Abstract
Herein, we developed a new pencil graphite ion-selective electrode strategy for the broadly used erectile dysfunction medication, sildenafil citrate (SC, vitamin V), for its automated potentiometry and potentiometric titration profiling in marketed tablets and human urine samples. The method was based on ion-pair complexation between SC and sodium tetraphenylborate (Na-TPB) or phosphotungstic acid (PTA), embedded into a pencil-fabricated graphite sensor electrode coated with poly(vinyl chloride, PVC) matrix, which is pre-plasticized with two different pre-studied plasticizers. The modern fabricated electrodes have a proven fast near-Nernstian response for SC over the concentration range of 1.0 × 10−6 to 1.0 × 10−2 and 1.0 × 10−5 to 1.0 × 10−2 M, with LODs of 6.5 × 10−7 and 5.5 × 10−6 over a pH 3–6 for (SC-TPB)- and (SC-PTA)-based membrane sensors, of O-nitrophenyl octyl ether (O-NPOE) and dioctyl phthalate (DOP), respectively. The selectivity coefficients for different interferents, including many inorganic cations, sugars, and/or nitrogenous compounds, were tested and confirmed. Applications of the proposed method were conducted on the determination of SC in its tablets and urine samples under the proper conditions. The percent recovery values were compared with those obtained by an official method and showed an RSD ≤ 0.3% (n = 5).
1. Introduction
Viagra is an anti-impotence drug with an active ingredient, sildenafil citrate (SC, vitamin V), and its own chemical nomenclature is 1-[4-ethoxy-3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo-[4,3-d] pyrimidin-5-yl) phenyl sulphonyl]-4-methylpiperazine citrate, Figure 1 [1,2]. Physiologically, the erection of the penis is caused by the release of nitric oxide (NO) in the corpus cavernosum, which follows sexual stimulation. Then NO activates a guanylate cyclase that increases the levels of cyclic guanosine monophosphate (cGMP), potentates smooth muscle relaxation in the corpus cavernosum, and is accompanied by the inflow of blood [3]. However, SC has no direct relaxant effect on the isolated human corpus cavernosum but potentiates the effect of NO, after inhibiting phosphodiesterase type 5 (PDE5), which is the responsible enzyme for the degradation of cGMP in the corpus cavernosum [4,5]. SC is used daily with a maximum dose of 100 mg for all categories of patients’ inabilities, whatever the causes of their erectile dysfunction. The elderly, those with hepatic or renal impairments, and/or those receiving cytochrome P450 enzyme CYP3A4 inhibitors could be treated with SC as well [6]. Moreover, the efficacy of SC is a dose-related improvement in the form of its biological duration of erections and hardness and the frequency of the patient’s abilities.
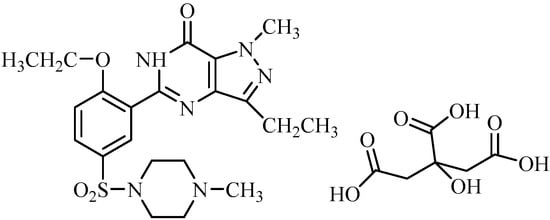
Figure 1.
The chemical structure of sildenafil citrate, SC.
Different developed analytical methods were described for the determination of SC in its oral pharmaceutical tablets, namely high-performance liquid chromatographic (HPLC) [7,8,9,10,11,12,13], spectrophotometric [2,14,15], and electroanalytical techniques (voltammetry) [16,17] and potentiometry [18].
The increasing use of ion sensors and their excellent efficiency and reliability in the medical analysis is putting increasing pressure on analytical chemists to develop new sensors for fast, accurate, reproducible, and selective determinations of various species. Furthermore, a dependable and specific assay is critical for determining the disposition, tolerance, and safety of a drug. Potentiometry with ion-selective sensors is frequently used in the field of pharmaceutical and biological analysis due to its convenience and good sensitivity, which encouraged analytical researchers to develop modern selective sensors for fast, accurate, reproducible, and selective determinations of various drug species [18,19].
Pencil graphite electrodes are considered a versatile form of ion-selective electrodes, characterized by their small size, which results from the absence of the internal filling solution, a rapid response time, and a long lifetime [20,21]. These features enable them to be used in biological systems’ determinations, with no need for further sample pretreatment steps, in the form of extraction or filtration because of their ability to be used with turbid or colored solutions [20,21,22,23,24].
