Vestibular Rehabilitation Improves Gait Quality and Activities of Daily Living in People with Severe Traumatic Brain Injury: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Interventions
2.4. Vestibular Rehabilitation (VRg Only)
2.5. Conventional Balance Rehabilitation (CRg only)
2.6. Blinding Randomization
2.7. Clinical Outcome Measures
2.8. Instrumental Assessment
2.9. Gait quality Instrumental Assessment
2.10. Statistical Analysis
3. Results
3.1. Clinical Assessment
3.2. Instrumental Assessment
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic brain injury: An overview of epidemiology, pathophysiology, and medical management. Med. Clin. N. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Demographics and clinical assessment working group of the international and interagency initiative toward common data elements for research on traumatic brain injury and psychological health. Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, F.; Compagnone, C.; Korsic, M.; Servadei, F.; Kraus, J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir. 2006, 148, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Majdan, M.; Plancikova, D.; Brazinova, A.; Rusnak, M.; Nieboer, D.; Feigin, V.L.; Maas, A. Epidemiology of traumatic brain injuries in Europe: A cross-sectional analysis. Lancet Public Health 2016, 1, e76–e83. [Google Scholar] [CrossRef]
- Dang, B.; Chen, W.; He, W.; Chen, G. Rehabilitation treatment and progress of traumatic brain injury dysfunction. Neural Plast. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- May, M.; Milders, M.; Downey, B.; Whyte, M.; Higgins, V.; Wojcik, Z.; Amin, S.; O’Rourke, S. Social behavior and impairments in social cognition following traumatic brain injury. J. Int. Neuropsychol. Soc. 2017, 23, 400–411. [Google Scholar] [CrossRef]
- Ponsford, J.L.; Spitz, G.; Cromarty, F.; Gifford, D.; Attwood, D. Costs of care after traumatic brain injury. J. Neurotrauma 2013, 30, 1498–1505. [Google Scholar] [CrossRef]
- Teasdale, G.; Maas, A.; Lecky, F.; Manley, G.; Stocchetti, N.; Murray, G. The Glasgow Coma Scale at 40 years: Standing the test of time. Lancet Neurol. 2014, 13, 844–854. [Google Scholar] [CrossRef]
- Artman Laura, K.; McMahon Brian, T. Functional limitations in TBI and their relationship to job maintenance following work re-entry. J. Vocat. Rehabil. 2013, 39, 13–21. [Google Scholar] [CrossRef]
- Bose, P.; Hou, J.; Thompson, F.J. Traumatic Brain Injury (TBI)-induced spasticity: Neurobiology, treatment, and rehabilitation. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015. [Google Scholar]
- Formisano, R.; Saltuari, L.; Sailer, U.; Birbarmer, G.; Gerstenbrand, F. Post-traumatic cerebellar syndrome. New Trends Clin. Neuropharmacol. 1987, 1, 115–118. [Google Scholar]
- Klima, D.; Morgan, L.; Baylor, M.; Reilly, C.; Gladmon, D.; Davey, A. Physical performance and fall risk in persons with traumatic brain injury. Percept. Mot. Ski. 2019, 126, 50–69. [Google Scholar] [CrossRef] [PubMed]
- Basford, J.R.; Chou, L.S.; Kaufman, K.R.; Brey, R.H.; Walker, A.; Malec, J.F.; Moessner, A.M.; Brown, A.W. An assessment of gait and balance deficits after traumatic brain injury. Arch. Phys. Med. Rehabil. 2003, 84, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Fino, P.C.; Peterka, R.J.; Hullar, T.E.; Murchison, C.; Horak, F.B.; Chesnutt, J.C.; King, L.A. Assessment and rehabilitation of central sensory impairments for balance in mTBI using auditory biofeedback: A randomized clinical trial. BMC Neurol. 2017, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Bruijn, S.M.; van Dieën, J.H. Control of human gait stability through foot placement. J. R. Soc. Interface 2018, 15, 20170816. [Google Scholar] [CrossRef] [PubMed]
