Parasitic Effects on Electrical Bioimpedance Systems: Critical Review
Abstract
1. Introduction
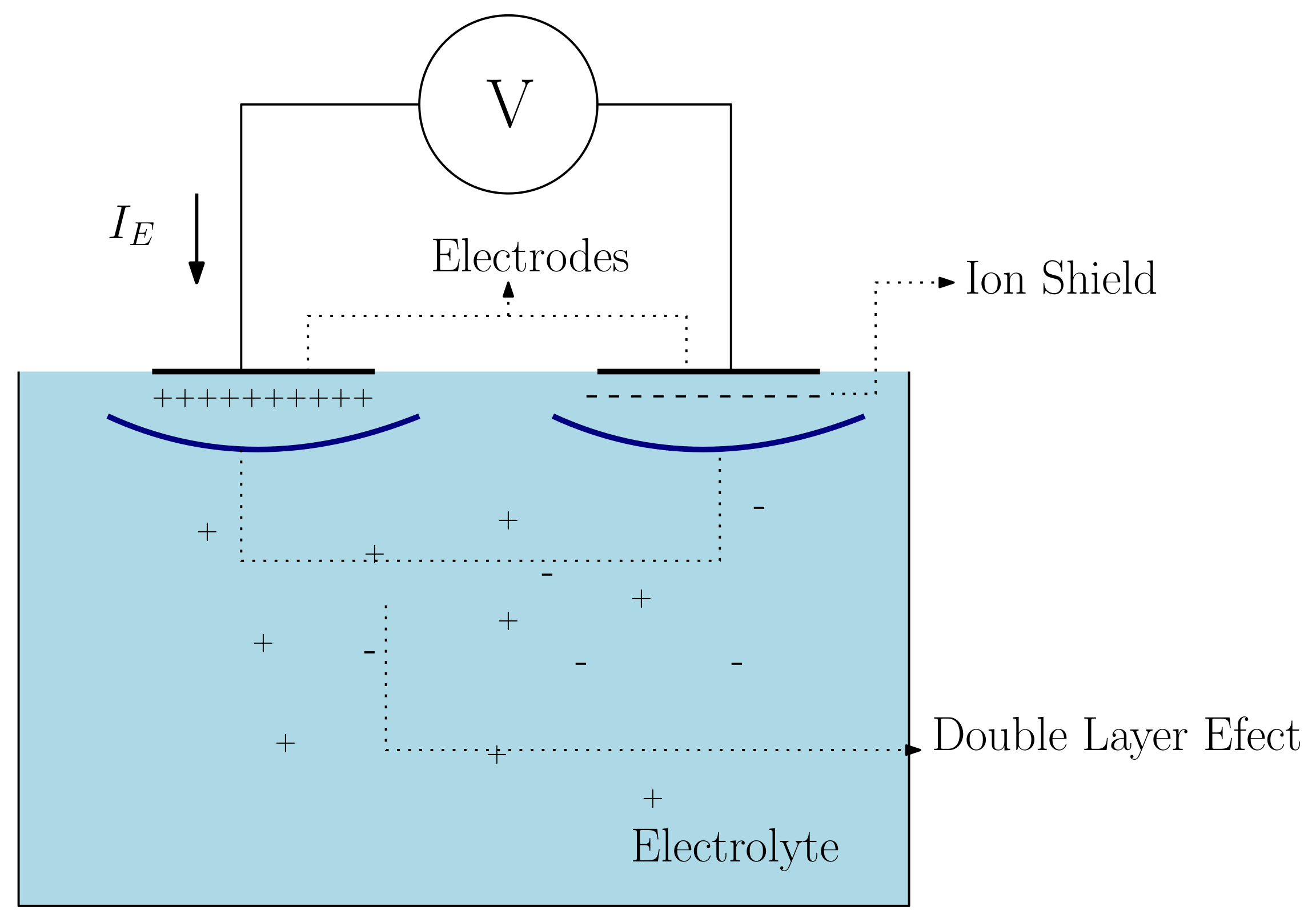
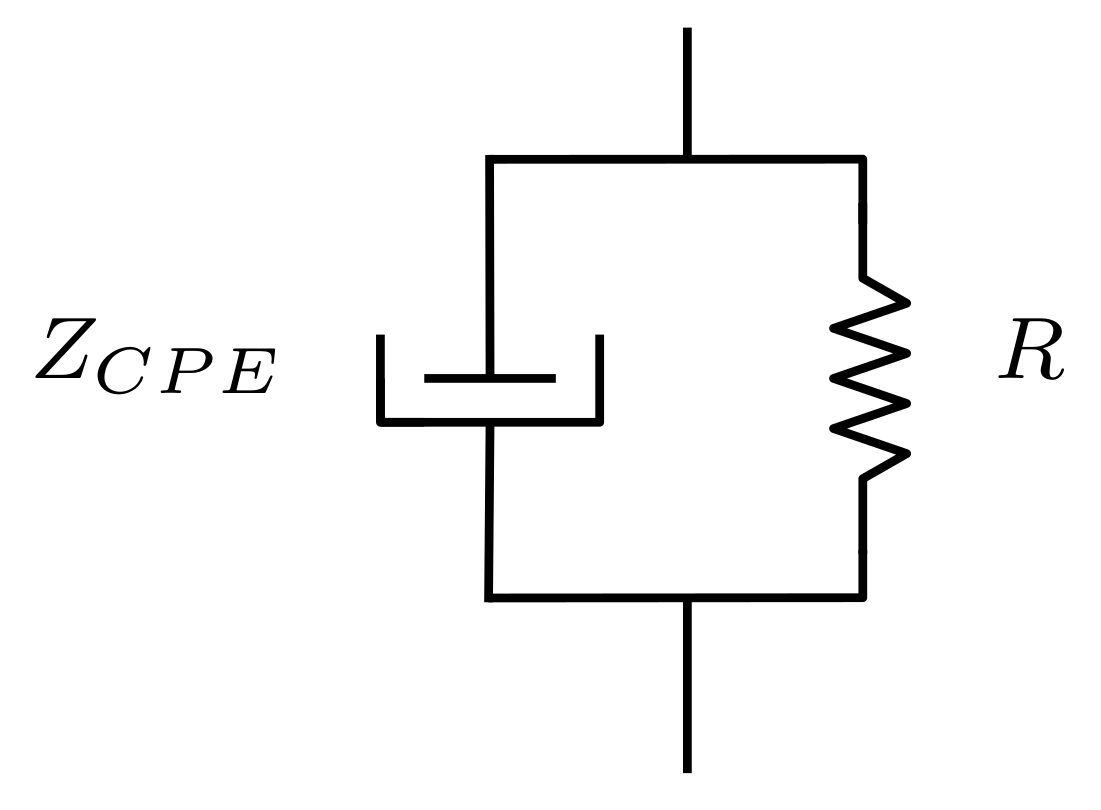
2. Electrode Capacitance Effects
3. Parasitic Capacitance in Measurement Systems
4. Parasitic Capacitance Reduction Techniques
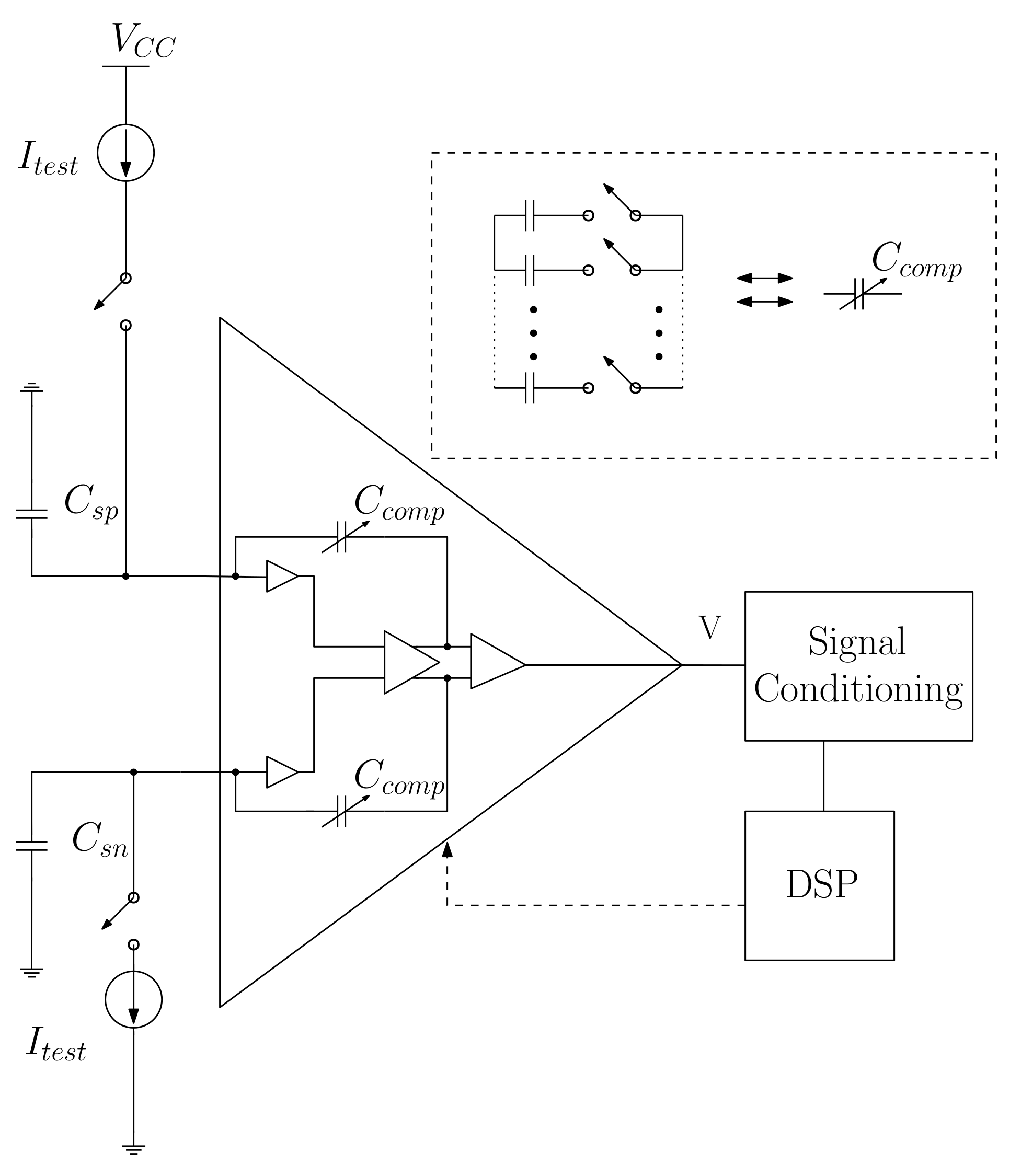
4.1. Compensating the Voltage Measured in the Charge
- Using the installed instrumentation’s electronics by discarding the use of new operational amplifiers in the measurement system and by reducing the area and the production costs of the integrated circuit;
- Compensating for the system’s low influence () in the amplifier’s Power Source Rejection Ratio (PSRR), Common Mode Rejection Ratio (CMRR) and Total Harmonic Distortion (THD). In addition, open loop gain and frequency band remain unchanged;
- Increasing the input impedance by two decades in the range [49], considering that it can reach 100 GΩ at low frequencies;
- Reducing power consumption of the amplifier.
- This compensation model disregards the intrinsic capacitances of transistors. These capacitances reduce the transconductance characteristics of the circuit at relatively high frequencies (10 kHz);
- Temperature drift affects the open-loop gain of the OpAmp due to internal changes in the transistors’ transconductance. Furthermore, MOS transistors’ transconductances are a function of the drain current [43], which subsequently influences the open-loop gain of the OpAmp. These changes affect the linearity of the compensation capacitance , which impedes full compensation for a wide range of parasitic capacitances;
- Influence from the output impedance of sources. The impedance of sources during the calibration stage can introduce over/under compensation for parasitic capacitances given that the input impedance magnitude is within the GΩ range.
4.2. Excitation Current Compensation
- Removal of parasitic capacitance effects on systems where physical removal is not a viable option ;
- Reduced cost since implementation occurs at the computational level in a processing system;
- Error linked to estimates. When the value is precisely known, then the effect of the parasitic capacitance is fully ruled out, assuming the measurement system is unaffected by the action of .
- The method critically depends on prior knowledge of , since errors associated with its estimate spread errors in .