The objective of the present work is to develop a new, accurate, sensitive, time, and duration cost-saving potentiometric method for the determination of SC. The methods utilized the convenient pencil graphite two sensor electrodes depending on the difference in the active pH range for each sensor. Initially, we developed and validated the potentiometric method based on ion-pair complexometric reactions between the target analyte, SC, and two different complexing agents, namely sodium tetraphenylborate (Na-TPB) and/or phosphotungstic acid (PTA), as the electroactive phases. Second, preprepared plasticized poly(vinyl chloride, PVC) membranes were coated into graphite rods and were highly sensitive, selective, reproducible, and accurate toward any of the resulting reaction mixtures of SC-TPB and/or SC-PTA in their solid states. Furthermore, O-nitrophenyl octyl ether (O-NPOE) is classically used as a plasticizer with PVC; many reports have highlighted that dioctyl phthalate (DOP) showed good results with PVC, especially if used in pencil graphite support electrodes [20,24,25,26]. Thus, both O-NPOE and DOP were tested in our study. Moreover, the present method was conducted to determine the SC in its oral tablets and spikes in human urine.
2. Materials and Methods
2.1. Apparatus
The potentiometric measurements were made at 25 ± 5 °C using a Hanna microprocessor ion analyzer pH/mV meter (model 8417) with the proposed SC-TPB or SC-PTA graphite membrane sensor in conjunction with a double-junction Ag/AgCl reference electrode (Orion 900200), dimensions: 110 × 12 mm (cap dia 16 mm) and containing 10% KCl solution in the outer compartment. A circulator thermostat Model C-100 (Cambridge, England), was used to control the temperature.
2.2. Materials
Sildenafil citrate (SC, vit. V) was supplied as a gift from Pfizer, Cairo, Egypt (Pfizer Co., Cairo, Egypt, under the authority of Pfizer Inc., New York, NY, USA). Sodium tetraphenylborate (Na-TPB) and tetrahydrofuran (THF) were purchased from Fluka (Buchs, Switzerland). Phosphotungstic acid (PTA), dioctyl phthalate (DOP), O-nitrophenyl octyl ether (O-NPOE), high molecular mass PVC, and chloranil were obtained from (Sigma Aldrich, St. Louis, MO, USA). All other chemicals used were of analytical grade unless otherwise stated, and doubly-deionized water was used throughout.
2.3. Pharmaceutical Preparation
Viagra® tablets (Batch No: EU/1/98/077/015, Pfizer Co., Egypt), under the authority of Pfizer INC., USA) were labeled to contain 50 mg of sildenafil citrate/tablet.
2.4. Preparation of the Solutions and Solids
2.4.1. Preparation of SC Stock Solution
The stock solution of SC (10−2 M) in acetate buffer (0.1 mM and pH = 5) was prepared by dissolving an accurately weighed 0.667 g of the pure powder into a 100 mL volumetric flask containing an amount of acetate buffer. After being mixed well, the contents were completed into the mark with the same solvent. Thus, the working solutions of the desired concentrations were prepared by serial dilution in the same solvent.
2.4.2. Preparation of Na-TPB and PTA Standard Solutions
A 10−2 M solution of Na-TPB or PTA was prepared by dissolving the accurately weighed amounts into a small amount of double distilled water and then made up to the mark of 100 mL of the volumetric flask by the same solvent.
2.4.3. Preparation of the Expected Interfering Ions Solution
Solutions (10−3 M) of the standard interferants such as NaCl, KCl, CaCl2, NH4Cl, ascorbic acid, glycine, glucose, lactose, fructose, maltose, starch, sucrose, p-aminophenol, ephedrine HCl, and p-aminobenzoic acid were prepared by dissolving the appropriate masses into the 100 mL portion of double distilled water.
2.4.4. Preparation of the Form of the Ion-Pair Complexes
SC-ion pairs using Na-TPB (SC-TPB) or PTA (SC-PTA) were prepared by mixing 50 mL of the target analyte (10−2 M) with 50 mL of any of the Na-TPB and/or PTA reagents, both in a concentration of 10−2 M. The formed complexes’ precipitates were filtered off, thoroughly washed with distilled water, and allowed to dry in the open air.
