Development of a New Voltammetric Method for Aceclofenac Determination on Glassy Carbon Electrode Modified with Hierarchical Nanocomposite
Abstract
:1. Introduction
2. Experimental
2.1. Apparatus
2.2. Chemicals and Glassware
2.3. Sample Preparation
2.3.1. Tablets
2.3.2. Lyophilizate Urine
2.3.3. Plasma
2.4. Fabrication of the eCNF/CNT/NiCo-GCE Sensor
2.4.1. Preparation of the Nanocomposite
Electrospinning
Thermal Treatment
2.4.2. Modification Process
2.5. Measurement Procedure
- Cleaning of the GCE surface: E = 1102 mV; t = 3 s;
- Accumulation: Eacc = 500 mV; tacc = 20 s;
- Rest period: 3 s;
- Registration of ACL voltammograms in the potential range: 500–1100 mV.
3. Results
3.1. Sensor Characterization
3.1.1. Volume of Modifier
3.1.2. Repeatability and Reproducibility
3.1.3. Stability of Sensor
3.2. Aceclofenac Behavior on the Surface of eCNF/CNT/NiCo-GCE
The Mechanism of the Electrochemical Reaction
3.3. Supporting Electrolyte Optimization
3.3.1. The Type of Supporting Electrolyte
3.3.2. The Concentration of Supporting Electrolyte
3.4. DPV Parameters Optimization
3.5. Accumulation Potential and Time Optimization
3.6. Interferences Study
3.7. Analytical Performance
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brogden, R.N.; Wiseman, L.R. Aceclofenac: A Review of its Pharmacodynamic Properties and Therapeutic Potential in the Treatment of Rheumatic Disorders and in Pain Management. Drugs 1996, 52, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Dooley, M.; Spencer, C.M.; Dunn, C.J. Aceclofenac: A reappraisal of its use in the management of pain and rheumatic disease. Drugs 2001, 61, 1351–1378. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Cloutier, J.-M.; Pelletierl, J.-P. Effects of Aceclofenac and Diclofenac on Synovial Inflammatory Factors in Human Osteoarthritis. Clin. Drug Investig. 