- In practical terms, is determined at fixed , frequency, which leads to compensation of errors outside the frequency at which is determined. This factor can lead to error propagation in the spectral intervals around .
4.3. Compensation by Negative Impedance Converters
- The parasitic capacitance of cables and multiplexers can be compensated, even when there is no access to the measurement system’s instrumentation;
- Low cost and easy implementation;
- It can be used in any electrode type, be it of excitation or reading.
- The NIC circuit is only capable of compensating the fixed capacitances. In cases where the parasitic capacitance changes due to frequency and/or voltage, there is a risk of introducing systematic errors to measurements due to over/under compensation;
- Using NIC can trigger instability at high frequencies.
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niari, M.R.; Eshghi, K.; Valilai, O.F. Adaptive capacity management in cloud manufacturing hyper-network platform: Case of COVID-19 equipment production. Int. J. Manag. Sci. Eng. Manag. 2022, 17, 239–258. [Google Scholar] [CrossRef]
- Ferrigno, G.; Cucino, V. Innovating and transforming during COVID-19: Insights from Italian firms. R D Manag. 2021, 51, 325–338. [Google Scholar] [CrossRef]
- Hanisch, M.; Rake, B. Repurposing without purpose? Early innovation responses to the COVID-19 crisis: Evidence from clinical trials. R D Manag. 2021, 51, 393–409. [Google Scholar] [CrossRef]
- Liu, W.; Beltagui, A.; Ye, S. Accelerated innovation through repurposing: Exaptation of design and manufacturing in response to COVID-19. R D Manag. 2021, 51, 410–426. [Google Scholar] [CrossRef]
- Adler, A.; Amato, M.B.; Arnold, J.H.; Bayford, R.; Bodenstein, M.; Böhm, S.H.; Brown, B.H.; Frerichs, I.; Stenqvist, O.; Weiler, N.; et al. Whither lung EIT: Where are we, where do we want to go and what do we need to get there? Physiol. Meas. 2012, 33, 679–694. [Google Scholar] [CrossRef]
- Frerichs, I.; Amato, M.B.P.; van Kaam, A.H.; Tingay, D.G.; Zhao, Z.; Grychtol, B.; Bodenstein, M.; Gagnon, H.; Böhm, S.H.; Teschner, E.; et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: Consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 2017, 72, 83–93. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, D.; Bardill, A.; de Gelidi, S.; Bayford, R.; Demosthenous, A. A High Frame Rate Wearable EIT System Using Active Electrode ASICs for Lung Respiration and Heart Rate Monitoring. IEEE Trans. Circuits Syst. I Regul. Pap. 2018, 65, 3810–3820. [Google Scholar] [CrossRef]
- Tomicic, V.; Cornejo, R. Lung monitoring with electrical impedance tomography: Technical considerations and clinical applications. J. Thorac. Dis. 2019, 11, 3122–3135. [Google Scholar] [CrossRef]
- Krause, U.; Becker, K.; Hahn, G.; Dittmar, J.; Ruschewski, W.; Paul, T. Monitoring of Regional Lung Ventilation Using Electrical Impedance Tomography After Cardiac Surgery in Infants and Children. Pediatr. Cardiol. 2014, 35, 990–997. [Google Scholar] [CrossRef]
- Ivanovs, A.; Nikitenko, A.; Di Castro, M.; Torims, T.; Masi, A.; Ferre, M. Multisensor Low-Cost System for Real Time Human Detection and Remote Respiration Monitoring. In Proceedings of the Third IEEE International Conference on Robotic Computing (IRC), Naples, Italy, 25–27 February 2019; pp. 254–257. [Google Scholar] [CrossRef]
- Frerichs, I.; Schiffmann, H.; Hahn, G.; Hellige, G. Non-invasive radiation-free monitoring of regional lung ventilation in critically ill infants. Intensive Care Med. 2001, 27, 1385–1394. [Google Scholar] [CrossRef]
