Identification of Early Heat and Water Stress in Strawberry Plants Using Chlorophyll-Fluorescence Indices Extracted via Hyperspectral Images
Abstract
1. Introduction
2. Materials and Methods
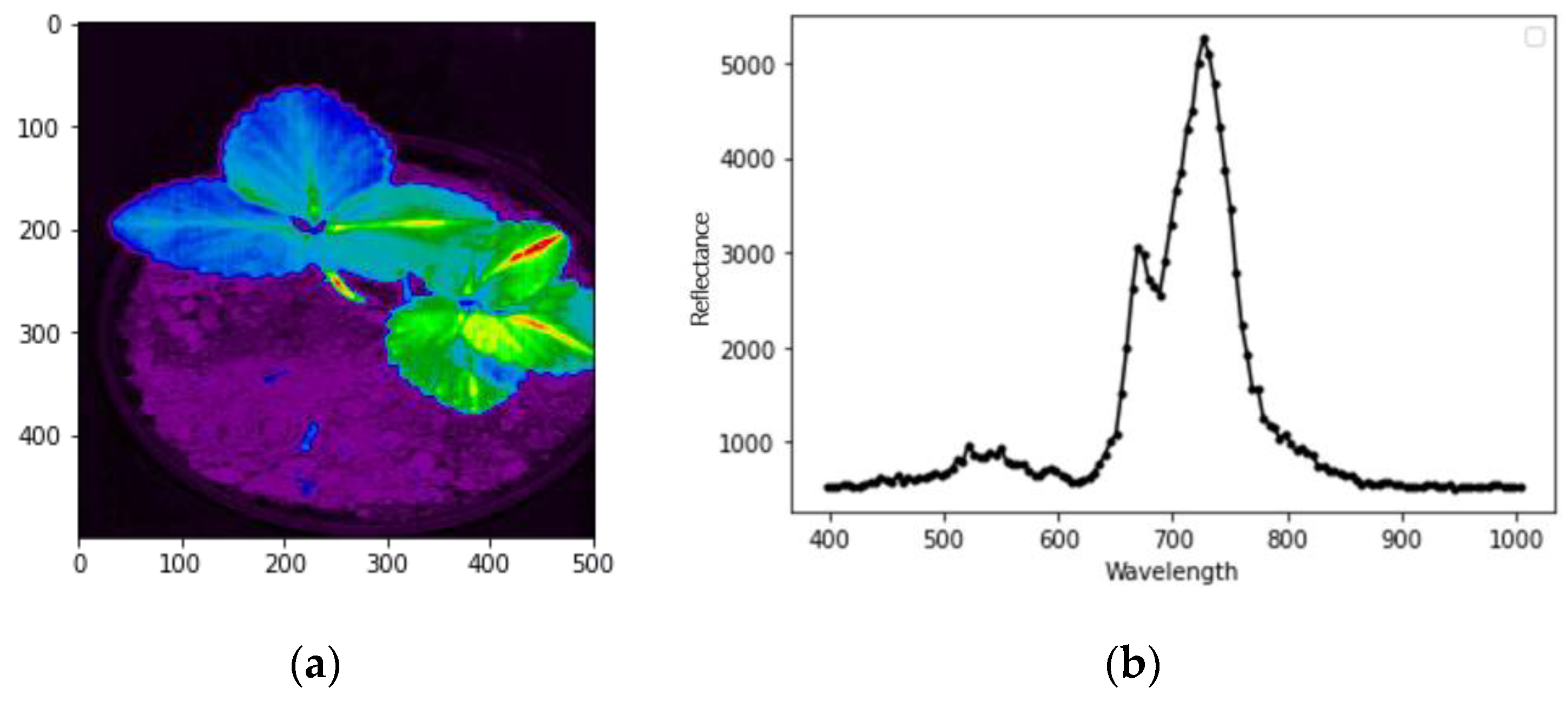
2.1. Imaging
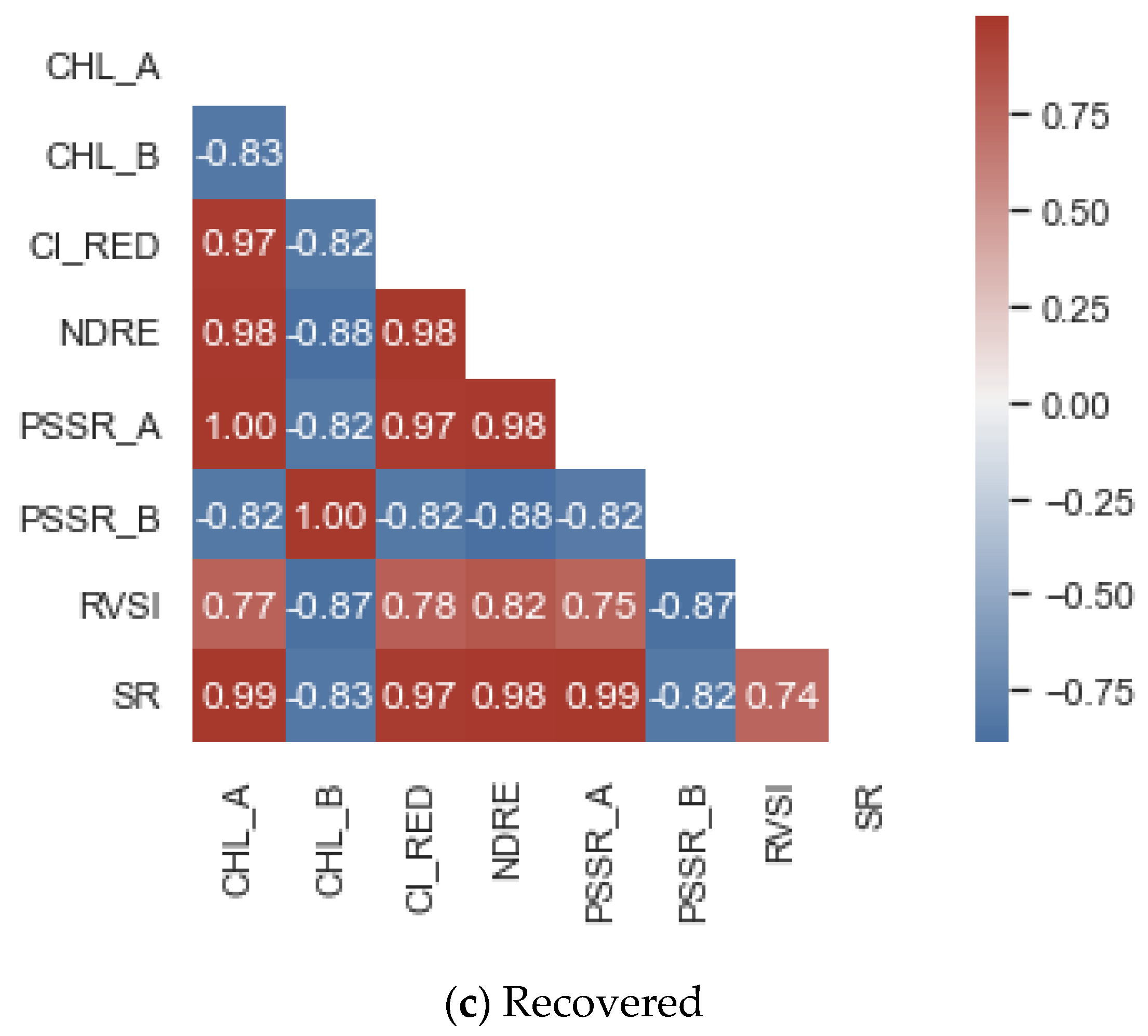
2.2. Chlorophyll-Fluorescence Indices
2.2.1. Pigment-Specific Normalized Difference for Chlorophyll A (PSNDa)
2.2.2. Pigment-Specific Normalized Difference for Chlorophyll B (PSNDb)
2.2.3. Pigment-Specific Simple Ratio for Chlorophyll A (PSSRa)
2.2.4. Pigment-Specific Simple Ratio for Chlorophyll B (PSSRb)
2.2.5. Chlorophyll Index at Red Edge (CI-RedEdge)
2.2.6. Normalized-Difference Red-Edge Index (NDRE)
2.2.7. Simple Ratio (SR)
2.2.8. Red-Edge Vegetation-Stress Index (RVSI)
2.3. System Configuration and Packages Used
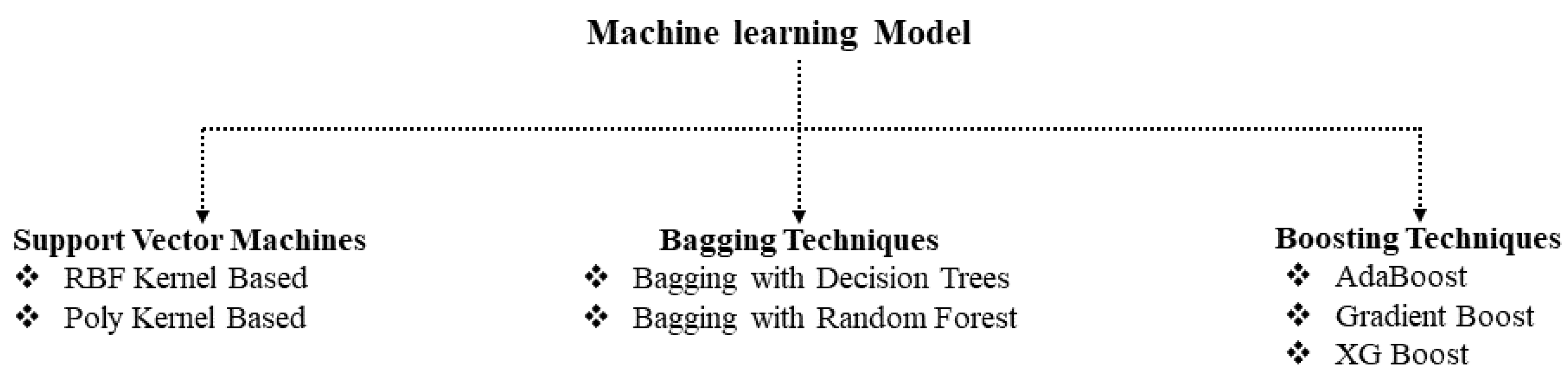
2.4. Machine-Learning Methods
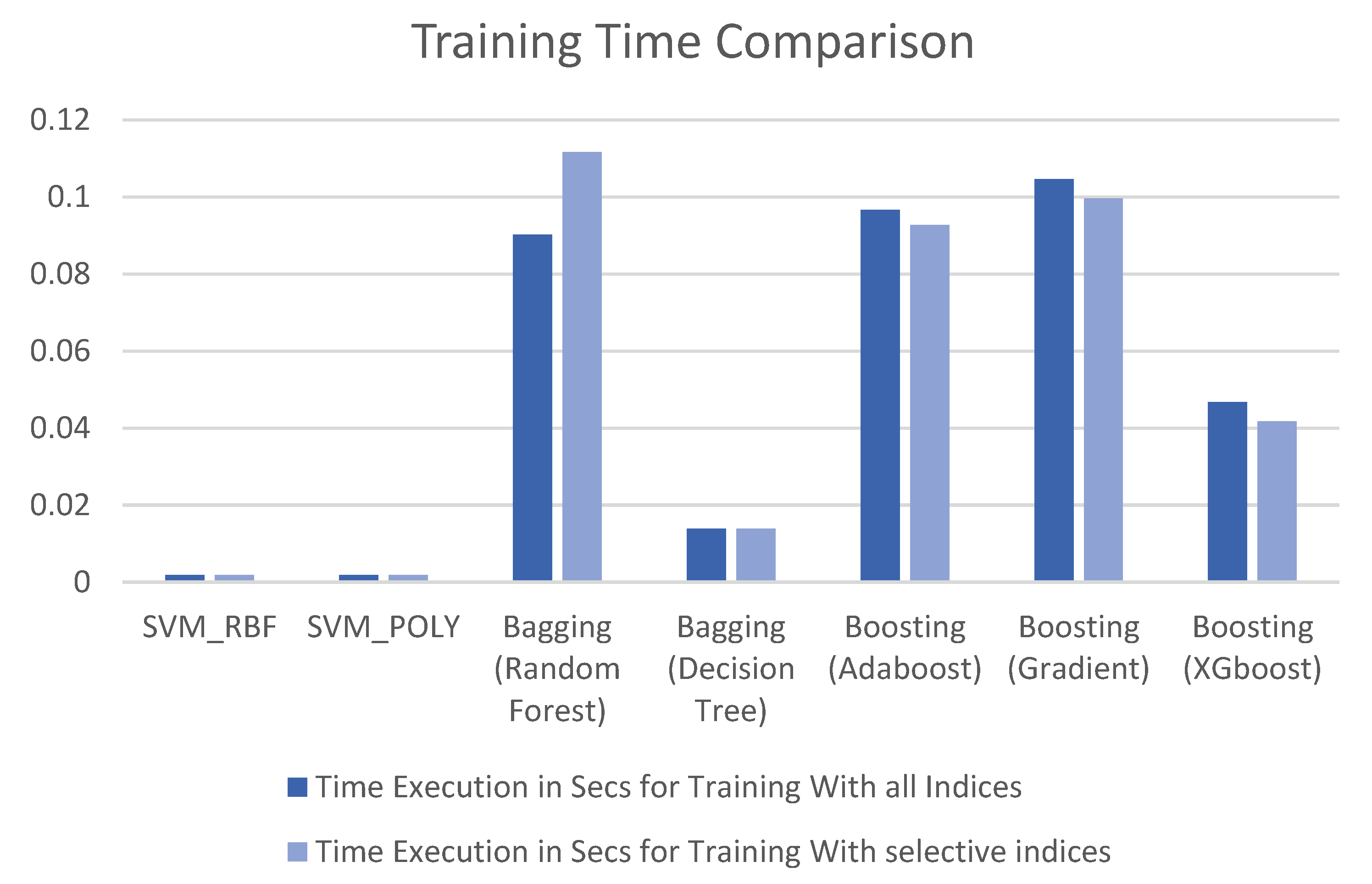
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kesici, M.; Gulen, H.; Ergin, S.; Turhan, E.; Ipek, A.; Koksal, N. Heat-Stress Tolerance of Some Strawberry (Fragaria × Ananassa) Cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 244–249. [Google Scholar] [CrossRef]
- Rodríguez, E.; Arqués, J.L.; Rodríguez, R.; Nuñez, M.; Medina, M.; Talarico, T.L.; Casas, I.A.; Chung, T.C.; Dobrogosz, W.J.; Axelsson, L.; et al. The Effect of Climate Change on Abiotic Plant Stress: A Review. Intech 1989, 32, 137–144. [Google Scholar]
- Kadir, S.; Sidhu, G.; Al-Khatib, K. Strawberry (Fragaria Xananassa Duch.) Growth and Productivity as Affected by Temperature. HortScience 2006, 41, 1423–1430. [Google Scholar] [CrossRef]