- Del Din, S.; Kirk, C.; Yarnall, A.J.; Rochester, L.; Hausdorff, J.M. Body-worn sensors for remote monitoring of parkinson’s disease motor symptoms: Vision, state of the art, and challenges ahead. J. Park. Dis. 2021, 11, S35–S47. [Google Scholar] [CrossRef] [PubMed]
- Beck, Y.; Herman, T.; Brozgol, M.; Giladi, N.; Mirelman, A.; Hausdorff, J.M. SPARC: A new approach to quantifying gait smoothness in patients with Parkinson’s disease. J. Neuroeng. Rehabil. 2018, 15, 49. [Google Scholar] [CrossRef]
- Garcia, F.D.V.; da Cunha, M.J.; Schuch, C.P.; Schifino, G.P.; Balbinot, G.; Pagnussat, A.S. Movement smoothness in chronic post-stroke individuals walking in an outdoor environment—A cross-sectional study using IMU sensors. PLoS ONE 2021, 16, e0250100. [Google Scholar] [CrossRef]
- Hess, R.J.; Brach, J.S.; Piva, S.R.; VanSwearingen, J.M. Walking skill can be assessed in older adults: Validity of the Figure-of-8 Walk Test. Phys. Ther. 2010, 90, 89–99. [Google Scholar] [CrossRef]
- Belluscio, V.; Bergamini, E.; Tramontano, M.; Formisano, R.; Buzzi, M.G.; Vannozzi, G. Does curved walking sharpen the assessment of gait disorders? An instrumented approach based on wearable inertial sensors. Sensors 2020, 20, 5244. [Google Scholar] [CrossRef]
- Wong, S.S.T.; Yam, M.-S.; Ng, S.S.M. The Figure-of-Eight Walk test: Reliability and associations with stroke-specific impairments. Disabil. Rehabil. 2013, 35, 1896–1902. [Google Scholar] [CrossRef]
- Belluscio, V.; Bergamini, E.; Iosa, M.; Tramontano, M.; Morone, G.; Vannozzi, G. The iFST: An instrumented version of the Fukuda Stepping Test for balance assessment. Gait Posture 2018, 60, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, M.; Russo, V.; Spitoni, G.F.; Ciancarelli, I.; Paolucci, S.; Manzari, L.; Morone, G. Efficacy of vestibular rehabilitation in patients with neurologic disorders: A systematic review. Arch. Phys. Med. Rehabil. 2020, 102, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, M.; Grasso, M.G.; Soldi, S.; Casula, E.P.; Bonnì, S.; Mastrogiacomo, S.; D’Acunto, A.; Porrazzini, F.; Caltagirone, C.; Koch, G. Cerebellar intermittent theta-burst stimulation combined with vestibular rehabilitation improves gait and balance in patients with multiple sclerosis: A preliminary double-blind randomized controlled trial. Cerebellum 2020, 19, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, M.; Cinnera, A.M.; Manzari, L.; Tozzi, F.F.; Caltagirone, C.; Morone, G.; Pompa, A.; Grasso, M.G. Vestibular rehabilitation has positive effects on balance, fatigue and activities of daily living in highly disabled multiple sclerosis people: A preliminary randomized controlled trial. Restor. Neurol. Neurosci. 2018, 36, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, M.; Bergamini, E.; Iosa, M.; Belluscio, V.; Vannozzi, G.; Morone, G. Vestibular rehabilitation training in patients with subacute stroke: A preliminary randomized controlled trial. NeuroRehabilitation 2018, 43, 247–254. [Google Scholar] [CrossRef]
- Tramontano, M.; Medici, A.; Iosa, M.; Chiariotti, A.; Fusillo, G.; Manzari, L.; Morelli, D. The effect of vestibular stimulation on motor functions of children with cerebral palsy. Mot. Control 2017, 21, 299–311. [Google Scholar] [CrossRef]
- Gurley, J.M.; Hujsak, B.D.; Kelly, J.L. Vestibular rehabilitation following mild traumatic brain injury. NeuroRehabilitation 2013, 32, 519–528. [Google Scholar] [CrossRef]