2.4.5. Preparation of Ion-Selective Membranes
The ion-selective membranes (sensor electrodes) selective to any of Na-TPB and/or PTA, as an electroactive phase, were prepared by mixing 190.0 mg of PVC with 350.0 mg of any of the plasticizer of DOP or O-NPOE into 5 mL of THF. Thus, the component of each mixture was mix-vortex until obtaining the homogeneous mixture, which then was evaporated at room temperature until a concentrated mixture was obtained. Then it was transferred into a small tube (3 mL in volume). Six graphite rods (3 mm diameter and 10 cm long), prepared from spectroscopic grade graphite, were used as a conducting substrate [23] and were dipped separately into the obtained membrane-coating mixture; then, the rest of the THF solvent was left to evaporate. Thus, thin membranes were formed on the graphite surfaces, and this step was repeated several trials until a suitable membrane with a thickness of 0.2 cm was obtained. Finally, the prepared sensor electrodes were conditioned by soaking them into the SC standard solution (10−2 M) for 2 h just before instrumental measurements and were stored in the same solution when not in use.
2.5. Analytical Procedures
2.5.1. Sensors’ Calibration Study
Each of the prepared graphite sensor electrodes was calibrated by immersing it in conjunction with the reference electrode in a 50 mL beaker containing 10 mL of SC solution (10−2 M) in a concentration ranging from 1.0 × 10−6 to 1.0 × 10−2 M (n = 5) at a pH of 5 using acetate buffer (as mentioned above) with a continues stirring. Then the potential readings were recorded after stabilization to ±0.5 mV. The calibration curves for both ion-pair complexing agents were constructed by plotting the recorded potential versus the logarithmic SC concentration.
It is noteworthy that the sensor was soaked in 10−2 M SC solution for 2 h before and stored in the same solution after ending the measurement steps.
2.5.2. Selectivity of the Prepared Sensors Study
The selectivity coefficients of the SC-TPB and SC-PTA ion-selective sensor electrodes (ISEs) were determined employing separate solution methods (SSM) [18,19,20,21,22,23]. Aliquots (10 mL) of 1.0 × 10−2 M SC solution were adjusted to pH 5.0 with acetate buffer, and the SC-TPB or SC-PTA sensors were immersed in the test solution, and the potential was measured. The potentials of 1.0 × 10−3 M solutions of the interferents adjusted to pH 5.0 were measured. The selectivity coefficients were measured by employing a separate solution method (SSM) with the rearranged Nicolsky equation [18,23,24]:
where E1 and E2 are the measured potentials of 1.0 × 10−2 M SC alone and in the presence of 1.0 × 10−3 M of the interfering substances, respectively, z1 and z2 are the charges of the SC and interfering species (B). S and A are the slope and intercept of the electrode calibration plot, respectively.
2.5.3. Determination of SC in Viagra® Tablets
Three tablets of Viagra®, 50 mg, were pulverized and homogenized carefully. A weight that is equivalent to the average weight of one tablet was dissolved in double distilled water by shaking for 30 min with a mechanical shaker. After filtration, the filtrate was made up to 50 mL in a volumetric calibrated flask. Next, the pH of the solution was adjusted to pH 5, vortex-mixed, and finally, the potentials of the samples were determined using any of the prepared graphite sensor electrodes in the triplicate measurements (n = 3), as described in Section 2.5.1.
2.5.4. Spectrophotometric Method (Reported) for Determination of SC
An aliquot of SC solution containing 2.5 mg was mixed with 7 mL of chloranil (5 × 10−3 M) in a 10 mL volumetric flask. The content of the flask was completed with acetonitrile and mixed. Then the absorbance of the resulting violet color was measured at 548 nm against an experimental blank under the same conditions [15].
2.5.5. Determination of SC by a Potentiometric Titration
To test the simple, practical applicability of the prepared graphite-O-NPOE membrane of SC-TPB and SC-PTA, sensor electrodes were used as indicator electrodes for the titration of 5.0 mL of 1.0 × 10−2 M of SC solution with 1.0 × 10−2 M of Na-TPB solution.
2.5.6. Analysis Procedure of Spiked Human Urine Samples
The prepared graphite sensor electrodes were used for the determination of the standard SC in urine samples (n = 5) using a standard addition method to conduct the reliability and the required bio-sensitivity. Standard SC was used to spike urine samples (2.0 mL of various working solutions). Then, the developed method in Section 2.5.1 was carried out against a urine blank.
2.5.7. Standard Addition Method
Virtually, the standard addition (or spiking) step was developed by adding a different known volume of a known concentration test solution (1.0 × 10−2 M, 1.0–10.0 mL) of SC at pH 5 into a 25 mL measuring flask. Next, the potentials displayed by this test solution were measured before and after the addition of a fixed 1.0 mL aliquot of standard SC solution (1.0 × 10−2 M). The change in the electrode potential (ΔE, mV) was then recorded and used for determining SC.
