MEMSbased Double-Stacked Tower Biosensor Array with Integrated Readout Circuitry for Detection of Salivary pH as a Diagnostic Biomarker Applied for Chronic Periodontal Disease
Abstract
:1. Introduction
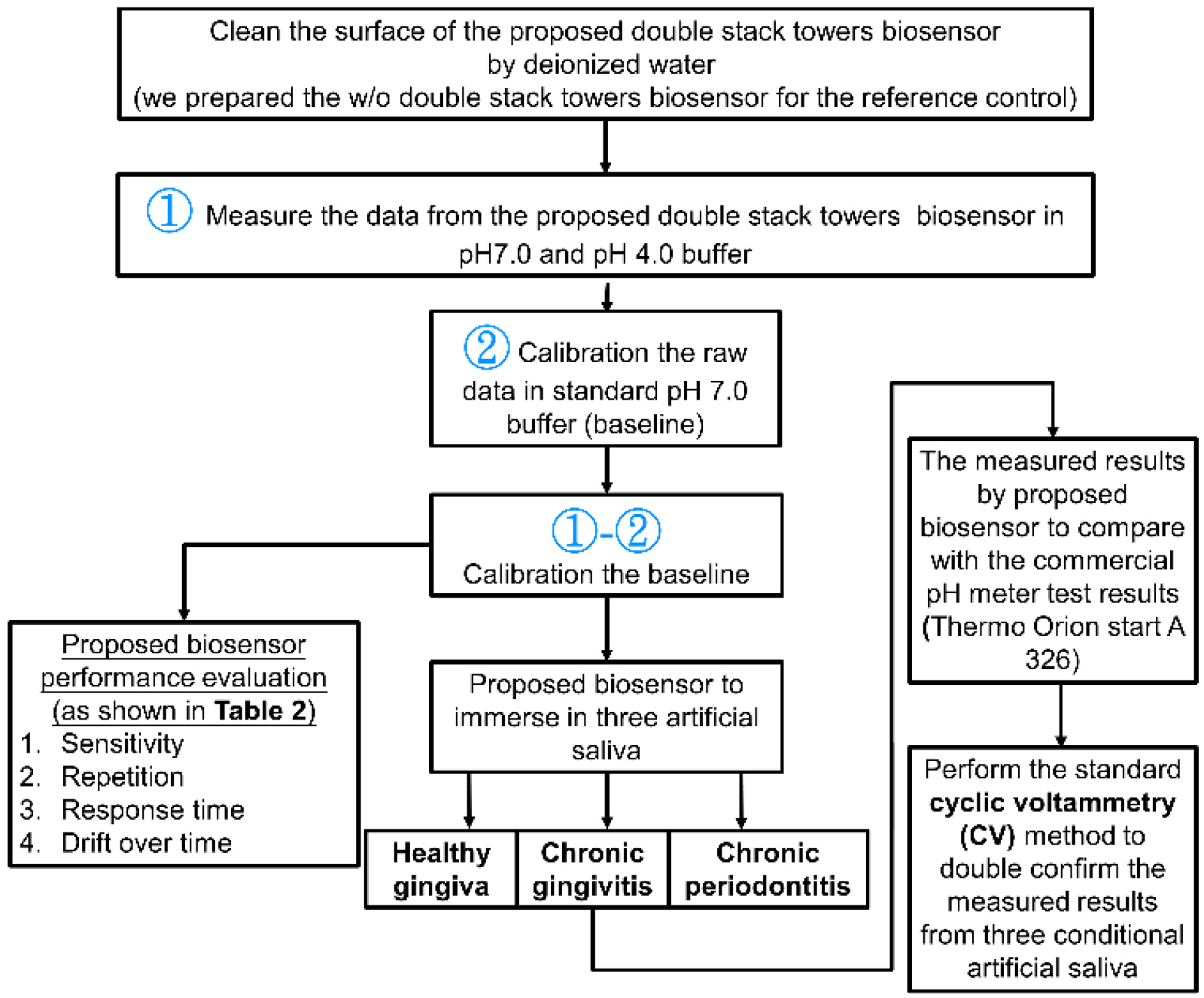
2. Materials and Methods
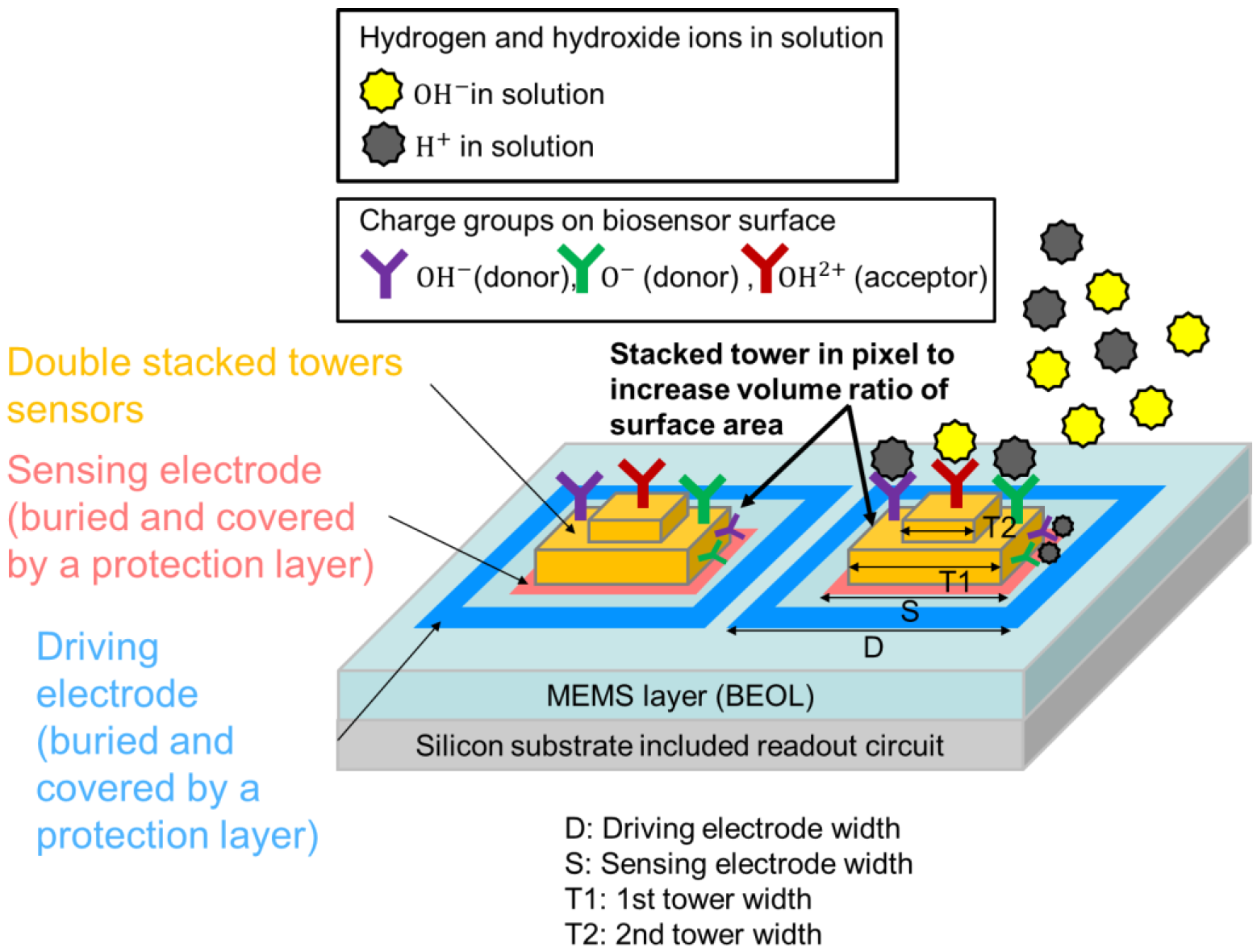
2.1. Double Stacked Tower Biosensor Pixel Design and MEMS Fabrication Flow
2.1.1. Proposed Pixel Structure
2.1.2. The Operational Principle of Electrical Field Change for Ion Binding
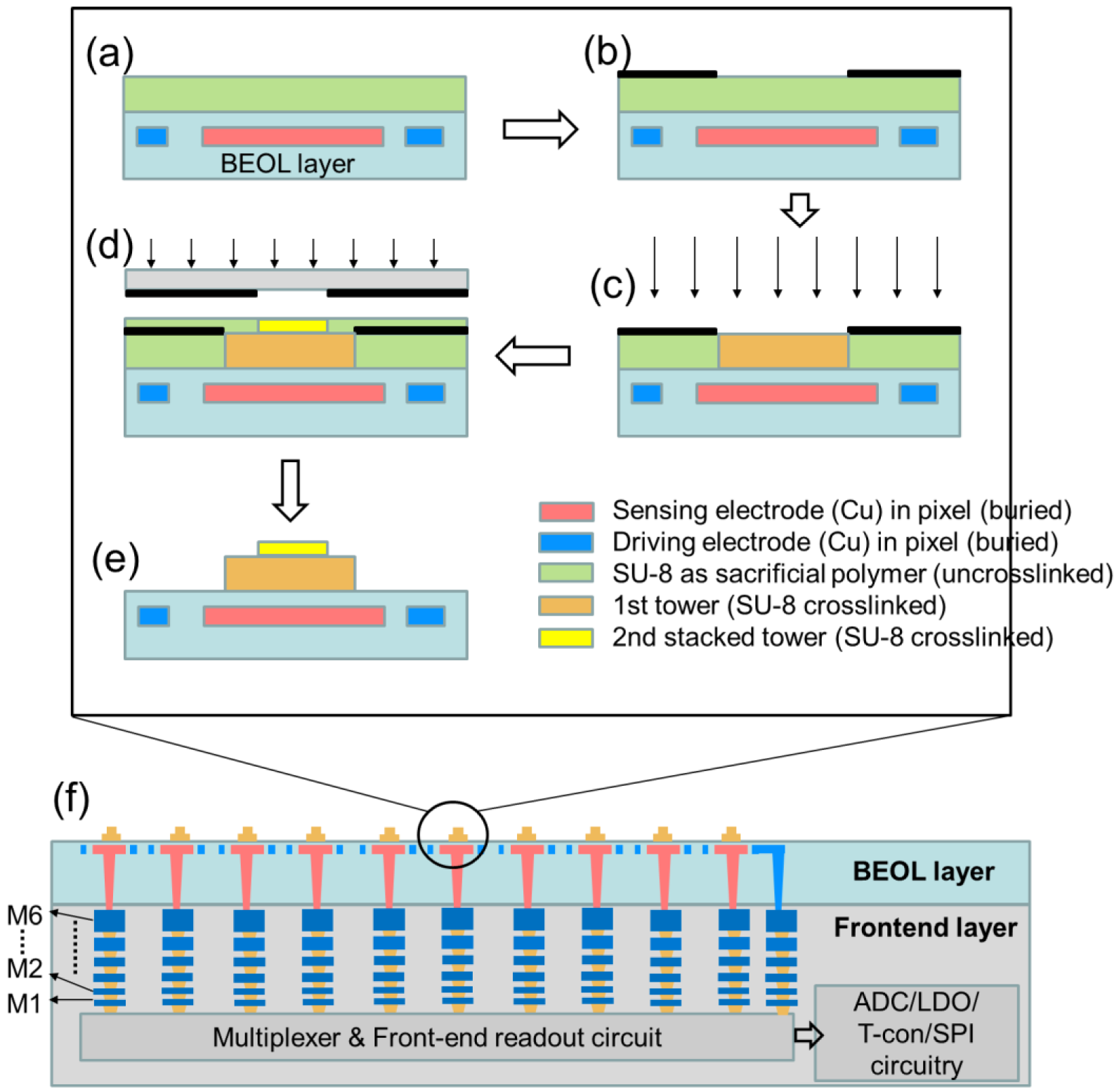
2.1.3. Fabrication Flow of Stacked Tower Biosensor Array
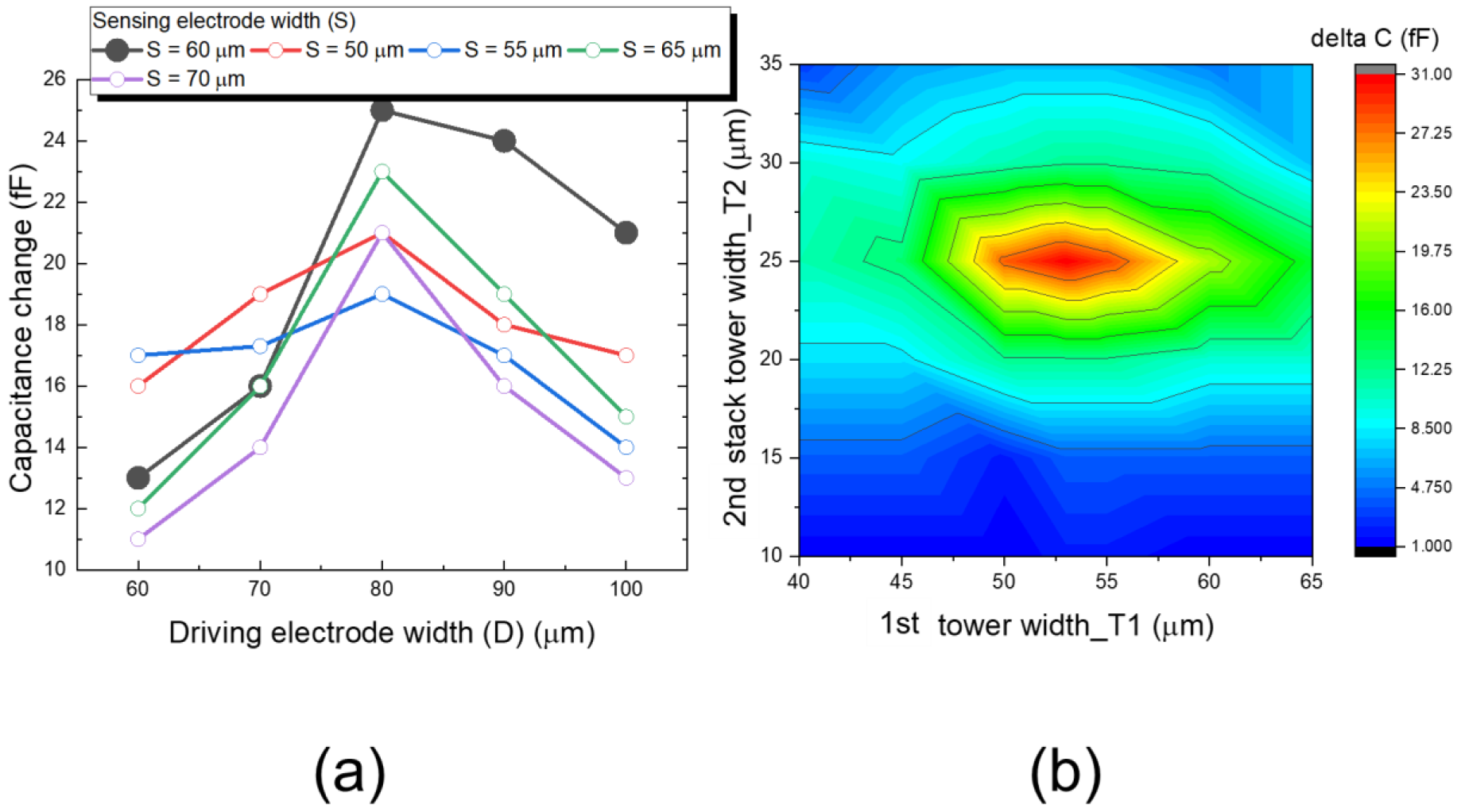
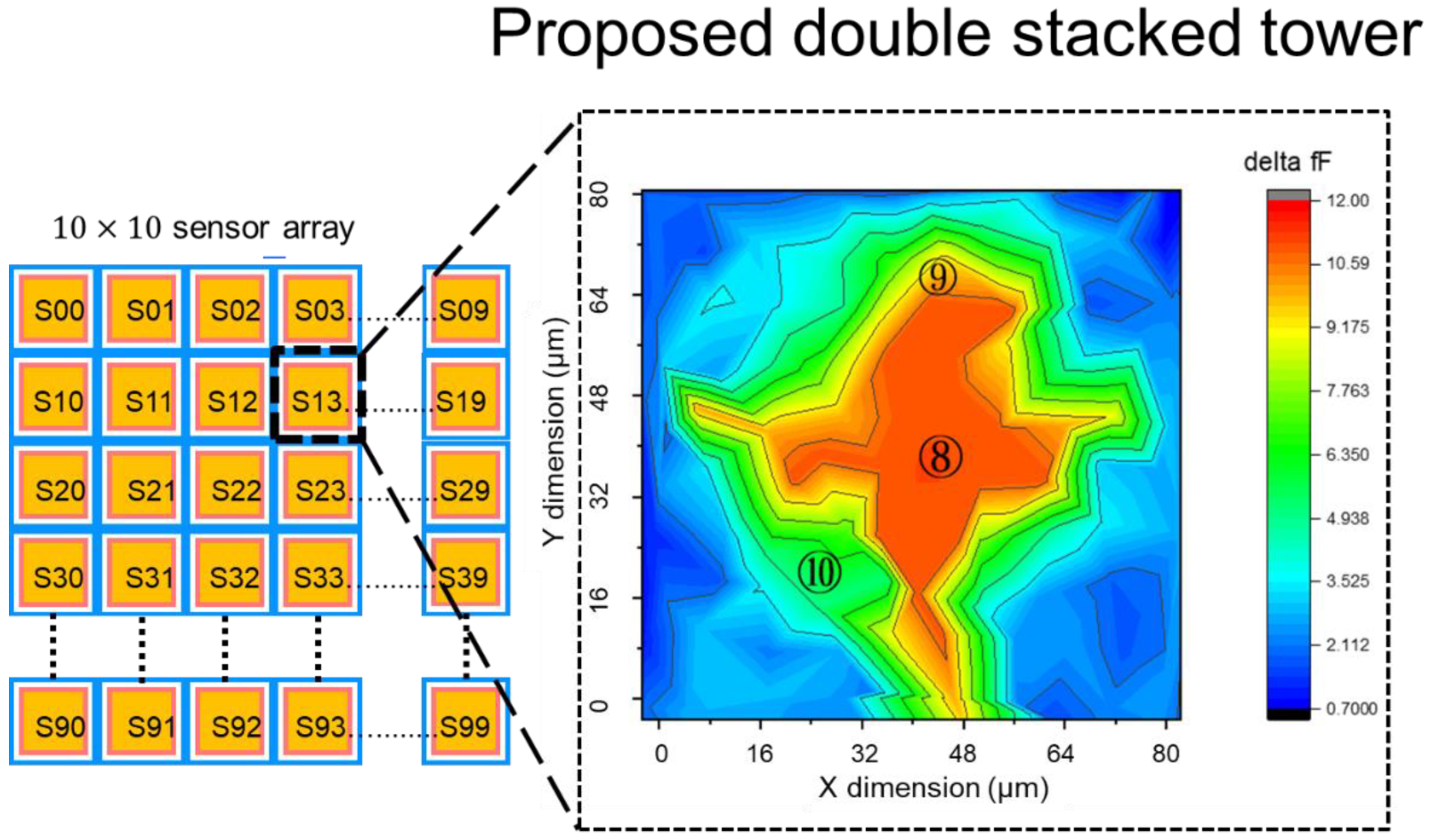
2.1.4. Pixel Layout and Sensitivity Evaluation of Double Stacked Tower Biosensor
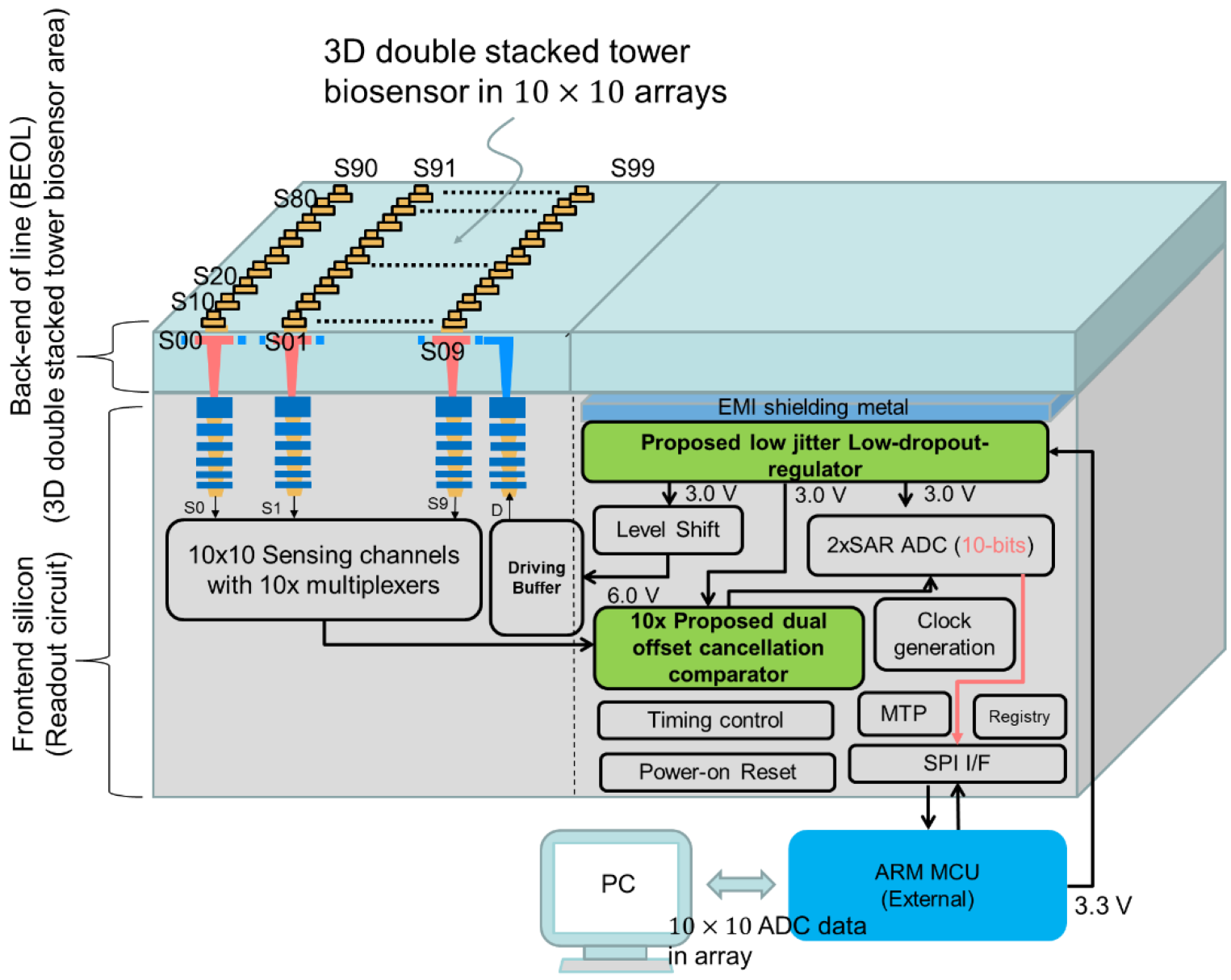
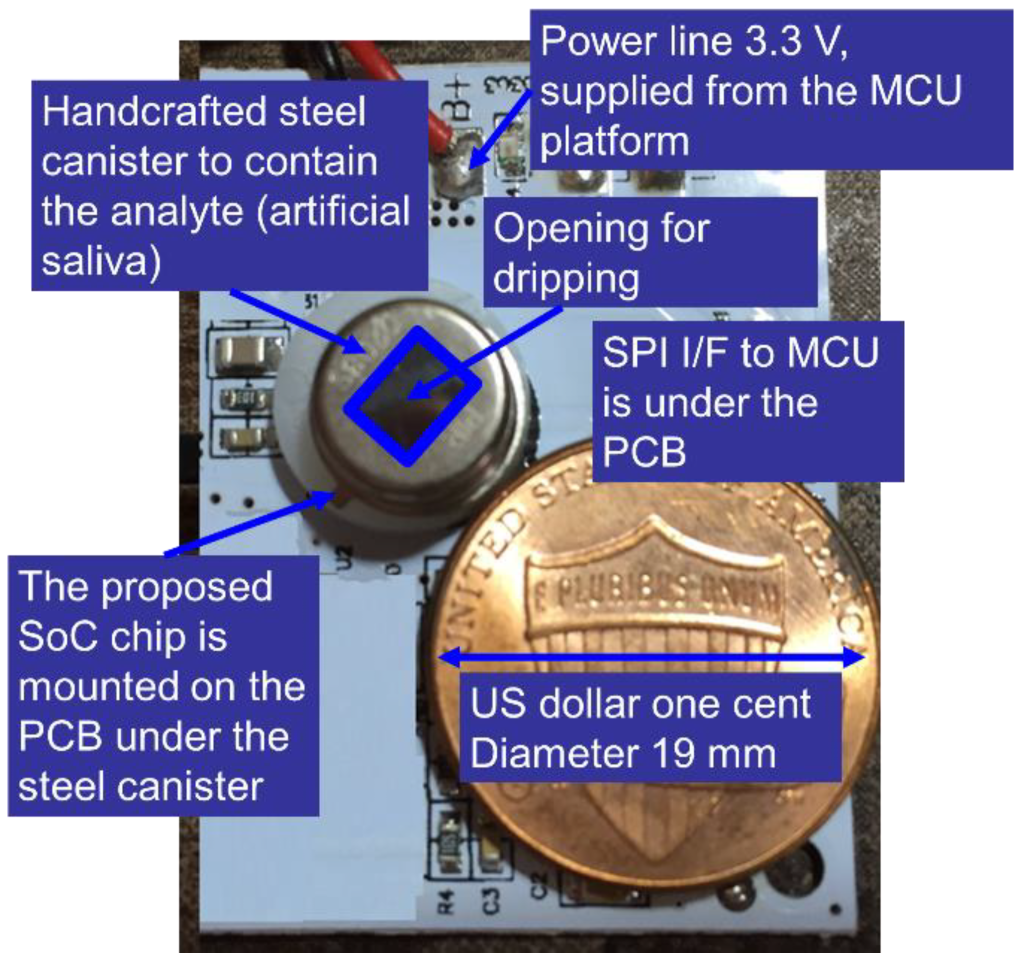
2.2. System Architecture
2.2.1. System Architecture of Frontend Circuits
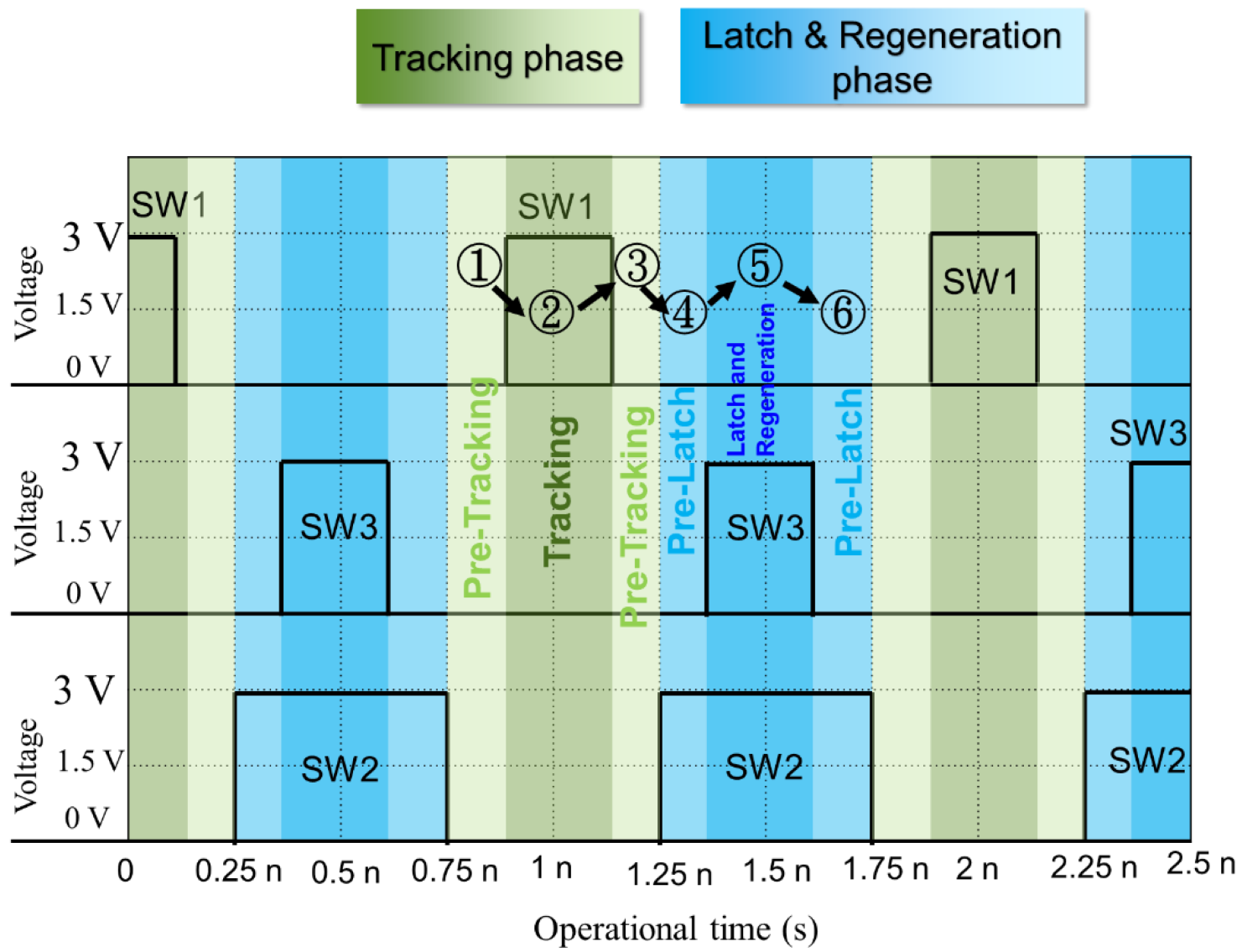
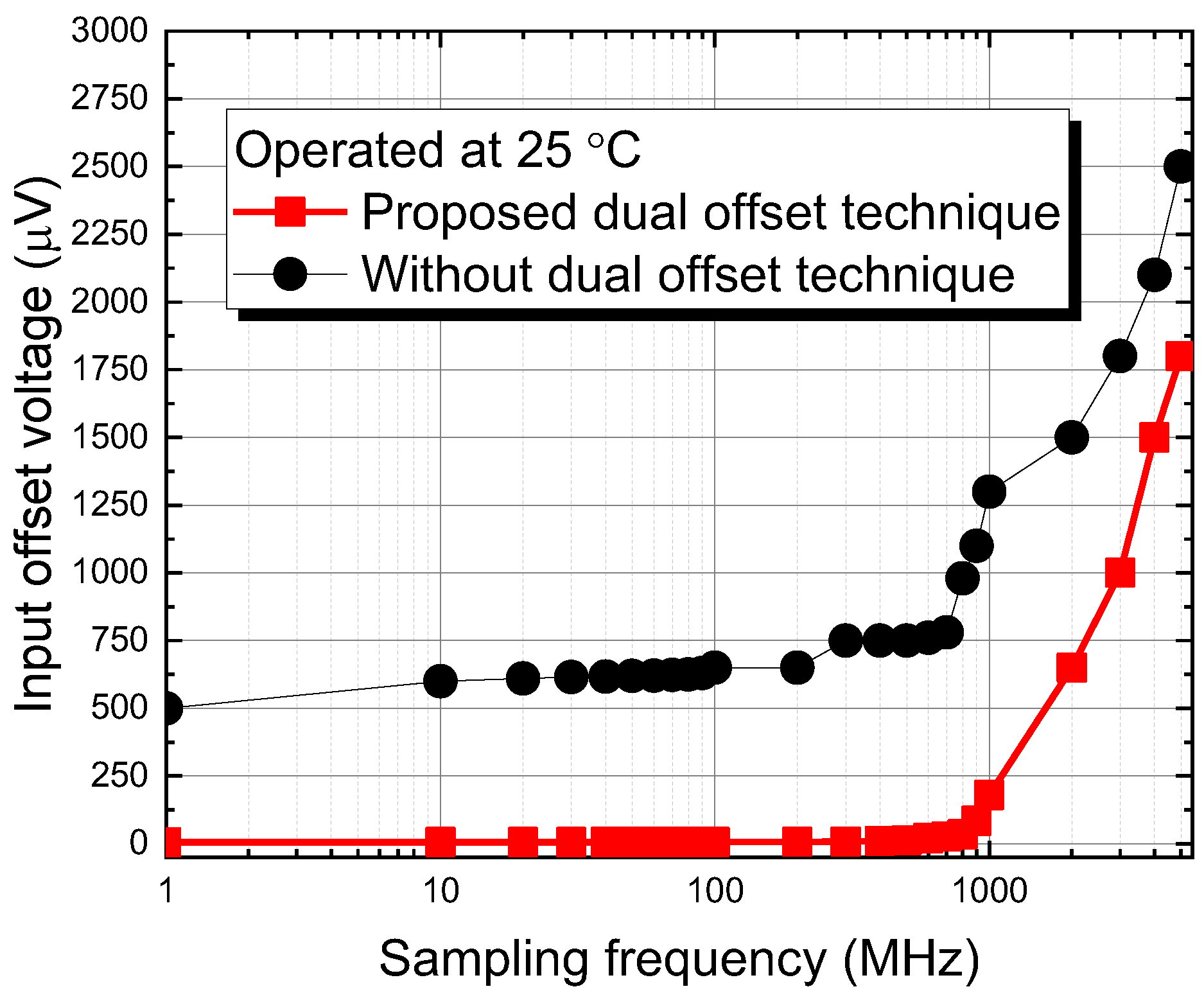
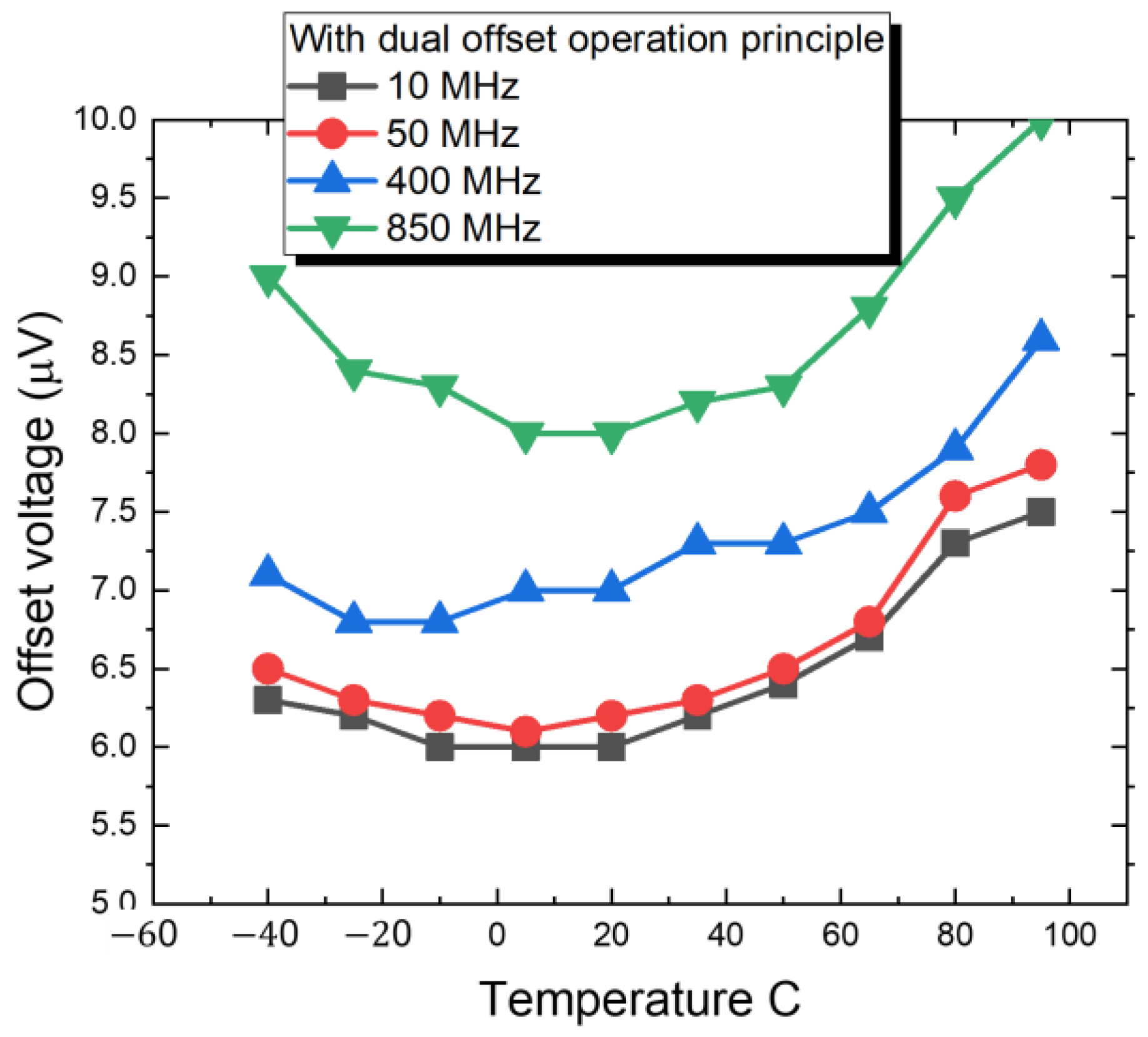
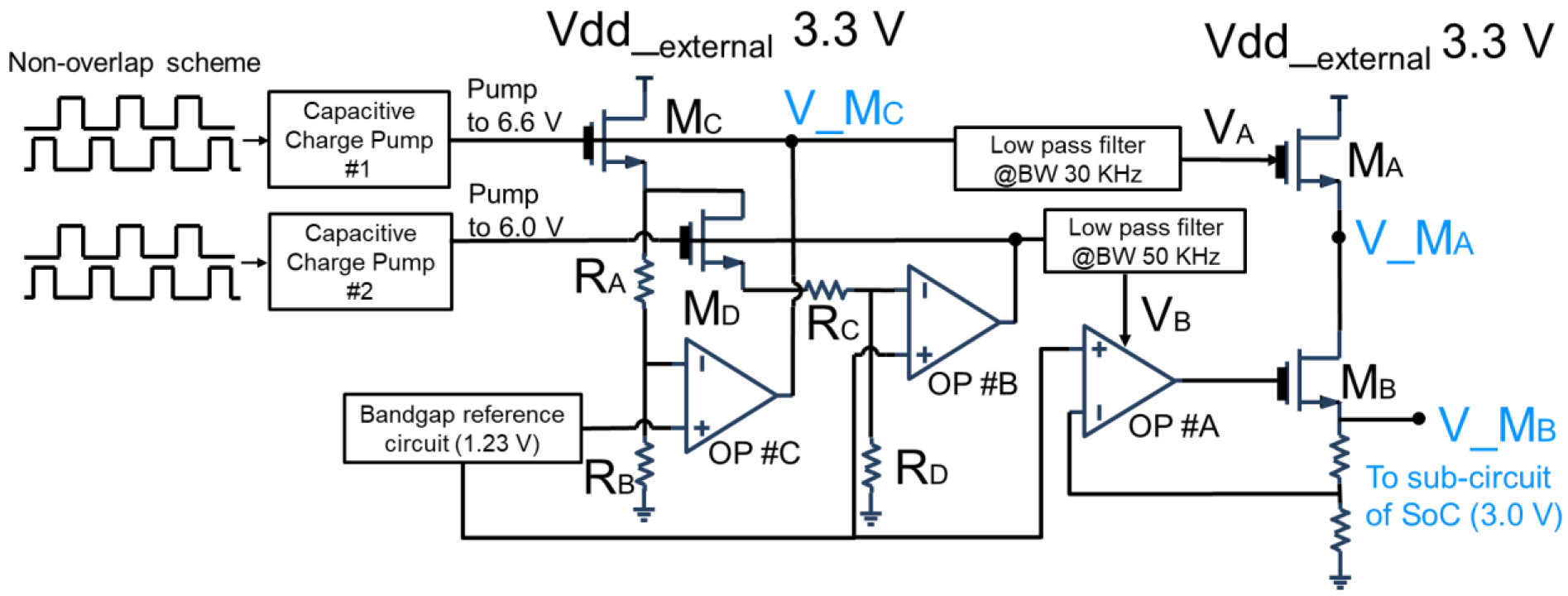
2.2.2. Design of Dual Offset Cancellation Circuit