- Gottshall, K. Vestibular rehabilitation after mild traumatic brain injury with vestibular pathology. NeuroRehabilitation 2011, 29, 167–171. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, Revised ed.; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Gouvier, W.D.; Blanton, P.D.; Laporte, K.K.; Nepomuceno, C. Reliability and validity of the Disability Rating Scale and the Levels of Cognitive Functioning Scale in monitoring recovery from severe head injury. Arch. Phys. Med. Rehabil. 1987, 68, 94–97. [Google Scholar] [CrossRef]
- Semlyen, J.K.; Summers, S.J.; Barnes, M.P. Traumatic brain injury: Efficacy of multidisciplinary rehabilitation. Arch. Phys. Med. Rehabil. 1998, 79, 678–683. [Google Scholar] [CrossRef]
- Tramontano, M.; Bonnì, S.; Cinnera, A.M.; Marchetti, F.; Caltagirone, C.; Koch, G.; Peppe, A. Blindfolded balance training in patients with parkinson’s disease: A sensory-motor strategy to improve the gait. Park. Dis. 2016, 2016, 7536862. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonnì, S.; Ponzo, V.; Tramontano, M.; Cinnera, M.; Caltagirone, C.; Koch, G.; Peppe, A. Neurophysiological and clinical effects of blindfolded balance training (BBT) in Parkinson’s disease patients: A preliminary study. Eur. J. Phys. Rehabil. Med. 2019, 55, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Curcio, A.; Temperoni, G.; Tramontano, M.; De Angelis, S.; Iosa, M.; Mommo, F.; Cochi, G.; Formisano, R. The effects of aquatic therapy during post-acute neurorehabilitation in patients with severe traumatic brain injury: A preliminary randomized controlled trial. Brain Inj. 2020, 34, 1630–1635. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Baldwin, M.; Polissar, N.L.; Gruber, W. predicting the probability for falls in community-dwelling older adults. Phys. Ther. 1997, 77, 812–819. [Google Scholar] [CrossRef]
- Herman, T.; Inbar-Borovsky, N.; Brozgol, M.; Giladi, N.; Hausdorff, J.M. The Dynamic Gait Index in healthy older adults: The role of stair climbing, fear of falling and gender. Gait Posture 2009, 29, 237–241. [Google Scholar] [CrossRef]
- Berg, W.P.; Alessio, H.M.; Mills, E.M.; Tong, C. Circumstances and consequences of falls in independent community-dwelling older adults. Age Ageing 1997, 26, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Howe, J.A.; Inness, E.L.; Venturini, A.; Williams, J.I.; Verrier, M.C. The Community Balance and Mobility Scale-a balance measure for individuals with traumatic brain injury. Clin. Rehabil. 2006, 20, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Belluscio, V.; Bergamini, E.; Tramontano, M.; Bustos, A.O.; Allevi, G.; Formisano, R.; Vannozzi, G.; Buzzi, M.G. Gait quality assessment in survivors from severe traumatic brain injury: An instrumented approach based on inertial sensors. Sensors 2019, 19, 5315. [Google Scholar] [CrossRef]
- Simonetti, E.; Villa, C.; Bascou, J.; Vannozzi, G.; Bergamini, E.; Pillet, H. Gait event detection using inertial measurement units in people with transfemoral amputation: A comparative study. Med. Biol. Eng. Comput. 2019, 58, 461–470. [Google Scholar] [CrossRef]
- Summa, A.; Vannozzi, G.; Bergamini, E.; Iosa, M.; Morelli, D.; Cappozzo, A. Multilevel upper body movement control during gait in children with cerebral palsy. PLoS ONE 2016, 11, e0151792. [Google Scholar] [CrossRef]
- Melendez-Calderon, A.; Shirota, C.; Balasubramanian, S. Estimating movement smoothness from inertial measurement units. Front. Bioeng. Biotechnol. 2021, 8, 558771. [Google Scholar] [CrossRef] [PubMed]
- Menz, H.B.; Lord, S.R.; Fitzpatrick, R.C. Acceleration patterns of the head and pelvis when walking on level and irregular surfaces. Gait Posture 2003, 18, 35–46. [Google Scholar] [CrossRef]