- Klamkowski, K.; Treder, W. Morphological and Physiological Responses of Strawberry Plants to Water Stress. Agric. Conspec. Sci. 2006, 71, 159–165. [Google Scholar]
- Li, H.; Li, T.; Gordon, R.J.; Asiedu, S.K.; Hu, K. Strawberry Plant Fruiting Efficiency and Its Correlation with Solar Irradiance, Temperature and Reflectance Water Index Variation. Environ. Exp. Bot. 2010, 68, 165–174. [Google Scholar] [CrossRef]
- Cordoba-Novoa, H.A.; Pérez-Trujillo, M.M.; Cruz Rincón, B.E.; Flórez-Velasco, N.; Magnitskiy, S.; Moreno Fonseca, L.P. Shading Reduces Water Deficits in Strawberry (Fragaria X Ananassa) Plants during Vegetative Growth. Int. J. Fruit Sci. 2022, 22, 725–740. [Google Scholar] [CrossRef]
- Zulini, L.; Fischer, C.; Bertamini, M. Chlorophyll Fluorescence as a Tool for Evaluation of Viability in Freeze-Stressed Grapevine Buds. Photosynthetica 2010, 48, 317–319. [Google Scholar] [CrossRef]
- Liu, F.; Savić, S.; Jensen, C.R.; Shahnazari, A.; Jacobsen, S.E.; Stikić, R.; Andersen, M.N. Water Relations and Yield of Lysimeter-Grown Strawberries under Limited Irrigation. Sci. Hortic. 2007, 111, 128–132. [Google Scholar] [CrossRef]
- Ledesma, N.A.; Nakata, M.; Sugiyama, N. Effect of High Temperature Stress on the Reproductive Growth of Strawberry Cvs. “Nyoho” and “Toyonoka”. Sci. Hortic. 2008, 116, 186–193. [Google Scholar] [CrossRef]
- Abdelrahman, M. Growth and Productivity of Strawberry Cultivars at High Temperatures (Heat Stress, Fragaria); Kansas State University: Manhattan, KS, USA, 1984. [Google Scholar]