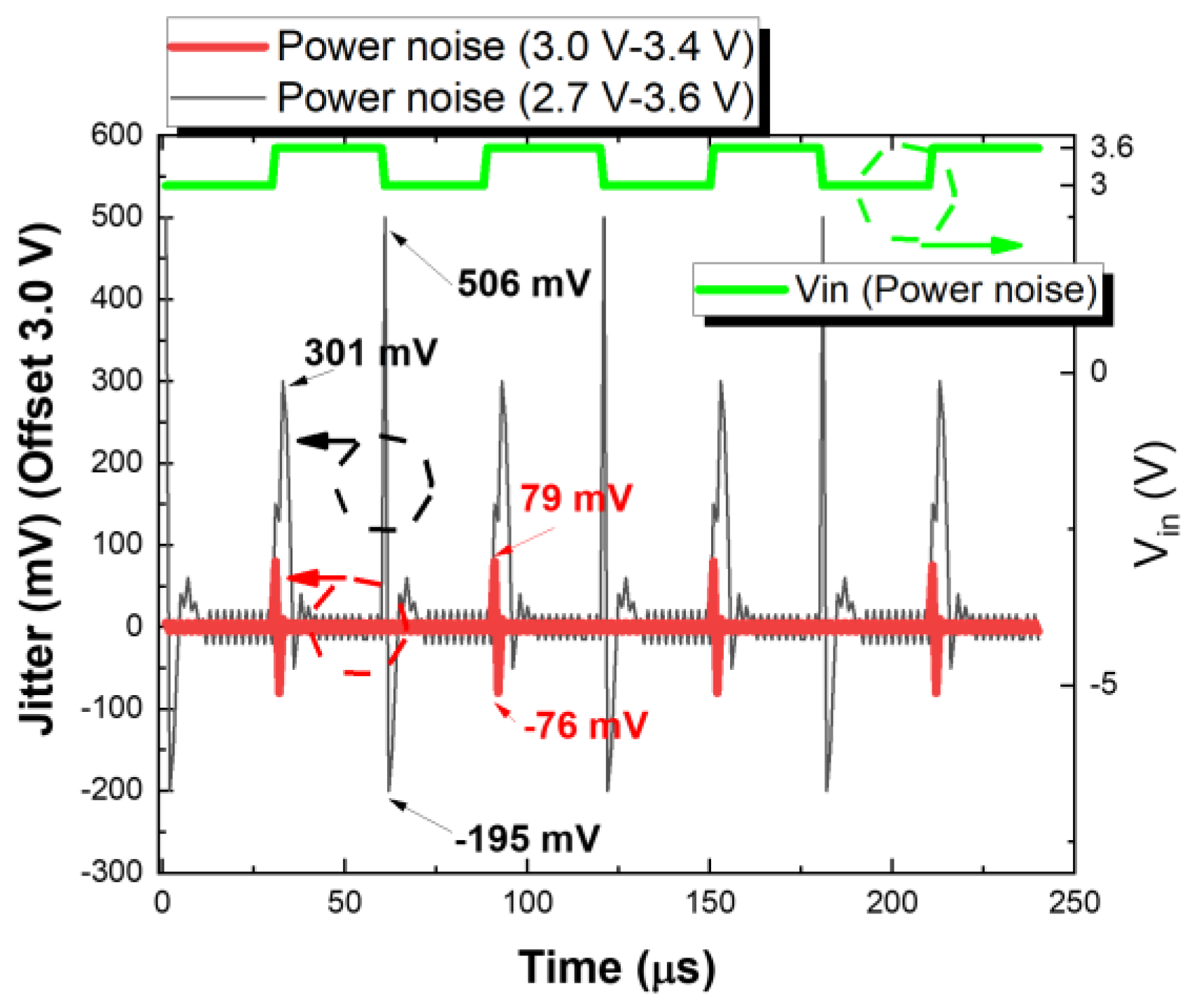
2.2.3. Design of Low-Jitter Dropout Regulator
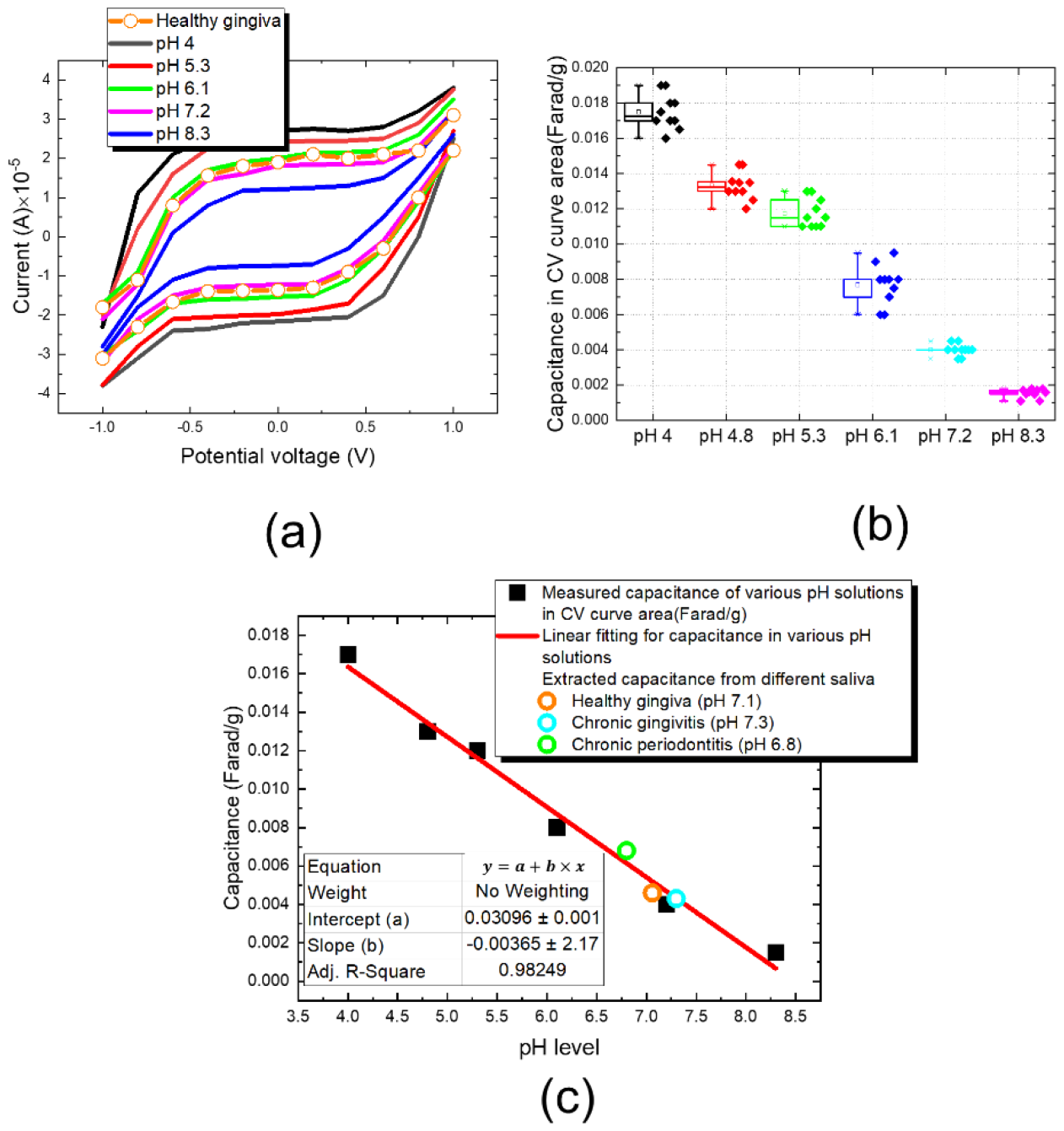
3. Results
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takahashi, N.; Schachtele, C.F. Effect of pH on the growth and proteolytic activity of Porphyromonas gingivalis and Bacteroides Intermedius. J. Dent. Res. 1990, 69, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Saito, K.; Schachtele, C.F.; Yamada, T. Acid tolerance and acid-neutralizing activity of Porphyromonas gingivalis, Prevotella intermedia and Fusobacterium nucleatum. Oral Microbiol. Immunol. 1997, 12, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, E.O.; Erdemir, A. The detection of salivary minerals in smokers and non-smokers with chronic periodontitis by the inductively coupled plasma-atomic emission spectrophotometry technique. J. Periodontol. 2006, 77, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Zuabi, O.; Machtei, E.E.; Ben-Aryeh, H.; Ardekian, L.; Peled, M.; Laufer, D. The effect of smoking and periodontal treatment on salivary composition in patients with established periodontitis. J. Periodontol. 