- Brown, B. Electrical impedance tomography (EIT): A review. J. Med Eng. Technol. 2003, 27, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Grimnes, S.; Martinsen, O. Bioimpedance and Bioelectricity Basics, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2014; Volume 4. [Google Scholar]
- Bertemes-Filho, P. Tissue Characterisation Using an Impedance Spectroscopy Probe. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2002. [Google Scholar]
- Jones, D. Constraints on tetrapolar tissue impedance measurements. Electron. Lett. 2001, 37, 1515–1517. [Google Scholar] [CrossRef]
- Newell, J.; Isaacson, D.; Mueller, J. Electrical Impedance Tomography. IEEE Trans. Med. Imaging 2002, 21, 553–554. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Bolonchuk, W.W.; Hall, C.B.; Siders, W.A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J. Appl. Physiol. 1986, 60, 1327–1332. [Google Scholar] [CrossRef]
- Jaffrin, M.Y.; Morel, H. Body fluid volumes measurements by impedance: A review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Med. Eng. Phys. 2008, 30, 1257–1269. [Google Scholar] [CrossRef]
- Kassanos, P. Bioimpedance Sensors: A Tutorial. IEEE Sens. J. 2021, 21, 22190–22219. [Google Scholar] [CrossRef]
- Koessler, L.; Colnat-Coulbois, S.; Cecchin, T.; Hofmanis, J.; Dmochowski, J.P.; Norcia, A.M.; Maillard, L.G. In-vivo measurements of human brain tissue conductivity using focal electrical current injection through intracerebral multicontact electrodes. Hum. Brain Mapp. 2017, 38, 974–986. [Google Scholar] [CrossRef]
- Cherepenin, V.; Karpov, A.; Korjenevsky, A.; Kornienko, V.; Kultiasov, Y.; Ochapkin, M.; Trochanova, O.; Meister, J. Three-dimensional EIT imaging of breast tissues: System design and clinical testing. IEEE Trans. Med Imaging 2002, 21, 662–667. [Google Scholar] [CrossRef]
- Tucker, A.S.; Fox, R.M.; Sadleir, R.J. Biocompatible, High Precision, Wideband, Improved Howland Current Source with Lead-Lag Compensation. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 63–70. [Google Scholar] [CrossRef]
- McEwan, A.; Cusick, G.; Holder, D.S. A review of errors in multi-frequency EIT instrumentation. Physiol. Meas. 2007, 28, S197–S215. [Google Scholar] [CrossRef]
- Wu, H.; Yang, Y.; Bagnaninchi, P.-O.; Jia, J. Calibrated Frequency-Difference Electrical Impedance Tomography for 3D Tissue Culture Monitoring. IEEE Sens. J. 2019, 18, 7813–7821. [Google Scholar] [CrossRef]
- Caytak, H.; Boyle, A.; Adler, A.; Bolic, M. Bioimpedance Spectroscopy Processing and Applications. Encycl. Biomed. Eng. 2014, 254–257. [Google Scholar] [CrossRef]
- Ayllon, D.; Gil-Pita, R.; Seoane, F. Detection and Classification of Measurement Errors in Bioimpedance Spectroscopy. PLoS ONE 2016, 11, e0156522. [Google Scholar] [CrossRef] [PubMed]
- Da Mata, A.M.M.; de Moura, B.F.; Martins, M.F.; Palma, F.H.S.; Ramos, R. Parasitic capacitances estimation of an Electrical Impedance Tomography data acquisition system by Bayesian inference. Measurement 2021, 174, 108992. [Google Scholar] [CrossRef]
- Aliau-Bonet, C.; Pallas-Areny, R. On the Effect of Body Capacitance to Ground in Tetrapolar Bioimpedance Measurements. IEEE Trans. Biomed. Eng. 2012, 59, 3405–3411. [Google Scholar] [CrossRef]
- Cornish, B.H.; Jacobs, A.; Thomas, B.J.; Ward, L.C. Optimizing electrode sites for segmental bioimpedance measurements. Physiol. Meas. 1999, 20, 241–250. [Google Scholar] [CrossRef]
- Hong, H.; Rahal, M.; Demosthenous, A.; Bayford, R.H. Comparison of a new integrated current source with the modified Howland circuit for EIT applications. Physiol. Meas. 2009, 30, 999–1007. [Google Scholar] [CrossRef]