- Hasanuzzaman, M. Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives II: Mechanisms of Adaptation and Stress Amelioration; Springer Nature: Cham, Switzerland, 2020; ISBN 9789811521720. [Google Scholar]
- ElMasry, G.; Wang, N.; ElSayed, A.; Ngadi, M. Hyperspectral Imaging for Nondestructive Determination of Some Quality Attributes for Strawberry. J. Food Eng. 2007, 81, 98–107. [Google Scholar] [CrossRef]
- Hennessy, A.; Clarke, K.; Lewis, M. Hyperspectral Classification of Plants: A Review of Waveband Selection Generalisability. Remote Sens. 2020, 12, 113. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Couture, J.J. Hyperspectral Assessment of Plant Responses to Multi-Stress Environments: Prospects for Managing Protected Agrosystems. Plants People Planet 2020, 2, 244–258. [Google Scholar] [CrossRef]
- Park, E.; Kim, Y.S.; Omari, M.K.; Suh, H.K.; Faqeerzada, M.A.; Kim, M.S.; Baek, I.; Cho, B.K. High-Throughput Phenotyping Approach for the Evaluation of Heat Stress in Korean Ginseng (Panax Ginseng Meyer) Using a Hyperspectral Reflectance Image. Sensors 2021, 21, 5634. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wu, G.; Tian, C.; Li, N.; Yang, H.; Bai, Y.; Zhang, B. Hyperspectral Imaging for Early Identification of Strawberry Leaves Diseases with Machine Learning and Spectral Fingerprint Features. Infrared Phys. Technol. 2021, 118, 103898. [Google Scholar] [CrossRef]
