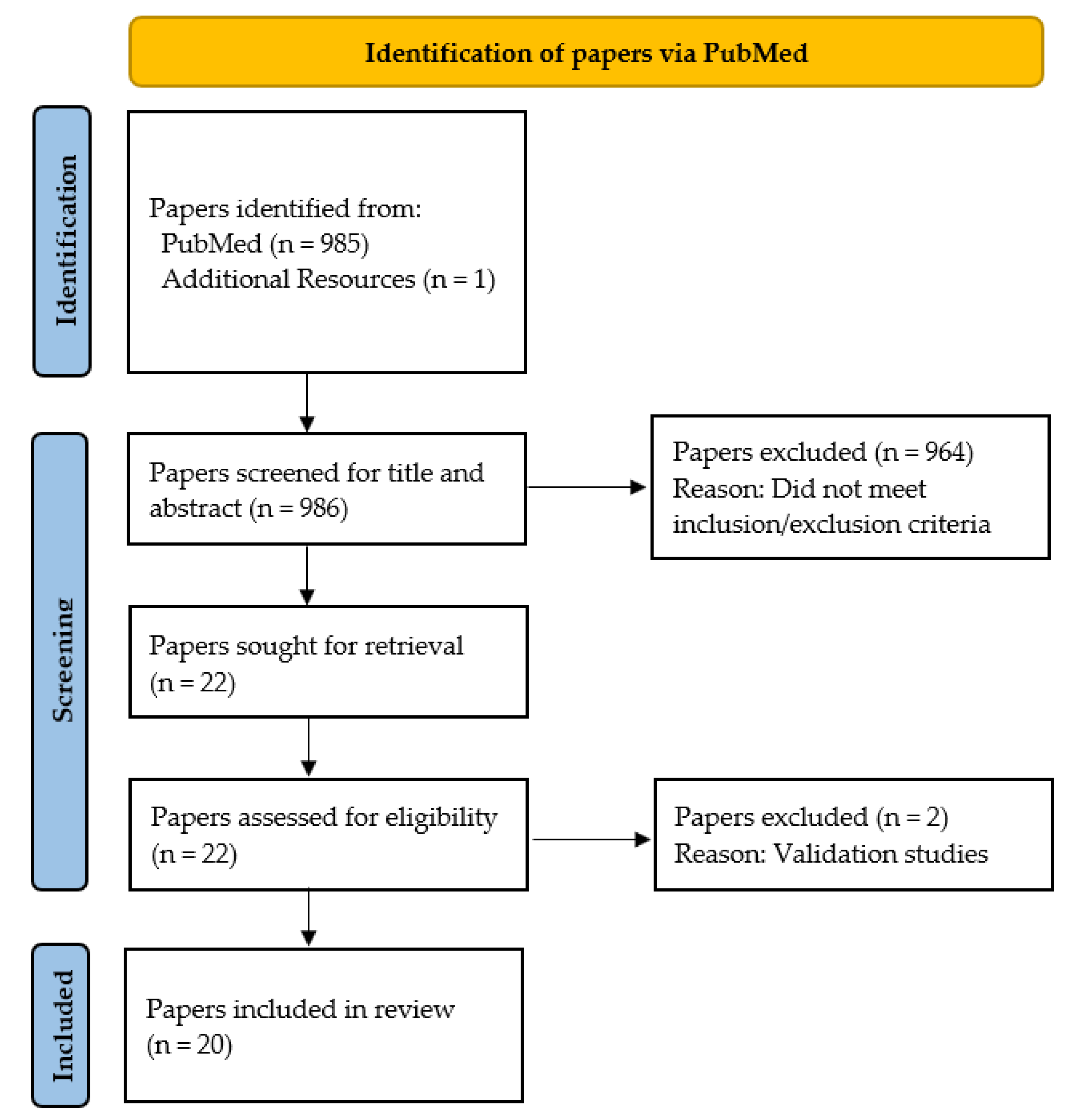

| Concept | Search Query | |
|---|---|---|
| Diabetic Foot | “Diabetic Neuropathies” [MeSH Terms] OR “Diabetic Foot” [MeSH Terms] OR “Foot Ulcer” [MeSH Terms] OR “Peripheral Arterial Disease” [MeSH Terms]) | |
| AND | Gait and Balance | “Gait” [MeSH Terms] OR “Walking” [MeSH Terms] OR “Postural Balance” [MeSH Terms]) |
| Study Title Country | Study Design Participants | Tasks | Sensor Type (Manufacturer) Placement Sampling Frequency | Key Measures | Findings |
|---|---|---|---|---|---|
| Menz et al., 2004 [30] Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy Australia | Observational DPN n = 30 (22 men; 8 women) Age (years) = 73.5 ± 8.3 BMI (kg/m2) = 28.2 ± 6.0 Duration of DM (years) = 12.3 ± 8.4 HbA1c (%) = 7.6 ± 1.3 VPT (Volts) = 37.6 ± 11.4 HC n = 30 (22 men; 8 women) Age (years) = 73.9 ± 9.0 BMI (kg/m2) = 25.6 ± 3.4 | Gait 20 m Two surface conditions
| 3D Accelerometer (NA) n = 2 Head (n = 1) Sacrum (n = 1) Frequency not reported | Gait speed Cadence Step length Step time variability Smoothness (harmonic ratio) | ↓ Gait speed, cadence, step length, and smoothness on both surfaces in DPN vs. HC ↑ Step time variability on irregular surface in DPN vs. HC |
| Allet et al., 2009 [31] Gait alterations of diabetic patients while walking on different surfaces Switzerland | Observational DPN n = 15 (sex ratio not reported) Age (years) = 61.29 ± 6.52 Height (m) = 1.67 ± 0.08 Weight (kg) = 86.94 ± 9.13 Duration of DM (years) = 8.83 ± 4.60 Blood sugar level not reported VPT (Scale) = 2.63 ± 1.58 DM (without neuropathy) n = 15 (sex ratio not reported) Age (years) = 55.83 ± 8.20 Height (m) = 1.72 ± 0.12 Weight (kg) = 90.30 ± 22.15 Duration of DM (years) = 9.87 ± 7.78 Blood sugar level not reported VPT (Scale) = 5.65 ± 1.14 HC n = 15 (sex ratio not reported) Age (years) = 57.42 ± 4.31 Height (m) = 1.73 ± 0.10 Weight (kg) = 79.93 ± 11.53 VPT (Scale) = 6.80 ± 0.86 | Gait Distance not reported Three surface conditions
| IMU (BioAGM, Lausanne, Switzerland) n = 4 Shin (n = 2; right and left) Thigh (n = 2; right and left) Frequency = 200 Hz | Gait speed Cadence Stride length Stance phase Double support Gait cycle time Step time variability | ↓ Gait speed, cadence, and stride length in DPN vs. HC, but not DPN vs. DM ↑ Stance phase, double support, gait cycle time, and stride time variability on all surfaces in DPN vs. HC, but not DPN vs. DM |
| Allet et al., 2010 [32] An exercise intervention to improve diabetic patients’ gait in a real-life environment Switzerland | Interventional (RCT): IG (Exercise intervention 60 min per session; two sessions per week; 12 weeks) DPN n = 35 (sex ratio not reported) Age (years) = 63.0 ± 8.0 BMI (kg/m2) = 30.5 ± 6.0 Disease duration not reported Blood sugar level not reported VPT (Scale) = 3.2 ± 1.3 CG (No treatment or advice) DPN n = 36 (sex ratio not reported) Age (years) = 64.0 ± 8.9 BMI (kg/m2) = 31.5 ± 5.3 Disease duration not reported Blood sugar level not reported VPT (Scale) = 3.3 ± 1.3 | Gait Distance not reported Two surface conditions