1999, 70, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, S.; Tan, D.S.; Thomson, D.J.; Freund, M.S.; Bridges, G.E. A wireless passive sensor for temperature compensated remote ph monitoring. IEEE Sens. J. 2013, 13, 2428–2436. [Google Scholar] [CrossRef]
- Zdrachek, E.; Bakker, E. Potentiometric Sensing. Anal. Chem. 2019, 91, 2–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, L.; Llorella, A.; Esquivel, J.P.; Sabaté, N. Paper-Based Batteries as Conductivity Sensors for Single-Use Applications. ACS Sens. 2020, 5, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Khanna, V.K. Remedial and adaptive solutions of ISFET non-ideal behaviour. Sens. Rev. 2013, 33, 228–237. [Google Scholar] [CrossRef]
- Ahmed, N.M.; Sabah, F.A.; Al-Hardan, N.H.; Almessiere, M.A.; Mohammad, S.M.; Lim, W.F.; Jumaah, M.; Islam, A.K.M.S.; Hassan, Z.; Quah, H.J.; et al. Development of EGFET-based ITO pH sensors using epoxy free membrane. Semicond. Sci. Technol. 2021, 36, 045027. [Google Scholar] [CrossRef]
- Jung, S.H.; Seo, Y.M.; Gu, T.; Jang, W.; Kang, S.G.; Hyeon, Y.; Hyun, S.H.; Lee, J.H.; Whang, D. Super-Nernstian pH Sensor Based on Anomalous Charge Transfer Doping of Defect-Engineered Graphene. Nano Lett. 2020, 21, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.J.; Jaselskis, B.; Moore, C.E. Development of the Glass Electrode and the pH Response. J. Chem. Educ. 2013, 90, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Scholz, F. Nikolsky’s ion exchange theory versus Baucke’s dissociation mechanism of the glass electrode. J. Solid State Electrochem. 2011, 15, 67–68. [Google Scholar] [CrossRef]
- McMurray, H.N.; Douglas, P.; Abbot, D. Novel thick-film pH sensors based on ruthenium dioxide-glass composites. Sens. Actuators B Chem. 1995, 28, 9–15. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Chou, J.-C. Preparation and characterization of the titanium dioxide thin films used for pH electrode and procaine drug sensor by sol–gel method. Mater. Chem. Phys. 2009, 114, 542–548. [Google Scholar] [CrossRef]