3. Results
3.1. The Conduction Mechanism of the Graphite-Coated Sensor
With the aid of the modern fabricated graphite-coated sensor electrode, the internal boundary potential (graphite membrane) is dependent on the compound species/concentration present on the graphite-coated surfaces. The cationic conduction (Equations (2) and (3), Scheme 1) in the sensor could be attributed to the combination of the membrane TPB or PTA with SC ions in its solution as in the following [23,24]:
where Na-TPB or PTA is embedded in the membrane, and SC-TPB or SC-PTA is the reaction product at the membrane surface. Therefore, the charge transfer across the membrane is carried out by ions coupled to the electronic charge at the graphite surface. Thus, the accumulation of SC ions at the membrane surface will alter its potential.
SC + Na-TPB → SC-TPB
SC + PTA → SC-PTA
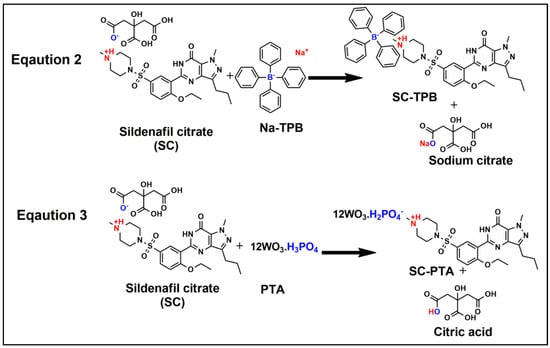
Scheme 1.
The detailed mechanism of the cationic conduction sensing of SC by Na-TPB and PTA fabricated PVC graphite sensors, indicated by chemical structures.
3.2. Sensor Electrode Behavior Study
The general working characteristics of the electrodes were evaluated by performing regular calibrations in SC solutions, as in Figure 2. Moreover, Table 1 shows the performed preliminary studies on the influence of a plasticizer choice as it affects electrode performances. However, the results of the determinations of the SC-TPB or SC-PTA plasticized with O-NPOE were compared with those obtained in the case of DOP, and their performance characteristics were evaluated according to the IUPAC recommendations [23,24,25,26]. Of the two tested plasticizers, O-NPOE shows the highest total potential change. These obtained data were attributed to the high dielectric constant of O-NPOE, and the high extractability of the formed SC-TPB and/or SC-PTA ion-pair complexes into the sensor matrix compared with another plasticizer (ε values are 24 and 5.2 of O-NPOE and DOP, respectively) [27]. Therefore, PVC plasticized with O-NPOE was used for the next experimental studies.
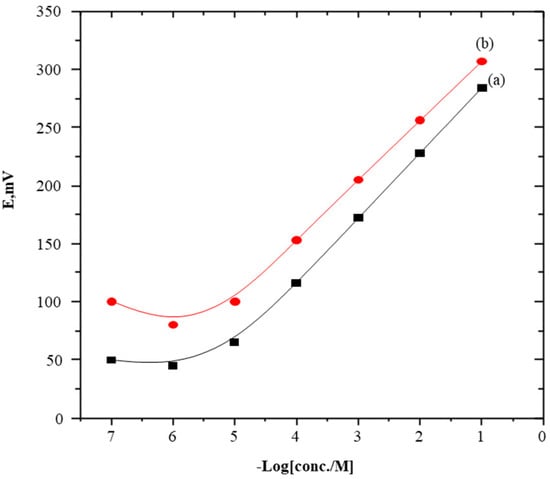
Figure 2.
Potentiometric response of the PVC-O-NPOE membrane sensors plasticized (a) SC-TPB and (b) SC-PTA.

Table 1.
Potentiometric response characteristics performance of the SC-TPB and SC-PTA with PVC plasticized with O-NPOE and DOP.