- Gaggero, P.O.; Adler, A.; Brunner, J.; Seitz, P. Electrical impedance tomography system based on active electrodes. Physiol. Meas. 2012, 33, 831–847. [Google Scholar] [CrossRef]
- Oh, T.I.; Lee, K.H.; Kim, S.M.; Koo, H.; Woo, E.J.; Holder, D. Calibration methods for a multi-channel multi-frequency EIT system. Physiol. Meas. 2007, 28, 1175–1188. [Google Scholar] [CrossRef]
- Saulnier, G. Electrical Impedance Tomography: Methods, History and Applications; Chapter 2–EIT Instrumentation; IOP Publishing: Bristol, UK, 2004. [Google Scholar]
- McAdams, E.T. A Study of Electrode-Tissue Impedances Encountered in Cardiac Pacing. Ph.D. Thesis, University of Leeds, Leeds, UK, 1987. [Google Scholar]
- Breiter, M.; Kleinerman, M.; Delahay, P. Structure of the Double Layer and Electrode Processes. J. Am. Chem. Soc. 1958, 80, 5111–5117. [Google Scholar] [CrossRef]
- Conway, B.E.; Bockris, J.O.; Ammar, I.A. The dielectric constant of the solution in the diffuse and Helmholtz double layers at a charged interface in aqueous solution. Trans. Faraday Soc. (RSC Publ.) 1951, 47, 756–766. [Google Scholar] [CrossRef]
- Schmickler, W. Electronic Effects in the Electric Double Layer. Chem. Rev. 1996, 96, 3177–3200. [Google Scholar] [CrossRef]
- Parsegian, V. Van Der Waals Forces: A Handbook for Biologists, Chemists, Engineers, and Physicists; Cambridge University Press: Cambridge, UK, 2005; pp. 1–380. [Google Scholar] [CrossRef]
- Bera, T.K. Bioelectrical Impedance Methods for Noninvasive Health Monitoring: A Review. J. Med. Eng. 2014, 2014, 381251. [Google Scholar] [CrossRef]
- Karki, B.; Wi, H.; McEwan, A.; Kwon, H.; Oh, T.I.; Woo, E.J.; Seo, J.K. Evaluation of a multi-electrode bioimpedance spectroscopy tensor probe to detect the anisotropic conductivity spectra of biological tissues. Meas. Sci. Technol. 2014, 25, 075702. [Google Scholar] [CrossRef]
- Buendia, R.; Seoane, F.; Gil-Pita, R. Experimental validation of a method for removing the capacitive leakage artifact from electrical bioimpedance spectroscopy measurements. Meas. Sci. Technol. 2010, 21, 115802. [Google Scholar] [CrossRef]
- Jackson, J.D. Classical Electrodynamics, 3rd ed.; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Gray, P.R.; Paul, J.H.; Stephen, H.L.; Robert, G.M. Analysis and Design of Analog Integrated Circuits, 5th ed.; Wiley Publishing: Hoboken, NJ, USA, 2009. [Google Scholar]
- Morcelles, K.F.; Sirtoli, V.G.; Bertemes-Filho, P.; Vincence, V.C. Howland current source for high impedance load applications. Rev. Sci. Instruments 2017, 88, 114705. [Google Scholar] [CrossRef] [PubMed]
- Bertemes-Filho, P.; Felipe, A.; Vincence, V. High Accurate Howland Current Source: Output Constraints Analysis. Circuits Syst. 2013, 4, 451–458. [Google Scholar] [CrossRef]
- Zhang, F.; Teng, Z.; Zhong, H.; Yang, Y.; Li, J.; Sang, J. Wideband mirrored current source design based on differential difference amplifier for electrical bioimpedance spectroscopy. Biomed. Phys. Eng. Express 2018, 4, 025032. [Google Scholar] [CrossRef]
- Bertemes-Filho, P.; Vincence, V.C.; Santos, M.M.; Zanatta, I.X. Low power current sources for bioimpedance measurements: A comparison between Howland and OTA-based CMOS circuits. J. Electr. Bioimpedance 2012, 3, 66–73. [Google Scholar] [CrossRef]
- Kusche, R.; Kaufmann, S.; Ryschka, M. Dry electrodes for bioimpedance measurements—design, characterization and comparison. Biomed. Phys. Eng. Express 2018, 5, 015001. [Google Scholar] [CrossRef]
- Chang, C.; Onabajo, M. Instrumentation amplifier input capacitance cancellation for biopotential and bioimpedance measurements. In Proceedings of the 2014 IEEE 57th International Midwest Symposium on Circuits and Systems (MWSCAS), College Station, TX, USA, 3–6 August 2014; pp. 539–542. [Google Scholar]