- Na, Y.W.; Jeong, H.J.; Lee, S.Y.; Choi, H.G.; Kim, S.H.; Rho, I.R. Chlorophyll Fluorescence as a Diagnostic Tool for Abiotic Stress Tolerance in Wild and Cultivated Strawberry Species. Hortic. Environ. Biotechnol. 2014, 55, 280–286. [Google Scholar] [CrossRef]
- Aasen, H.; Van Wittenberghe, S.; Medina, N.S.; Damm, A.; Goulas, Y.; Wieneke, S.; Hueni, A.; Malenovský, Z.; Alonso, L.; Pacheco-Labrador, J.; et al. Sun-Induced Chlorophyll Fluorescence II: Review of Passive Measurement Setups, Protocols, and Their Application at the Leaf to Canopy Level. Remote Sens. 2019, 11, 927. [Google Scholar] [CrossRef]
- Mo, C.; Kim, M.S.; Kim, G.; Cheong, E.J.; Yang, J.; Lim, J. Detecting Drought Stress in Soybean Plants Using Hyperspectral Fluorescence Imaging. J. Biosyst. Eng. 2015, 40, 335–344. [Google Scholar] [CrossRef]
- Bauriegel, E.; Giebel, A.; Herppich, W.B. Hyperspectral and Chlorophyll Fluorescence Imaging to Analyse the Impact of Fusarium Culmorum on the Photosynthetic Integrity of Infected Wheat Ears. Sensors 2011, 11, 3765–3779. [Google Scholar] [CrossRef]
- Delalieux, S.; Auwerkerken, A.; Verstraeten, W.W.; Somers, B.; Valcke, R.; Lhermitte, S.; Keulemans, J.; Coppin, P. Hyperspectral Reflectance and Fluorescence Imaging to Detect Scab Induced Stress in Apple Leaves. Remote Sens. 2009, 1, 858–874. [Google Scholar] [CrossRef]
- Zhao, T.; Nakano, A.; Iwaski, Y.; Umeda, H. Application of Hyperspectral Imaging for Assessment of Tomato Leaf Water Status in Plant Factories. Appl. Sci. 2020, 10, 4665. [Google Scholar] [CrossRef]