| Gyroscope (NA) n = 4 Shin (n = 2; right and left) Thigh (n = 2; right and left) Frequency = 200 Hz | Gait speed Cadence Stride length Stance time Gait cycle time Step time variability Time points: Baseline 12-week 6-month | All gait and balance variables were similar between IG and CG at baseline ↑ Gait speed, cadence, and stride length in IG at 12-week and 6-month vs. baseline on both surfaces ↓ Gait cycle time and stance time in IG at 12-week and 6-month vs. baseline on both surfaces |
| Crews et al., 2012 [33] Impact of strut height on offloading capacity of removable cast walkers USA | Observational DPN with diabetic foot risk classification: Grade 1 (n = 8) Grade 3 (n = 1) Grade 4 (n = 2) n = 11 (7 men, 4 women) Age (years) = 51.4 ± 10.0 BMI (kg/m2) = 33.9 ± 7.3 Duration of DM (years) = 14.5 ± 9 Blood sugar level not reported VPT value not provided | Gait 20 m Four shoe conditions
| IMU (BioAGM, Lausanne, Switzerland) n = 5 Shin (n = 2; right and left) Thigh (n = 2; right and left) Lumbar region (n = 1) Frequency = 200 Hz | Gait speed Stride length Stride time Double support Gait speed variability | ↓ Gait speed and stride length in ankle-high RCW and knee-high RCW vs. standard athletic shoe ↑ Stride time, double support, and gait speed variability in ankle-high RCW and knee-high RCW vs. standard athletic shoe |
| Najafi et al., 2013a [34] The impact of footwear and walking distance on gait stability in diabetic patients with peripheral neuropathy USA | Observational DPN n = 12 (8 men; 4 women) Age (years) = 60 ± 12 BMI (kg/m2) = 33.2 ± 6.4 Duration of DM (years) = 10 ± 13 Blood sugar level not reported VPT (Volts; right foot) = 56 ± 25 VPT (Volts; left foot) = 61 ± 29 HC n = 8 (6 men; 2 women) Age (years) = 60 ± 6 BMI (kg/m2) = 27.0 ± 3.2 VPT (Volts; right foot) = 19 ± 4 VPT (Volts; left foot) = 20 ± 3 | Gait Four conditions (two distance × two footwear)
| IMU (BioSensics, Newton, MA, USA) n = 5 Shin (n = 2; right and left) Thigh (n = 2; right and left) Lower back (n = 1) Frequency = 100 Hz | Gait initiation steps Gait initiation speed Gait speed Stride length Stride time Double limb support Gait speed variability CoM sway | All variables were similar between DPN and HC in the short-distance condition regardless of footwear conditions ↓ Gait initiation speed, gait speed, and stride length in DPN vs. HC in the long-distance condition regardless of footwear conditions ↑ Gait initiation steps, stride time, double-limb support, and gait-speed variability in the short-distance condition regardless of footwear conditions CoM sway was similar between DPN and HC in all conditions |
| Najafi et al., 2013b [35] A novel plantar stimulation technology for improving protective sensation and postural control in patients with diabetic peripheral neuropathy: A double-blinded, randomized study USA | Interventional (RCT) IG (Electrical plantar stimulation; 30 min per treatment; 5 treatments per week; 6 weeks) DPN n = 25 (sex ratio not reported) Age (years) = 61.6 ± 8.3 BMI not reported Disease duration not reported HbA1c (%) = 7.6 ± 1.6 VPT (Volts) = 46.8 ± 23 CG (Sham stimulation) DPN n = 29 (sex ratio not reported) Age (years) = 61.4 ± 8.2 BMI not reported Disease duration not reported HbA1c (%) = 7.1 ± 1.5 VPT (Volts) = 37.6 ± 22 | Quiet standing Two conditions