3.3. Effect of pH
The effect of pH on the SC sensor electrodes containing the SC-TPB and SC-PAT with PVC plasticized with O-NPOE was investigated by measuring the response to 1 × 10−3, 1 × 10−4, and 1 × 10−5 M of SC solutions at pHs that ranged from 1.0–10. The pH is adjusted using HCl and /or NaOH. Figure 3A,B show that the two sensor electrodes exhibited a stable response over the pH range of 3–6 and 3.3–6, respectively, for SC-TPB and SC-PTA sensor electrodes. At lower pH values, the SC contains more than one positive site (nitrogen centers), which increases the potential response and controls the membrane response and stability. However, at the higher pH values, the potential readings for both sensors decreased sharply due to the precipitation or hydrolysis of the measured SC samples. Thus, if the electrode was applied for the determination of SC in samples with a pH higher than 6, the pH of the sample should be adjusted using HCl.
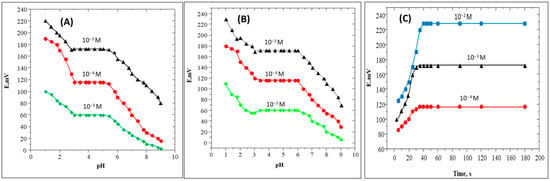
Figure 3.
(A–C): The effect of pH on O-NPOE plasticized PVC electrodes containing the (A) SC-TPB and (B) SC-PTA on SC sensing, while (C) shows the average dynamic response time of the SC-TPB sensor electrode towards SC.
3.4. Response Time Study
The response time was studied by measuring the steady state potential of 10−4, 10−3, and 10−2 M solutions of SC for 3 min. As can be seen in Figure 3C, less than 30 s was the average dynamic response time required to reach the maximum response for SC-TPB sensor electrodes with PVC plasticized with O-NPOE. Moreover, it is noted that the response time is more rapid when the measured solutions are lower in concentration; however, 30 s is still optimum for all the measured SC solutions.
3.5. Effect of the Diverse (Interfering Ions) Ions on the SC Sensor Electrode
The ions and substances that might exist in biological fluids or SC pharmaceutics, including ions, sugars, amino acids, and different cations, were all tested as possible interferents, and the results are listed in Table 2.

Table 2.
Selectivity coefficients of various interfering species for SC-TPB and SC-PTA.
A reasonably accurate selectivity toward SC in the presence of the common nitrogenous compounds such as amino acids and amines and some inorganic cations was observed. The results confirmed that there was no serious interference by various pharmaceutical excipients, diluents, and active ingredients commonly used in the drug tablet formulations, namely lactose, maltose, glucose, starch, talc powder, mannitol, and magnesium stearate, at a concentration as high as a 10–fold molar excess over SC, Table 2.
The other advantage that could be added to the present work is that the plasticized PVC membrane sensors behave in different ways compared to the liquid membrane sensors. The ion exchange sites are poorly mobile, and the coefficients of such a system is given by the equation:
where UD and UB and KD and KB are the mobilities and molar distribution coefficients of the SC and the interfering species (B) in the membrane phase and between the aqueous phase and the PVC membrane, respectively.
As reported for the PVC surface membranes, the ions’ presence undergoes mobility restriction owing to their complexation with long-chain complexing agents [18,26]. Hence, the partition coefficients of the SC and the existence of the interfering ions between the membrane and the measured aqueous phase are the main reasons for the observed sensor selectivity.
3.6. Determination of SC by Potentiometric Titration
The applicability of the present method was checked using a simple potentiometric titration technique. The results obtained (Table 3 and Figure 4) demonstrated that SC reacts with Na-TPB in a molar ratio of 1:1, and the titration curve was symmetrical with a very well-defined potential jump of about 340 mV, which indicates the high sensitivity of the prepared graphite-O-NPOE and graphite-DOP sensor electrodes as compared with the reported results [26]. Moreover, the method showed its suitability in the form of accuracy and precision, as indicated in Table 3.

Table 3.
The precision of the potentiometric titration of 5.0 mL of 1.0 × 10−2 M of SC solution with 1.0 × 10−2 M of Na-TPB solution using SC-TPB with PVC plasticized with O-NPOE or DOP.
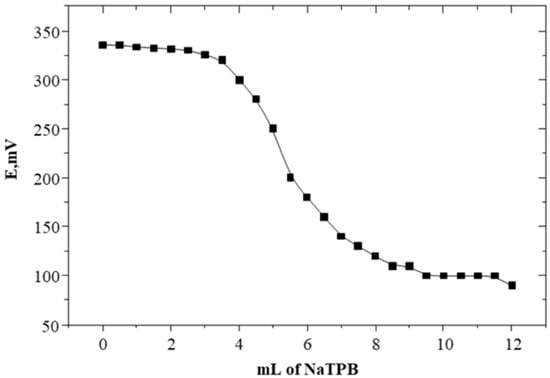
Figure 4.