- Zhu, N.; Wang, G.; Yang, G.; Dai, W. A Fast 2D Otsu Thresholding Algorithm Based on Improved Histogram. In Proceedings of the 2009 Chinese Conference on Pattern Recognition, (CCPR 2009) and the First CJK Joint Workshop on Pattern Recognition (CJKPR), Nanjing, China, 4–6 November 2009; pp. 319–323. [Google Scholar] [CrossRef]
- Blackburn, G.A. Relationships between Spectral Reflectance and Pigment Concentrations in Stacks of Deciduous Broadleaves. Remote Sens. Environ. 1999, 70, 224–237. [Google Scholar] [CrossRef]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.D.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T.; et al. Coincident Detection of Crop Water Stress, Nitrogen Status and Canopy Density Using Ground Based Multispectral Data. In Proceedings of the Fifth International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000. [Google Scholar]
- Roncagliolo, P.A.; García, J.G.; Muravchik, C.H. Optimized Carrier Tracking Loop Design for Real-Time High-Dynamics GNSS Receivers. Int. J. Navig. Obs. 2012, 50, 663–666. [Google Scholar] [CrossRef]
- Huntington, J.; Miller, J.J.; Merton, R.; Huntington, J. Early simulation results of the ARIES-1 satellite sensor for multi-temporal vegetation research derived from AVIRIS. In Proceedings of the Eighth Annual JPL Airborne Earth Science Workshop, Pasadena, CA, USA, 4–8 March 1996. [Google Scholar]
- Tien Bui, D.; Pradhan, B.; Lofman, O.; Revhaug, I. Landslide Susceptibility Assessment in Vietnam Using Support Vector Machines, Decision Tree, and Nave Bayes Models. Math. Probl. Eng. 2012, 2012, 974638. [Google Scholar] [CrossRef]





| Type | Count | Resolution |
|---|---|---|
| Controlled | 45 | 502 × 500 × 128 |
| stressed | 43 | 502 × 500 × 128 |