1997, 14, 226–232. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeong, C.K.; Choi, S.J.; Kim, S.B.; Lee, M.H.; Ko, G.I.; Sohn, D.H. Simultaneous determination of aceclofenac and diclofenac in human plasma by narrowbore HPLC using column-switching. J. Pharm. Biomed. Anal. 2000, 23, 775–781. [Google Scholar] [CrossRef]
- Chandra, P.; Rathore, A.S.; Lohidasan, S.; Mahadik, K.R. Application of HPLC for the simultaneous determination of aceclofenac, paracetamol and tramadol hydrochloride in pharmaceutical dosage form. Sci. Pharm. 2012, 80, 337–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, B.; Auge, D.; Rau, T.; Rietbrock, S.; Brune, K.; Werner, U. Simultaneous determination of aceclofenac and three of its metabolites in human plasma by high-performance liquid chromatography. Biomed. Chromatogr. 2003, 17, 268–275. [Google Scholar] [CrossRef]
- Kokilambigai, K.S.; Lakshmi, K.S. Utilization of green analytical chemistry principles for the simultaneous estimation of paracetamol, aceclofenac and thiocolchicoside by UV spectrophotometry. Green Chem. Lett. Rev. 2021, 14, 97–105. [Google Scholar] [CrossRef]
- El Kousy, N.M. Spectrophotometric and spectrofluorimetric determination of etodolac and aceclofenac. J. Pharm. Biomed. Anal. 1999, 20, 185–194. [Google Scholar] [CrossRef]
- Villar Navarro, M.; Ramos Payán, M.; Fernández-Torres, R.; Bello-López, M.A.; Callejón Mochón, M.; Guiráum Pérez, A. Capillary electrophoresis determination of nonsteroidal anti-inflammatory drugs in wastewater using hollow fiber liquid-phase microextraction. Electrophoresis 2011, 32, 2107–2113. [Google Scholar] [CrossRef]