Potentiometric titration of 5.0 mL of 1.0 × 10−2 M of SC solution with 1.0 × 10−2 M of Na-TPB solution using SC-TPB with PVC plasticized with O-NPOE.
3.7. Direct Determination of SC in the Viagra® Tablets
The prepared graphite sensor electrode showed acceptable working and indicator characteristics, suitable for SC potentiometric determination of SC in its pharmaceutics. Table 4 summarizes the results of the potentiometric analysis of SC in its Viagra tablets. The results of the proposed methods were compared with those obtained using the reference USP method [1]. The average recovery was 101.34 ± 0.65% with a relative standard deviation (RSD) of ±0.70% for the proposed sensor SC-TPB and 95.26 ± 0.51% and RSD of 1.07% for the proposed SC-PTA. The values presented in Table 4 reveal a good agreement between both methods, the proposed potentiometric and the spectrophotometric recommended by USP [2,15], and there was no difference between the two methods regarding accuracy and precision as revealed by the student’s t-test and the variance ratio F-test.

Table 4.
Determination of SC in Viagra® tablets using SC-TPB and SC-PTA with PVC plasticized with O-NPOE or DOP.
3.8. Analysis SC Spiked in Human Urine Samples
The proposed sensor electrodes were employed successfully for the determination of SC in spiked urine samples. The used urine samples’ pH was measured and was found to be slightly acidic (4–6), which lies in the optimum range of the sensor. Table 5 shows the obtained results, which indicated that the prepared sensor electrodes could detect the SC content in the spiked urine sample with high accuracy and precision. The values presented in Table 5 reveal a good agreement between both methods, the proposed potentiometric and the spectrophotometric recommended by USP [2,15].

Table 5.
Determination of SC in spiked human urine samples using SC-TPB and SC-PTA with PVC plasticized by O-NPOE or DOP.
3.9. Repeatability and Reproducibility Study
The results revealed that there were no changes in the potential response of the two SC-TPB and SC-PAT sensor electrodes over a long time after repeating each measurement five times. The prepared sensors are usable for at least 60 days without any change in their response characteristics after they were stored in 1.0 × 0−2 M of SC solution when not in use. The master membrane can be used after four months with the same response when stored below 4 °C, as the results indicated.
3.10. Comparison of the Performance of the Proposed Method with the Previously Reported Potentiometric Methods for SC Analysis
The performance of the proposed potentiometric method for the determination of SC was compared with the previously reported electrochemical methods in the literature [18,19,28,29,30,31]. As illustrated in Table 6, the current method is more sensitive than methods AdCSV [28] and FIA with an amperometric detector [29] and with a more realistic linear range than the FIA method [18]. Additionally, our method used simpler materials than the Ppy/Cit/Graphite [30] and FIA with amperometric detector [29] methods. Furthermore, the speed here is more rapid than all the previously reported methods, which gives us the advantages of simplicity, rapidity, sensitivity, reproducibility, and accuracy. In conclusion, this method is cheap, fast, and sensitive compared to most of the methods listed in Table 6, as it gives a realistic linear range from 1.0 × 10−6–1.0 × 10−2 M, and is safe, non-laborious, and not tedious. The sensor used in this method is made of natural graphite, as it is compatible with the application of sustainability standards compared to the other methods referred to in the literature. Furthermore, 95% of the final response was reached within about 15 s compared with the previous methods.

Table 6.
The comparison between the previous methods and the proposed method regarding the determination of vitamin V using electrochemical sensors.
4. Conclusions
A rapid and convenient study was utilized in the preparation of modern sensitive, selective graphite-sensitive electrodes used for the first time in the instrumental potentiometry and potentiometric titrimetric determination of SC in pure and pharmaceutical forms as well as in spiked human urine. The method had simple presteps of complex formation between Na-TPB and/or PTA and SC, and then loaded into two types of modern sensor electrodes using PVC with two available plasticizers. Both Na-TPB and PTA fabricated sensors showed similar analytical performance towards SC in terms of linearity and LOD; however, PTA is more cost-effective than Na-TPB; thus, it could be slightly preferred. The profiling of the proposed method was conducted for the determination of SC in its tablets, and urine samples with the % recovery values were compared with those obtained by an official method and showed an RSD ≤ 0.3% (n = 5).