| IMU (BioSensics, Newton, MA, USA) n = 2 Shin (n = 1) Lower back (n = 1) Frequency not reported | CoM sway area Time points: Baseline 2-week 4-week 6-week 6-month | All variables were similar between IG and CG at baseline. ↓ CoM sway area at weeks 2, 4, and 6 vs. baseline in IG ↑ CoM sway area at weeks 2, 4, and 6 vs. baseline in CG |
| Grewal et al., 2013 [36] Diabetic peripheral neuropathy and gait: Does footwear modify this association? USA | Observational DPN with active DFU n = 16 (sex ratio not reported) Age (years) = 58.3 ± 4.4 BMI (kg/m2) = 29.5 ± 3.7 Disease duration not reported Blood sugar level not reported VPT value not provided DPN without active DFU n = 15 (sex ratio not reported) Age (years) = 54.2 ± 11.3 BMI (kg/m2) = 31.2 ± 5.9 Disease duration not reported Blood sugar level not reported VPT value not provided HC n = 8 (sex ratio not reported) Age (years) = 59.6 ± 6 BMI (kg/m2) = 27 ± 3.2 | Gait 200 feet Habitual pace | IMU (BioSensics, Newton, MA, USA) Sensor placement not reported Sampling frequency not reported | Gait initiation steps Gait initiation distance Gait speed Stride length Gait cycle time Double stance Gait speed variability CoM sway Knee RoM | ↑ Gait initiation steps, gait speed variability in DPN groups vs. HC ↓ Knee RoM in DPN groups vs. HC |
| Kelly et al., 2013 [37] Fear of falling is prevalent in older adults with diabetes mellitus but is unrelated to level of neuropathy USA | Observational DPN n = 16 (10 men; 6 women) Age (years) = 73 ± 8 BMI (kg/m2) = 30.6 ± 5.7 Duration of DM (years) = 17 ± 11 HbA1c (%) = 8.9 ± 2.7 VPT (Volts) = 49.7 ± 21.9 DM without neuropathy n = 18 (5 men; 13 women) Age (years) = 62 ± 7 BMI (kg/m2) = 31.2 ± 5.9 Duration of DM (years) = 13 ± 13 HbA1c (%) = 7.2 ± 1.6 VPT (Volts) = 18.3 ± 4.5 | Gait 20 m Habitual pace | IMU (BioSensics, Newton, MA, USA) n = 5 Shin (n = 2; right and left) Thigh (n = 2; right and left) Lower back (n = 1) Frequency not reported | Gait initiation steps Gait speed Stride length Stride time Double stance Gait speed variability CoM sway | ↑ Gait initiation steps and double stance in DPN vs. DM without neuropathy Gait initiation steps and double stance were significantly correlated with VPT |
| Wrobel et al., 2014 [38] A novel shear reduction insole effect on the thermal response to walking stress, balance, and gait for diabetic neuropathy USA | Interventional DFO; immediate effect DPN n = 27 (14 men; 13 women) Age (years) = 65.1 BMI (kg/m2) = 33.9 Disease duration not reported Blood sugar level not reported VPT value not provided | Gait 200 steps Two conditions
Two conditions