- Vinay, M.M.; Nayaka, Y.A.; Purushothama, H.T.; Yathisha, R.O.; Basavarajappa, K.V.; Manjunatha, P. OH functionalized Multi-Walled Carbon Nanotube modified electrode as electrochemical sensor for the detection of Aceclofenac. Int. J. Environ. Anal. Chem. 2019, 99, 1553–1564. [Google Scholar] [CrossRef]
- Posac, J.R.; Vázquez, M.D.; Tascón, M.L.; Acuña, J.A.; de la Fuente, C.; Velasco, E.; Sánchez-Batanero, P. Determination of aceclofenac using adsorptive stripping voltammetric techniques on conventional and surfactant chemically modified carbon paste electrodes. Talanta 1995, 42, 293–304. [Google Scholar] [CrossRef]
- El-Henawee, M.; Saleh, H.; Hussien, E.M.; Derar, A.R. Differential Pulse Voltammetric strategy for Simultaneous Determination of Aceclofenac and Ezogabine in Biological Fluids at Edge-plane Pyrolytic Graphite Electrode. Anal. Bioanal. Electrochem. 2021, 13, 94–110. [Google Scholar]
- Manjunatha, P.; Arthoba Nayaka, Y.; Chethana, B.K.; Vidyasagar, C.C.; Yathisha, R.O. Development of multi-walled carbon nanotubes modified pencil graphite electrode for the electrochemical investigation of aceclofenac present in pharmaceutical and biological samples. Sens. Bio-Sens. Res. 2018, 17, 7–17. [Google Scholar] [CrossRef]
- Skrzypek, S.; Smarzewska, S.; Ciesielski, W. Determination of Blasticidin S in Spiked Rice Using SW Voltammetry with a Renewable Silver Amalgam Film Electrode. Electroanalysis 2012, 24, 1153–1159. [Google Scholar] [CrossRef]
- Brycht, M.; Skrzypek, S.; Robak, J.; Guzsvány, V.; Vajdle, O.; Zbiljić, J.; Nosal-Wiercińska, A.; Guziejewski, D.; Andrijewski, G. Ultra trace level determination of fenoxanil by highly sensitive square wave adsorptive stripping voltammetry in real samples with a renewable silver amalgam film electrode. J. Electroanal. Chem. 2015, 738, 69–76. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Wasąg, J. Determination of trace amounts of Ga (III) by adsorptive stripping voltammetry with in situ plated bismuth fi lm electrode. Talanta 2015, 144, 1091–1095. [Google Scholar] [CrossRef]