Author Contributions
A.M.E.H. and H.A.B.: conceptualization, methodology, investigation, formal analysis, visualization, validation, and writing of the original draft. M.A.E.H.: conceptualization, investigation, supervision, resources, funding acquisition, data curation, and writing review and editing. M.H.E.-M.: conceptualization, supervision, and writing review and editing. W.A.M. and S.A.: resources, funding acquisition, and writing, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Researchers Supporting Project (number RSP2022R516) from King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors are thankful to the Researchers Supporting Project (number RSP2022R516) at King Saud University, Riyadh, Saudi Arabia. The authors would like to thank the Deanship of Scientific Research at Shaqra University for supporting this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United States Pharmacopoeia DI, NF XXI ed.; USP Convention Inc.: Rockvile, MD, USA, 2001; pp. 2691–2694.
- Harikrishna, K.; Nagaralli, B.; Seetharamappa, J. Extractive spectrophotometric determination of sildenafil citrate (viagra) in pure and pharmaceutical formulations. J. Food Drug Anal. 2008, 16, 11–17. [Google Scholar] [CrossRef]
- Vardi, M.; Nini, A. Phosphodiesterase inhibitors for erectile dysfunction in patients with diabetes mellitus. Cochrane Database Syst. Rev. 2007, 2007, CD002187. [Google Scholar] [CrossRef] [PubMed]
- Kloner, R.A. Pharmacology and drug interaction effects of the phosphodiesterase 5 inhibitors: Focus on α-blocker interactions. Am. J. Cardiol. 2005, 96, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.J.; Freestone, S.; Allen, M.J.; Muirhead, G.J. Sildenafil citrate and blood-pressure–lowering drugs: Results of drug interaction studies with an organic nitrate and a calcium antagonist. Am. J. Cardiol. 1999, 83, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Langtry, H.D.; Markham, A. Sildenafil: A review of its use in erectile dysfunction. Drugs 1999, 57, 967–989. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, X.-s.; Yang, B.-y. Determination of Sildenafile citrate mixed illegally in Zhuangyao Bushen pill. West China J. Pharm. Sci. 2006, 21, 590. [Google Scholar]
- Aboul-Enein, H.Y.; Hefnawy, M.M. Rapid determination of sildenafil citrate in pharmaceutical preparations using monolithic silica HPLC column. J. Liq. Chromatogr. Relat. Technol. 2003, 26, 2897–2908. [Google Scholar] [CrossRef]
- Dinesh, N.; Vishukumar, B.; Nagaraja, P.; Gowda, N.M.; Rangappa, K. Stability indicating RP-LC determination of sildenafil citrate (Viagra) in pure form and in pharmaceutical samples. J. Pharm. Biomed. Anal. 2002, 29, 743–748. [Google Scholar] [CrossRef]
- Daraghmeh, N.; Al-Omari, M.; Badwan, A.; Jaber, A. Determination of sildenafil citrate and related substances in the commercial products and tablet dosage form using HPLC. J. Pharm. Biomed. Anal. 2001, 25, 483–492. [Google Scholar] [CrossRef]
- Dong, F.; Liao, J.; Yuan, Z. Determination of sildenafil in tablet by reversed-phase high-performance liquid chromatography. J. Instrum. Anal. 2000, 19, 53–54. [Google Scholar]
- Segall, A.; Vitale, M.; Perez, V.; Palacios, M.; Pizzorno, M. Reversed-phase HPLC determination of sildenafil citrate in the presence of its oxidative-induced degradation products. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 1377–1386. [Google Scholar] [CrossRef]
- Ergun, B.; Saracoglu, A.; Ilgin, S.; Atkosar, Z.; Kircali, K.; Altiokka, G. Validation of a Reversed-phase HPLC method for the analysis of sildenafil citrate in pharmaceutical preparations and in spiked human plasma. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 1539–1548. [Google Scholar] [CrossRef]
- Issa, Y.; El-Hawary, W.; Youssef, A.; Senosy, A. Spectrophotometric determination of sildenafil citrate in pure form and in pharmaceutical formulation using some chromotropic acid azo dyes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaalan, N.H. Utility of certain π acceptors for spectrophotometric determination of sildenafil citrate. Am. J. Appl. Sci. 2007, 4, 743–747. [Google Scholar] [CrossRef]