| Recovered | 43 | 502 × 500 × 128 |
| Models and Scores | Class | SVM | Bagging | Boosting | ||||
|---|---|---|---|---|---|---|---|---|
| RBF | Polynomial | Decision Tree | Random Forest | Adaboost | Gradient Boosting | XG Boosting | ||
| Precision | Controlled | 0.67 | 1.00 | 1.00 | 1.00 | 1.00 | 0.86 | 0.88 |
| Stressed | 0.56 | 0.50 | 0.44 | 0.80 | 0.35 | 0.58 | 0.64 | |
| Recovered | 0.73 | 0.71 | 0.62 | 1.00 | 0.00 | 0.86 | 0.86 | |
| Recall | Controlled | 0.46 | 0.15 | 0.85 | 0.92 | 0.92 | 0.46 | 0.54 |
| Stressed | 0.62 | 0.88 | 0.50 | 1.00 | 0.88 | 0.88 | 0.88 | |
| Recovered | 0.92 | 1.00 | 0.67 | 0.92 | 0.00 | 1.00 | 1.00 | |
| F1 Score | Controlled | 0.55 | 0.27 | 0.92 | 0.96 | 0.96 | 0.60 | 0.67 |
| Stressed | 0.59 | 0.64 | 0.47 | 0.89 | 0.50 | 0.70 | 0.74 | |
| Recovered | 0.81 | 0.83 | 0.64 | 0.96 | 0.00 | 0.92 | 0.92 | |
| Overall Accuracy | 0.67 | 0.64 | 0.70 | 0.94 | 0.57 | 0.76 | 0.79 | |
| Models and Scores | Class | SVM | Bagging | Boosting | ||||
|---|---|---|---|---|---|---|---|---|
| RBF | Polynomial | Decision Tree | Random Forest | Adaboost | Gradient Boosting | XG Boosting | ||
| Precision | Controlled | 0.67 | 1.00 | 1.00 | 0.91 | 1.00 | 0.92 | 0.86 |
| Stressed | 0.56 | 0.50 | 0.73 | 0.78 | 0.35 | 0.78 | 0.58 | |
| Recovered | 0.73 | 0.71 | 0.79 | 0.85 | 0.00 | 1.00 | 0.86 | |
| Recall | Controlled | 0.46 | 0.15 | 0.62 | 0.77 | 0.92 | 0.85 | 0.46 |
| Stressed | 0.62 | 0.88 | 1.00 | 0.88 | 0.88 | 0.88 | 0.88 | |
| Recovered | 0.92 | 1.00 | 0.92 | 0.92 | 0.00 | 1.00 | 1.00 | |