- Adamczyk, M.; Grabarczyk, M. Simple and Fast Simultaneously Determination of In (III) and Ti (IV) Using Lead Modified Glassy Carbon Electrode by Stripping Voltammetry. J. Electrochem. Soc. 2020, 167, 126515. [Google Scholar] [CrossRef]
- AlAqad, K.M.; Suleiman, R.; Al Hamouz, O.C.S.; Saleh, T.A. Novel graphene modified carbon-paste electrode for promazine detection by square wave voltammetry. J. Mol. Liq. 2018, 252, 75–82. [Google Scholar] [CrossRef]
- Smajdor, J.; Piech, R.; Pięk, M.; Paczosa-Bator, B. Sensitive Voltammetric Determination of Ethinyl Estradiol on Carbon Black Modified Electrode. J. Electrochem. Soc. 2017, 164, H885–H889. [Google Scholar] [CrossRef]
- Maringa, A.; Mugadza, T.; Antunes, E.; Nyokong, T. Characterization and electrocatalytic behaviour of glassy carbon electrode modified with nickel nanoparticles towards amitrole detection. J. Electroanal. Chem. 2013, 700, 86–92. [Google Scholar] [CrossRef]
- Han, L.; Zhang, X. Simultaneous voltammetry determination of dihydroxybenzene isomers by nanogold modified electrode. Electroanalysis 2009, 21, 124–129. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, J.; Wen, Y.; Zhang, J.; Ding, W. The electro-synthesized imprinted PEDOT film as a simple voltammetric sensor for highly sensitive and selective detection of vitamin K3 in poultry drug samples. Synth. Met. 2017, 230, 79–88. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Lian, J. Electrochemical synthesis of polyaniline nano-networks on p-aminobenzene sulfonic acid functionalized glassy carbon electrode. Its use for the simultaneous determination of ascorbic acid and uric acid. Biosens. Bioelectron. 