| IMU (BioSensics, Newton, MA, USA) Sensor placement not reported Frequency not reported | Gait Gait initiation steps Gait initiation speed Gait initiation double stance Gait speed Stride length Stride time Double stance Gait speed variability CoM sway Quiet standing CoM sway area | ↓ Gait initiation double stance for DFO vs. standard shoe during habitual walking |
| Grewal et al., 2015 [39] Sensor-based interactive balance training with visual joint movement feedback for improving postural stability in diabetics with peripheral neuropathy: A randomized controlled trial USA | Interventional (RCT) IG (Balance training exercise with real-time visual feedback; twice a week; 4 weeks) DPN n = 19 (male = 8, female = 11) Age (years) = 62.58 ± 7.98 BMI (kg/m2) = 31.78 ± 7.53 Duration of DM (years) = 17.17 ± 10.08 HbA1c (mmol/mol) = 65.23 ± 19.65 VPT (Volts) = 34.28 ± 8.16 CG (Not specified) DPN n = 16 (male = 8, female = 8) Age (years) = 64.90 ± 8.50 BMI (kg/m2) = 29.58 ± 4.24 Duration of DM (years) = 17.40 ± 9.42 HbA1c (mmol/mol) = 65.40 ± 29.91 VPT (V) = 33.52 ± 6.16 | Quiet standing Two condition
| IMU (BioSensics, Newton, MA, USA) n = 2 Shin (n = 1) Lower back (n = 1) Frequency = 100 Hz | CoM sway Ankle sway Hip sway Time points: Baseline 4-week | ↓ CoM, ankle and hip sway at 4-week vs. baseline in IG during eyes open |
| Toosizadeh et al., 2015 [40] The influence of diabetic-peripheral neuropathy on local postural muscle and central sensory feedback balance control USA | Observational DPN n = 18 (11 men; 7 women) Age (years) = 65 ± 8 BMI (kg/m2) = 29.3 ± 5.4 Duration of DM (years) = 19 ± 11 Blood sugar level not reported VPT (mV) = 34.6 ± 7.0 HC n = 18 (7 men; 11 women) Age (years) = 69 ± 3 BMI (kg/m2) = 27.0 ± 4.1 | Quiet standing Two conditions
| IMU (BioSensics, Newton, MA, USA) n = 2 Sensor placement not reported Frequency not reported | CoM sway Local-control balance Central-control balance | ↑ CoM sway, local-control balance, and central-control balance in DPN vs. HC for both conditions |
| Toosizadeh et al., 2016 [41] Alterations in gait parameters with peripheral artery disease: The importance of pre-frailty as a confounding variable USA | Observational PAD n = 17 (10 men; 7 women) Age (years) = 74 ± 8 BMI (kg/m2) = 26.8 ± 3.5 ABI = 0.83 ± 0.04 HC n = 24 (12 men; 12 women) Age (years) = 76 ± 7 BMI (kg/m2) = 27.9 ± 5.7 | Gait 25 steps Two conditions
| IMU (BioSensics, Newton, MA, USA) n = 5 Shin (n = 2; right and left) Thigh (n = 2; right and left) Lower back (n = 1) Frequency not reported | Gait initiation steps Gait initiation distance Gait speed Stride length Gait cycle time Double support Gait speed variability Trunk sway Knee RoM | ↑ Gait initiation steps, gait-initiation distance, and trunk sway in PAD vs. HC for both paces ↑ Gait speed in PAD vs. HC for both paces ↓ Stride length, ↑ Gait cycle time and double support in PAD vs. HC for habitual pace ↑ Knee RoM and gait-speed variability in PAD vs. HC for fast pace |