- Al-Hilli, S.; Willander, M. The pH response and sensing mechanism of n-type ZnO/electrolyte interfaces. Sensors 2009, 9, 7445–7480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigand, M.; Kemna, A. Relationship between Cole–Cole model parameters and spectral decomposition parameters derived from SIP data. Geophys. J. Int. 2016, 205, 1414–1419. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Zhang, X.; Ang, M.H. Permittivity, loss factor and Cole-Cole model of acrylic materials for dielectric elastomers. Results Phys. 2021, 29, 104781. [Google Scholar] [CrossRef]
- Aekbote, B.L.; Fekete, T.; Jacak, J.; Vizsnyiczai, G.; Ormos, P.; Kelemen, L. Surface-modified complex SU-8 microstructures for indirect optical manipulation of single cells. Biomed. Opt. Express 2016, 7, 45–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.X.; Du, H.; Lee, M.S. A 360-MHz 3-V CMOS PLL with 1-V peak–peak power supply noise tolerance. In Proceedings of the 1996 IEEE International Solid-State Circuits Conference. Digest of Technical Papers, San Francisco, CA, USA, 10 February 1996; pp. 134–135. [Google Scholar]
- Rajapaksha, R.D.A.A.; Hashim, U.; Fernando, C.A.N. Design, Fabrication and characterization of 1.0 μm Gap Al based interdigitated electrode for biosensors. Microsyst. Technol. 2017, 23, 4501–4507. [Google Scholar] [CrossRef]
- Manjakkal, L.; Sakthivel, B.; Gopalakrishnan, N.; Dahiya, R. Printed flexible electrochemical pH sensors based on CuO nanorods. Sens. Actuators B Chem. 2018, 263, 50–58. [Google Scholar] [CrossRef]
- Rani, R.A.; Syono, M.I.; Ramli, A.S. Multi-finger gate ISFET (Mf-ISFET) for pH sensor application. In Proceedings of the 2008 IEEE International Conference on Semiconductor Electronics, Johor Bahru, Malaysia, 25–27 November 2008; pp. 350–353. [Google Scholar]








| Analyte | Solution pH 7.2 | Solution pH 4.0 | |
|---|---|---|---|
| Parameters | |||
| RP_target | 18.2 KΩ | 13.8 KΩ | |
| R_target | 2.8 KΩ | 2.1 KΩ | |
| C_target | F | F | |
| α | 0.81 | 0.75 | |
| Functions | Principle | Linearity Range | Sensitivity | Response Time in Second | Repetition | Drift over Time | Analyte Target | Functional Type | |
|---|---|---|---|---|---|---|---|---|---|
| Prior Works | |||||||||