2008, 24, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Górska, A.; Paczosa-Bator, B.; Wyrwa, J.; Piech, R. New Electrochemical Sensor of Prolonged Application for Metformin Determination Based on Hydrated Ruthenium Dioxide-Carbon Black-Nafion Modified Glassy Carbon. Electroanalysis 2020, 32, 1875–1884. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, A.; Liu, Y. Development of a Glassy Carbon Electrode Modified with Graphene/Au Nanoparticles for Determination of Acetaminophen in Pharmaceutical Preparation. Russ. J. Electrochem. 2018, 54, 1141–1147. [Google Scholar] [CrossRef]
- Dong, Y.; Ding, Y.; Zhou, Y.; Chen, J.; Wang, C. Differential pulse anodic stripping voltammetric determination of Pb ion at a montmorillonites/polyaniline nanocomposite modified glassy carbon electrode. J. Electroanal. Chem. 2014, 717–718, 206–212. [Google Scholar] [CrossRef]
- Wong, A.; Santos, A.M.; Fatibello-Filho, O. Determination of piroxicam and nimesulide using an electrochemical sensor based on reduced graphene oxide and PEDOT:PSS. J. Electroanal. Chem. 2017, 799, 547–555. [Google Scholar] [CrossRef]
- Górska, A.; Zambrzycki, M.; Paczosa-Bator, B.; Piech, R. New Electrochemical Sensor Based on Hierarchical Carbon Nanofibers with NiCo Nanoparticles and Its Application for Cetirizine Hydrochloride Determination. Materials 2022, 15, 3648. [Google Scholar] [CrossRef]


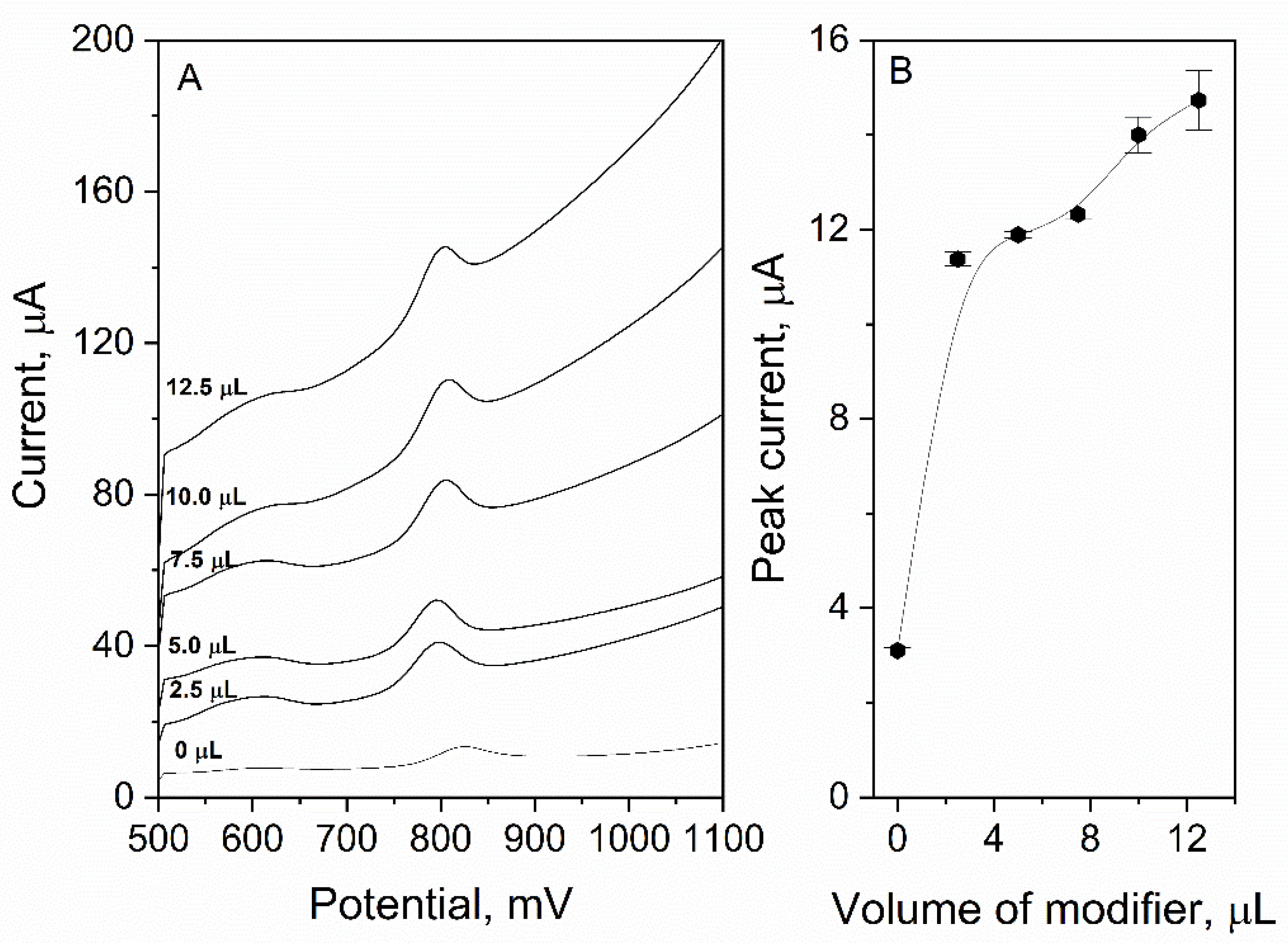
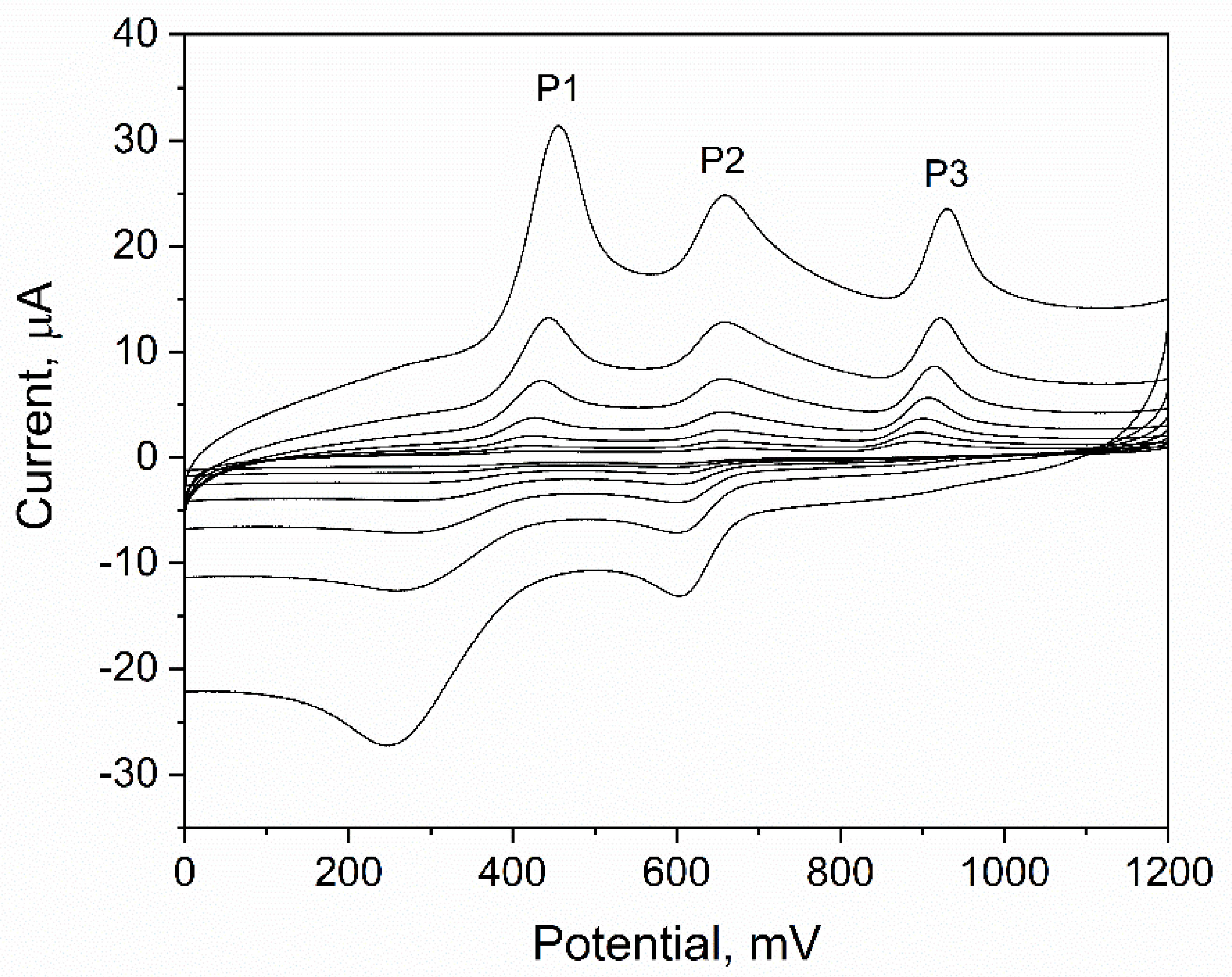
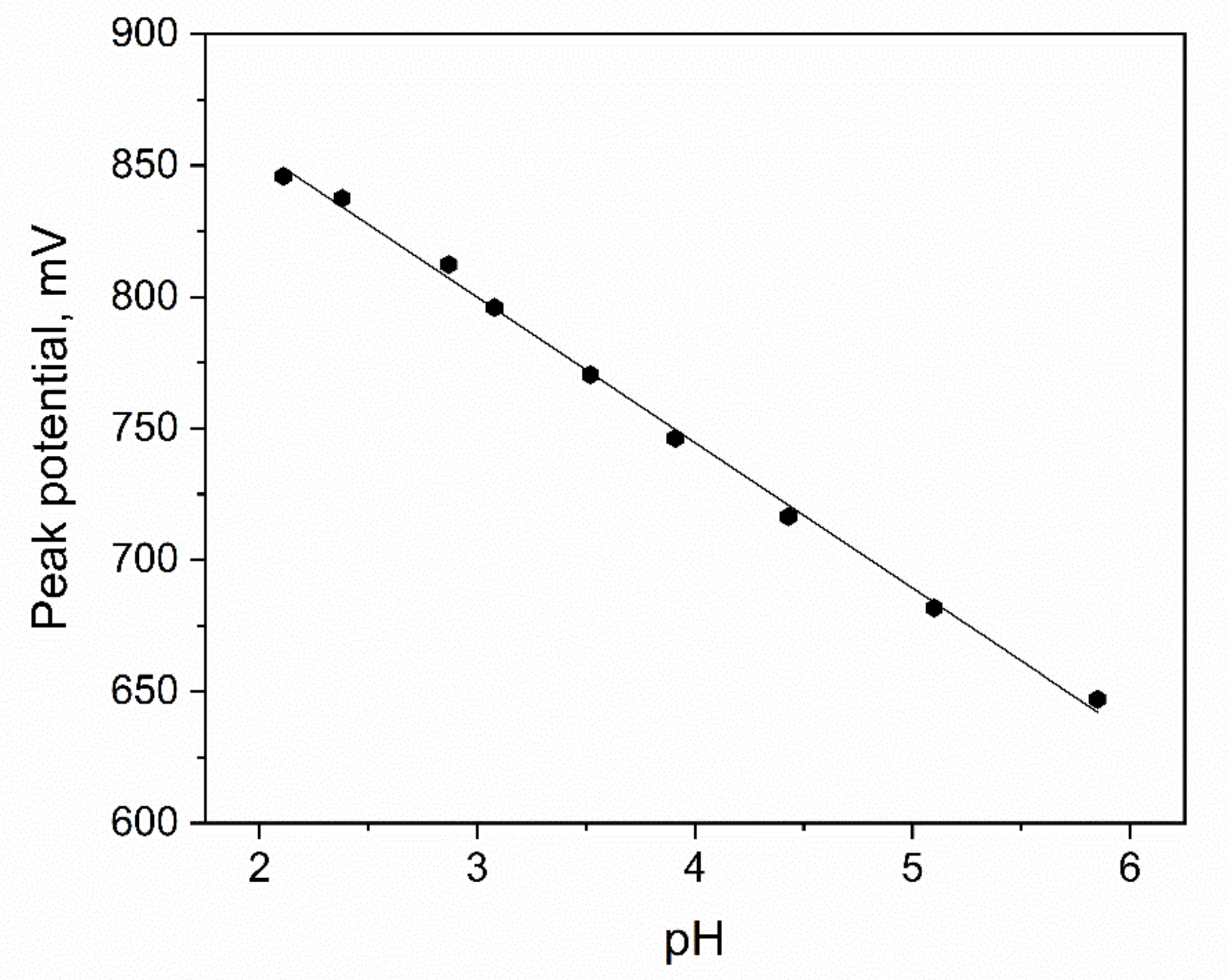


| Type of Working Electrode | LOD, nM | LOQ, nM | Reference |
|---|---|---|---|
| OH-MWCNT/CPE | 246 | Not specified | [10] |
| CMCPE | 19.3 | Not specified | [11] |
| EPG | Not specified | 70 | [12] |
| MCPGE | 2.6 | Not specified | [13] |