| Thiede et al., 2016 [42] Gait and balance assessments as early indicators of frailty in patients with known peripheral artery disease USA | Observational Pre-frail PAD n = 9 (4 men; 5 women) Age (years) = 74.4 ± 7.5 BMI (kg/m2) = 27.1 ± 3.1 ABI = 0.79 ± 0.14 Non-frail PAD n = 8 (6 men; 2 women) Age (years) = 73.4 ± 9.9 BMI (kg/m2) = 26.4 ± 4.1 ABI = 0.88 ± 0.12 Note: Fried criteria for frailty measurement | Gait 25 steps Three conditions
Two conditions
| IMU (BioSensics, Newton, MA, USA) n = 5 Shin (n = 2; right and left) Thigh (n = 2; right and left) Lower back (n = 1) Frequency not reported | Gait Gait speed Stride length Gait cycle time Double support Trunk sway Gait-speed variability Quiet standing CoM sway Ankle sway Hip sway | ↓ Gait speed, ↑ Gait cycle time, double support, gait-speed variability in pre-frail PAD vs. non-frail PAD for dual task walking ↑ Double support and trunk sway in pre-frail PAD vs. non-frail PAD for fast pace No significant difference in quiet standing |
| Najafi et al., 2017 [43] Using plantar electrical stimulation to improve postural balance and plantar sensation among patients with diabetic peripheral neuropathy: A randomized double blinded study USA and Qatar | Interventional (RCT) IG (Wearable plantar electrical stimulation; 1 h daily; 6 weeks; at home) DPN n = 17 (12 men; 5 women) Age (years) = 56 ± 11 BMI (kg/m2) = 28.7 ± 5.9 HbA1c (%) = 8.8 ± 1.9 Disease duration not reported VPT (Volts) = 41 ± 7 CG (Sham stimulation) DPN n = 11 (9 men; 2 women) Age (years) = 64 ± 10 BMI (kg/m2) = 31.5 ± 8.0 HbA1c (%) = 9.6 ± 2.2 Disease duration not reported VPT (Volts) = 40 ± 10 | Gait 10 m Two conditions
Two conditions
| IMU (BioSensics, Newton, MA, USA) n = 2 (gait) Shin (n = 2; right and left) n = 2 (quiet standing) Shin (n = 1) lower back (n = 1) Frequency not reported | Gait Gait speed Cadence Stride length Stride time Quiet standing CoM sway Ankle sway Hip sway Time points: Baseline 6-week | All variables were similar between IG and CG at baseline ↑ Gait speed, cadence, and stride length ↓ stride time at 6-week vs. baseline in IG ↓ Ankle sway at 6-week vs. baseline in IG |
| Esser et al., 2018 [44] Single sensor gait analysis to detect diabetic peripheral neuropathy: A proof of principle study UK | Observational DPN n = 17 (14 men; 3 women) Age (years) = 63 ± 9 BMI (kg/m2) = 33.6 ± 7.6 Duration of DM (years) = 24 ± 13 HbA1c (%) = 8.8 ± 1.0 HC n = 42 (30 men; 12 women) Age (years) = 61 ± 4 BMI (kg/m2) = 31.6 ± 3.9 | Gait 10 m Habitual pace | IMU (NA) n = 1 Lower back (n = 1) Frequency = 100 Hz | Gait speed Cadence Stride length Stride time | ↓ Gait speed, cadence, and stride length ↑ stride time in DPN vs. HC |
| Kang et al., 2019 [45] The effect of daily use of plantar mechanical stimulation through micro-mobile foot compression device installed in shoe insoles on vibration perception, gait, and balance in people with diabetic peripheral neuropathy USA | Interventional: Micro-mobile foot compression; 4 h daily; 4 weeks Severe DPN n = 30 (11 men; 19 women) Age (years) = 68.1 ± 9.7 BMI (kg/m2) = 33.4 ± 6.1 Disease duration not reported Blood sugar level not reported VPT (Volts) = 27.4 ± 12.6 | Gait 10 m Three conditions
Four conditions (two eyes conditions × two foot conditions)