- Berzas, J.; Rodriguez, J.; Castañeda, G.; Villaseñor, M. Voltammetric behavior of sildenafil citrate (Viagra) using square wave and adsorptive stripping square wave techniques: Determination in pharmaceutical products. Anal. Chim. Acta 2000, 417, 143–148. [Google Scholar] [CrossRef]
- Batista, E.F.; Sartori, E.R.; Medeiros, R.A.; Rocha-Filho, R.C.; Fatibello-Filho, O. Differential pulse voltammetric determination of sildenafil citrate (Viagra®) in pharmaceutical formulations using a boron-doped diamond electrode. Anal. Lett. 2010, 43, 1046–1054. [Google Scholar] [CrossRef]
- Hassan, S.S.; Elnemma, E.M.; Mahmoud, W.H.; Mohammed, A.H. Continuous potentiometric monitoring of viagra (sildenafil) in pharmaceutical preparations using novel membrane sensors. J. Appl. Electrochem. 2006, 36, 139–146. [Google Scholar] [CrossRef]
- Othman, A.; Rizk, N.; El-Shahawi, M. Polymer membrane sensors for sildenafil citrate (Viagra) determination in pharmaceutical preparations. Anal. Chim. Acta 2004, 515, 303–309. [Google Scholar] [CrossRef]
- Nashed, D.; Noureldin, I.; Sakur, A.A. New pencil graphite electrodes for potentiometric determination of fexofenadine hydrochloride and montelukast sodium in their pure, synthetic mixtures, and combined dosage form. BMC Chem. 2020, 14, 60. [Google Scholar] [CrossRef]
- Cattarall, R.; Henry, F. Coated wire ion-selective electrode. Anal. Chem. 1971, 43, 1905–1906. [Google Scholar] [CrossRef]
- Sakur, A.A.; Nashed, D.; Haroun, M.; Noureldin, I. Determination of prasugrel hydrochloride in bulk and pharmaceutical formulation using new ion selective electrodes. Res. J. Pharm. Technol. 2018, 11, 631–636. [Google Scholar] [CrossRef]
- Haroun, M.; Nashed, D.; Sakur, A.A. New electrochemical methods for the determination of Prasugrel using drug selective membranes. Int. J. Acadamic Sci. Res. 2017, 5, 30–36. [Google Scholar]
- Mansour, O.; Nashed, D.; Sakur, A.A. Determination of clopidogrel bisulphate using drug selective membranes. Res. J. Pharm. Technol. 2018, 11, 2017–2021. [Google Scholar] [CrossRef]
- Sakur, A.A.; Dabbeet, H.A.; Noureldin, I. Novel drug selective sensors for simultaneous potentiometric determination of both ciprofloxacin and metronidazole in pure form and pharmaceutical formulations. Res. J. Pharm. Technol. 2019, 12, 3377–3384. [Google Scholar] [CrossRef]
- Sakur, A.A.; Bassmajei, S.; Dabbeet, H. Novel moxifloxacin ion selective electrodes for potentiometric determination of moxifloxacin in pure form and pharmaceutical formulations. Int. J. Acadamic Sci. Res. 2015, 3, 66–75. [Google Scholar]
- Ma, T.S.; Hassan, S.S.M. Organic Analysis Using Ion-Selective Electrodes; Blackwell Science: Hoboken, NJ, USA, 1982. [Google Scholar]
- Sopha, H.; Hocevar, S.B.; Pihlar, B.; Ogorevc, B. Bismuth film electrode for stripping voltammetric measurement of sildenafil citrate. Electrochim. Acta 2012, 60, 274–277. [Google Scholar] [CrossRef]
- Júnior, A.; Luz, R.; Damos, F.; Santos, A.; Franco, D.; dos Santos, W. Determination of sildenafil citrate (Viagra®) in various pharmaceutical formulations by flow injection analysis with multiple pulse amperometric detection. J. Braz. Chem. Soc. 2012, 23, 1800–1806. [Google Scholar] [CrossRef]
- Ochiai, L.M.; Bindewald, E.H.; Mengarda, P.; Marcolino-Junior, L.H.; Bergamini, M.F. Disposable potentiometric citrate sensor based on polypyrrole-doped films for indirect determination of sildenafil in pharmaceuticals formulations. J. Appl. Polym. Sci. 2016, 133, 43762. [Google Scholar] [CrossRef]
- Frag, E.; Mohamed, G.; Alelaiwi, H. Electroanalytical determination of sildenafil in Viagra tablets using screen-printed and conventional carbon paste electrodes. J. Electroanal. Chem. 2011, 659, 121–127. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).