| F1 Score | Controlled | 0.55 | 0.27 | 0.76 | 0.83 | 0.96 | 0.88 | 0.60 |
| Stressed | 0.59 | 0.64 | 0.84 | 0.82 | 0.50 | 0.82 | 0.70 | |
| Recovered | 0.81 | 0.83 | 0.85 | 0.88 | 0.00 | 1.00 | 0.92 | |
| Overall Accuracy | 0.67 | 0.64 | 0.82 | 0.85 | 0.57 | 0.91 | 0.76 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poobalasubramanian, M.; Park, E.-S.; Faqeerzada, M.A.; Kim, T.; Kim, M.S.; Baek, I.; Cho, B.-K. Identification of Early Heat and Water Stress in Strawberry Plants Using Chlorophyll-Fluorescence Indices Extracted via Hyperspectral Images. Sensors 2022, 22, 8706. https://doi.org/10.3390/s22228706
Poobalasubramanian M, Park E-S, Faqeerzada MA, Kim T, Kim MS, Baek I, Cho B-K. Identification of Early Heat and Water Stress in Strawberry Plants Using Chlorophyll-Fluorescence Indices Extracted via Hyperspectral Images. Sensors. 2022; 22(22):8706. https://doi.org/10.3390/s22228706
Chicago/Turabian StylePoobalasubramanian, Mangalraj, Eun-Sung Park, Mohammad Akbar Faqeerzada, Taehyun Kim, Moon Sung Kim, Insuck Baek, and Byoung-Kwan Cho. 2022. "Identification of Early Heat and Water Stress in Strawberry Plants Using Chlorophyll-Fluorescence Indices Extracted via Hyperspectral Images" Sensors 22, no. 22: 8706. https://doi.org/10.3390/s22228706
APA StylePoobalasubramanian, M., Park, E.-S., Faqeerzada, M. A., Kim, T., Kim, M. S., Baek, I., & Cho, B.-K. (2022). Identification of Early Heat and Water Stress in Strawberry Plants Using Chlorophyll-Fluorescence Indices Extracted via Hyperspectral Images. Sensors, 22(22), 8706. https://doi.org/10.3390/s22228706