| IMU (BioSensics, Newton, MA, USA) n = 5 (gait) Shins (n = 2; right and left) Thigh (n = 2; right and left) Lower back (n = 1) Frequency not reported n = 2 (quiet standing) Shin (n = 1) lower back (n = 1) Frequency not reported | Gait Gait speed Stride length Stride time Double support Quiet standing CoM sway Ankle sway Hip sway Time points: Baseline 4-week | ↑ Gait speed and stride length at 4-week vs. baseline for habitual pace ↑ Gait speed and stride length ↓ stride time and double support at 4-week vs. baseline for dual task walking ↑ Gait speed ↓ double support at 4-week vs. baseline for fast pace ↓ CoM sway at 4-week vs. baseline for double stance eyes open and eyes closed |
| Kang et al., 2020 [46] Characteristics of the gait initiation phase in older adults with diabetic peripheral neuropathy compared to control older adults USA | Observational DPN n = 38 (20 men; 18 women) Age (years) = 72.6 ± 5.6 BMI (kg/m2) = 31.63 ± 6.07 Disease duration not reported Blood sugar level not reported VPT (Volts) = 32 V ± 14 HC n = 33 (13 men; 20 women) Age (years) = 77.9 ± 8.2 BMI (kg/m2) = 27.05 ± 4.23 | Gait 12 m Two conditions
| IMU (BioSensics, Newton, MA, USA) n = 5 Shins (n = 2; right and left) Thigh (n = 2; right and left) Lower back (n = 1) Frequency = 100 Hz | Gait-initiation steps Gait-initiation distance Gait speed CoM sway | ↑ Gait-initiation steps, gait-initiation distance, and CoM sway, ↓ gait speed in DPN vs. HC for both walking |
| Ling et al., 2020 [47] The impact of diabetic foot ulcers and unilateral offloading footwear on gait in people with diabetes USA | Observational DPN with DFU wearing unilateral offloading n = 12 (10 men; 2 women) Age (years) = 55.6 ± 2.7 BMI (kg/m2) = 30.9 ± 1.3 Blood sugar level not reported Disease duration not reported VPT not reported DPN without DFU n = 27 (20 men; 7 women) Age (years) = 64.3 ± 1.5 BMI (kg/m2) = 30.9 ± 1.0 Blood sugar level not reported Disease duration not reported VPT not reported HC n = 47 (22 men; 25 women) Age (years) = 62.9 ± 2.3 BMI (kg/m2) = 29.0 ± 0.9 | Gait 10 m Habitual pace | IMU (BioSensics, Newton, MA, USA) n = 5 Shins (n = 2; right and left) Thigh (n = 2; right and left) Lower back (n = 1) Frequency not reported | Gait speed Stride length Gait cycle time Double support Gait-speed variability Stride-length variability Double-support limp Step-length limp | ↓ Gait speed, and stride length, ↑ gait cycle time, double-support limp, and step- length limp in DPN with DFU wearing unilateral offloading vs. DPN without DFU and HC ↑ Double support, gait-speed variability, stride-length variability in DPN with DFU wearing unilateral offloading and DPN without DFU vs. HC |
| Du et al., 2021 [48] The feasibility and effectiveness of wearable sensor technology in the management of elderly diabetics with foot ulcer remission: A proof-of-concept pilot study with six cases China | Observational Longitudinal DM with recently recovered from DFU n = 6 (sex ratio not reported) Offloading footwear group (n = 3) Regular footwear group (n = 3) Age (years): between 55–80 Duration of DM: lasting for > 5 years Blood sugar level not reported VPT not reported | Gait 1 min Habitual walk Quiet standing Four conditions (two eyes conditions × two surface conditions)