- Chung, W.; Silverio, A.A.; Chang, S.; Zhou, M.; Tsai, V.F.S. A wideband current source for System-on-Chip Bio-Impedance Spectroscopy using a CCII drive and Pseudo-Resistor feedback. In Proceedings of the 2015 International Symposium on Bioelectronics and Bioinformatics (ISBB), Beijing, China, 14–17 October 2015; pp. 107–110. [Google Scholar]
- Mathews, R.J.; Freeborn, T.J. Modeling and experimental validation of parasitic capacitance effects on emulated bioimpedance measurements with high-impedance residuals. Int. J. Circuit Theory Appl. 2020, 48, 1057–1069. [Google Scholar] [CrossRef]
- Dutra, D.; Bertemes-Filho, P. Extracting parasite effects of electrical bioimpedance measurements. J. Electr. Bioimpedance 2018, 9, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Cole, K.S. Permeability and impermeability of cell membranes for ions. Cold Spring Harb. Symp. Quant. Biol. 1940, 8, 110. [Google Scholar] [CrossRef]
- McAdams, E.T.; Jossinet, J. Problems in Equivalent Circuit modelling of the Electrical Properties of Biological Tissues. Bioelectrochem. Bioenerg. 1996, 40, 147–152. [Google Scholar] [CrossRef]
- Grimnes, S.; Martinsen, O.G. Cole electrical impedance model - a critique and an alternative. IEEE Trans. Biomed. Eng. 2005, 52, 132. [Google Scholar] [CrossRef]
- Buendía, R. Hook Effect on Electrical Bioimpedance Spectroscopy Measurements. Analysis, Compensation and Correction. Master’s Thesis, University of Borås, Borås, Sweden, 2009. [Google Scholar]
- Ngo, C.; Munoz, C.; Leonhardt, S. Combination of Electrical Impedance Tomography and Forced Oscillation Technique: A new pulmonary diagnostic tool? In Proceedings of the 18th International Conference on Biomedical Applications of Electrical Impedance Tomography, Hanover, NH, USA, 21–24 June 2017; p. 21. [Google Scholar] [CrossRef]
- Bertemes-Filho, P.; Tanaka, H. An Adaptive Current Source using a Negative Impedance Converter (NIC) for Electrical Impedance Tomography (EIT). In Proceedings of the 17th International Conference of Mechanical Engineering (COBEM), Brazilian Society of Mechanical Sciences and Engineering, São Paulo City, Brazil, 10–14 November 2003; pp. 1–5. [Google Scholar]
- Tsunami, D.; McNames, J.; Colbert, A.; Pearson, S.; Hammerschlag, R. Variable frequency bioimpedance instrumentation. In Proceedings of the The 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; Volume 1, pp. 2386–2389. [Google Scholar]
- Bertemes-Filho, P.; Brown, B.; Wilson, A. A comparison of modified Howland circuits as current generators with current mirror type circuits. Physiol. Meas. 2000, 21, 1–6. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcôndes, D.W.C.; Paterno, A.S.; Bertemes-Filho, P. Parasitic Effects on Electrical Bioimpedance Systems: Critical Review. Sensors 2022, 22, 8705. https://doi.org/10.3390/s22228705
Marcôndes DWC, Paterno AS, Bertemes-Filho P. Parasitic Effects on Electrical Bioimpedance Systems: Critical Review. Sensors. 2022; 22(22):8705. https://doi.org/10.3390/s22228705
Chicago/Turabian StyleMarcôndes, David William Cordeiro, Aleksander Sade Paterno, and Pedro Bertemes-Filho. 2022. "Parasitic Effects on Electrical Bioimpedance Systems: Critical Review" Sensors 22, no. 22: 8705. https://doi.org/10.3390/s22228705
APA StyleMarcôndes, D. W. C., Paterno, A. S., & Bertemes-Filho, P. (2022). Parasitic Effects on Electrical Bioimpedance Systems: Critical Review. Sensors, 22(22), 8705. https://doi.org/10.3390/s22228705