| This work w/3D double stacked tower array + w/dual cancellation comparator | Electrical field | Wide pH 2.0–8.3 | 21 ADC count/pH (212 mV/pH) | 5 s | 98.9% | 0.5 mV | Chronic gingiva disease saliva | array pH Image (50-frame/s) | |
| This work w/o 3D double stacked tower array+ w/o dual cancellation comparator | Narrow Range 1: pH: 2–3 Range 2: pH: 3–8.3 | 6 ADC count/pH | 7 s | 96% | 0.5 mV | Chronic gingiva disease saliva | |||
| [20] Y-2017 | Electrical field | Narrow Range 1: pH: 4–6 Range 2: pH: 6–10 | 187 nA/pH | 6 s | 90% | 2nA([11] IDE NO:1–5) | Alkali and hydroxyl buffer | Analog current | |
| [21] Y-2018 | Electrical field | Narrow pH: 5–8 | 0.64 μF/pH | 13 s | 92% | 7.1 mV | Glucose, Na+. K+ | Analog voltage | |
| [10] Y-2020 | Transistor channel current | Narrow pH: 6–7.6 | 140 mV/pH | 20 s | 93% | 3.2 mV | Standard buffer | Analog voltage | |
| [11] Y-2019 | Transistor channel current | Wide pH: 7–12 | 49 mV/pH | 24 s | 91% | 4.2 mV | Standard buffer | Analog voltage | |