| IMU (BioSensics, Newton, MA, USA) n = 5 (gait) Shin (n = 2; right and left) Thigh (n = 2; right and left) Lower back (n = 1) Frequency = 100 Hz n = 2 (quiet standing) Shin (n = 1) lower back (n = 1) Frequency = 100 Hz | Gait: Gait speed Stride length Double support Swing phase Quiet standing: CoM sway Ankle sway Hip sway Timepoints: Baseline 1-week 1-month 4-month 6-month | ↑ Gait speed and stride length, ↓ double support in offloading footwear group Quiet standing remained similar |
| Lanzi et al., 2021 [49] Supervised exercise training improves 6 min walking distance and modifies gait pattern during pain-free walking condition in patients with symptomatic lower extremity peripheral artery disease Switzerland | Interventional: Supervised exercise training PAD n = 29 (15 men; 14 women) Age (years) = 65.4 ± 9.9 BMI (kg/m2) = 28.7 ± 6.2 ABI = 0.79 ± 0.14 | Gait 6 min walk test Habitual pace | IMU (GaitUp, Renens, Switzerland) n = 2 Sensor placement not specified Frequency not reported | Gait speed Stride length Stride time Stride frequency Double support Stance phase Swing phase Loading response Heel-strike pitch angle Toe-off pitch angle Max-heel clearance First max-toe clearance Second max-toe clearance Minimum toe clearance Time points: Baseline 3-month | ↑ Gait speed, stride length, swing phase, and loading response, ↓ stance phase at 3-month vs. baseline ↑ Toe off pitch angle at 3-month vs. baseline |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, G.E.; Stout, A.; Waldon, K.; Kang, S.; Killeen, A.L.; Crisologo, P.A.; Siah, M.; Jupiter, D.; Najafi, B.; Vaziri, A.; et al. Digital Biomarkers of Gait and Balance in Diabetic Foot, Measurable by Wearable Inertial Measurement Units: A Mini Review. Sensors 2022, 22, 9278. https://doi.org/10.3390/s22239278
Kang GE, Stout A, Waldon K, Kang S, Killeen AL, Crisologo PA, Siah M, Jupiter D, Najafi B, Vaziri A, et al. Digital Biomarkers of Gait and Balance in Diabetic Foot, Measurable by Wearable Inertial Measurement Units: A Mini Review. Sensors. 2022; 22(23):9278. https://doi.org/10.3390/s22239278
Chicago/Turabian StyleKang, Gu Eon, Angeloh Stout, Ke’Vaughn Waldon, Seungmin Kang, Amanda L. Killeen, Peter A. Crisologo, Michael Siah, Daniel Jupiter, Bijan Najafi, Ashkan Vaziri, and et al. 2022. "Digital Biomarkers of Gait and Balance in Diabetic Foot, Measurable by Wearable Inertial Measurement Units: A Mini Review" Sensors 22, no. 23: 9278. https://doi.org/10.3390/s22239278
APA StyleKang, G. E., Stout, A., Waldon, K., Kang, S., Killeen, A. L., Crisologo, P. A., Siah, M., Jupiter, D., Najafi, B., Vaziri, A., & Lavery, L. A. (2022). Digital Biomarkers of Gait and Balance in Diabetic Foot, Measurable by Wearable Inertial Measurement Units: A Mini Review. Sensors, 22(23), 9278. https://doi.org/10.3390/s22239278