| eCNF/CNT/NiCo-GCE | 0.7 | 2.1 | This work |
| Sample | ACL Added, mg | ACL Found ± s, mg, (Recovery, %) |
|---|---|---|
| Tablet a | 0 | 110 ± 4 |
| 100 | 220 ± 2 (103) | |
| 200 | 325 ± 7 (103) | |
| Sample | ACL added, µM | ACL found ± s, µM, (recovery, %) |
| Urine diluted 20× | 0 | ND |
| 0.2 | 0.19 ± 0.01 (98) | |
| 0.4 | 0.39 ± 0.02 (99) | |
| Urine diluted 10× | 0 | ND |
| 0.2 | 0.21 ± 0.01 (105) | |
| 0.4 | 0.42 ± 0.03 (105) | |
| Plasma diluted 33× | 0 | ND |
| 0.2 | 0.19 ± 0.02 (98) | |
| 0.4 | 0.42 ± 0.03 (105) | |
| Plasma diluted 20× | 0 | ND |
| 0.3 | 0.29 ± 0.02 (98) | |
| 0.6 | 0.63 ± 0.05 (104) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górska, A.; Paczosa-Bator, B.; Gaidukevič, J.; Piech, R. Development of a New Voltammetric Method for Aceclofenac Determination on Glassy Carbon Electrode Modified with Hierarchical Nanocomposite. Sensors 2022, 22, 8954. https://doi.org/10.3390/s22228954
Górska A, Paczosa-Bator B, Gaidukevič J, Piech R. Development of a New Voltammetric Method for Aceclofenac Determination on Glassy Carbon Electrode Modified with Hierarchical Nanocomposite. Sensors. 2022; 22(22):8954. https://doi.org/10.3390/s22228954
Chicago/Turabian StyleGórska, Anna, Beata Paczosa-Bator, Justina Gaidukevič, and Robert Piech. 2022. "Development of a New Voltammetric Method for Aceclofenac Determination on Glassy Carbon Electrode Modified with Hierarchical Nanocomposite" Sensors 22, no. 22: 8954. https://doi.org/10.3390/s22228954
APA StyleGórska, A., Paczosa-Bator, B., Gaidukevič, J., & Piech, R. (2022). Development of a New Voltammetric Method for Aceclofenac Determination on Glassy Carbon Electrode Modified with Hierarchical Nanocomposite. Sensors, 22(22), 8954. https://doi.org/10.3390/s22228954