| [22] Y-2017 | Transistor channel current | Narrow Range 1: pH: 4–6 Range 2: pH: 6–10 | 130 mV/pH | 12 s | 92% | 6.2 mV | Standard buffer | Analog voltage | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.-C. MEMSbased Double-Stacked Tower Biosensor Array with Integrated Readout Circuitry for Detection of Salivary pH as a Diagnostic Biomarker Applied for Chronic Periodontal Disease. Sensors 2022, 22, 8652. https://doi.org/10.3390/s22228652
Lin W-C. MEMSbased Double-Stacked Tower Biosensor Array with Integrated Readout Circuitry for Detection of Salivary pH as a Diagnostic Biomarker Applied for Chronic Periodontal Disease. Sensors. 2022; 22(22):8652. https://doi.org/10.3390/s22228652
Chicago/Turabian StyleLin, Wei-Cheng. 2022. "MEMSbased Double-Stacked Tower Biosensor Array with Integrated Readout Circuitry for Detection of Salivary pH as a Diagnostic Biomarker Applied for Chronic Periodontal Disease" Sensors 22, no. 22: 8652. https://doi.org/10.3390/s22228652
APA StyleLin, W.-C. (2022). MEMSbased Double-Stacked Tower Biosensor Array with Integrated Readout Circuitry for Detection of Salivary pH as a Diagnostic Biomarker Applied for Chronic Periodontal Disease. Sensors, 22(22), 8652. https://doi.org/10.3390/s22228652