- Latt, M.D.; Menz, H.B.; Fung, V.S.; Lord, S.R. Walking speed, cadence and step length are selected to optimize the stability of head and pelvis accelerations. Exp. Brain Res. 2008, 184, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Duarte, E.; Marco, E.; Muniesa, J.M.; Belmonte, R.; Aguilar, J.J.; Escalada, F. Early detection of non-ambulatory survivors six months after stroke. NeuroRehabilitation 2010, 26, 317–323. [Google Scholar] [CrossRef]
- Brach, J.S.; McGurl, D.; Wert, D.; VanSwearingen, J.M.; Perera, S.; Cham, R.; Studenski, S. Validation of a measure of smoothness of walking. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 136–141. [Google Scholar] [CrossRef]
- Courtine, G.; Schieppati, M. Human walking along a curved path. I. Body trajectory, segment orientation and the effect of vision. Eur. J. Neurosci. 2003, 18, 177–190. [Google Scholar] [CrossRef]
- Godi, M.; Giardini, M.; Schieppati, M. Walking along curved trajectories. Changes with age and parkinson’s disease. hints to rehabilitation. Front. Neurol. 2019, 10, 532. [Google Scholar] [CrossRef]
- Tramontano, M.; Piermaria, J.; Morone, G.; Reali, A.; Vergara, M.; Tamburella, F. Postural changes during exteroceptive thin plantar stimulation: The effect of prolonged use and different plantar localizations. Front. Syst. Neurosci. 2019, 13, 49. [Google Scholar] [CrossRef]
- Tramontano, M.; Morone, G.; Curcio, A.; Temperoni, G.; Medici, A.; Morelli, D.; Caltagirone, C.; Paolucci, S.; Iosa, M. Maintaining gait stability during dual walking task: Effects of age and neurological disorders. Eur. J. Phys. Rehabil. Med. 2017, 53, 7–13. [Google Scholar] [CrossRef]
- Iosa, M.; De Sanctis, M.; Summa, A.; Bergamini, E.; Morelli, D.; Vannozzi, G. Usefulness of magnetoinertial wearable devices in neurorehabilitation of children with cerebral palsy. Appl. Bionics Biomech. 2018, 2018, 5405680. [Google Scholar] [CrossRef]
- Miller Koop, M.; Ozinga, S.J.; Rosenfeldt, A.B.; Alberts, J.L. Quantifying turning behavior and gait in Parkinson’s disease using mobile technology. IBRO Rep. 2018, 5, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Manzari, L.; Princi, A.A.; De Angelis, S.; Tramontano, M. Clinical value of the video head impulse test in patients with vestibular neuritis: A systematic review. Eur. Arch. Otorhinolaryngol. 2021, 278, 4155–4167. [Google Scholar] [CrossRef] [PubMed]
- Manzari, L.; Koch, G.; Tramontano, M. Selective asymmetry of ocular vestibular-evoked myogenic potential in patients with acute utricular macula loss. J. Int. Adv. Otol. 2021, 17, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Manzari, L.; De Angelis, S.; Princi, A.A.; Galeoto, G.; Tramontano, M. The clinical use of the Suppression Head Impulse Paradigm in patients with vestibulopathy: A systematic review. Healthcare 2022, 10, 1182. [Google Scholar] [CrossRef] [PubMed]

| VRg (#15) | CRg (#15) | p Value | ||
|---|---|---|---|---|
| Age (years) | 34.7 ± 12.8 | 36.8 ± 12.9 | 0.663 | |
| Sex N (%) | Male | 7 (46.7) | 12 (80) | 0.128 |
| Female | 8 (53.3) | 3 (20) | ||
| Time since trauma (months) | 11.6 ± 7.3 | 9.3 ± 6.1 | 0.364 | |
| ABC Scale | 74.1 ± 11.8 | 72.6 ± 15.1 | 0.983 | |
| CIQ | 12.3 ± 9.9 | 10.7 ± 3.3 | 0.708 | |
| DGI | 16.9 ± 5.1 | 17.9 ± 4.5 | 0.678 | |
| BBS | 47.7 ± 5.8 | 44.2 ± 10.6 | 0.519 | |
| DHI | 34.5 ± 17.1 | 32.5 ± 15.5 | 0.546 | |
| CB&M | 37.7 ± 24.2 | 33.7 ± 23.0 | 0.740 | |
| VRg | CRg | |||||||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T0 | T1 | T2 | T3 | |
| DGI | 16.9 ± 5.1 | 20.1 ± 3.8 ** | 20.8 ± 3.4 ** | 21.6 ± 3.4 ** | 17.9 ± 4.5 | 20.3 ± 4.1 ** | 20.9 ± 4.0 ** | 21.6 ± 3.3 ** |
| BBS | 47.7 ± 5.8 | 49.8 ± 6.0 ** | 50.3 ± 5.3 ** | 51.1 ± 5.6 ** | 44.2 ± 10.6 | 47.9 ± 9.6 ** | 49.5 ± 8.3 ** | 49.2 ± 8.5 ** |
| DHI | 34.5 ± 17.1 | 20.9 ± 12.9 ** | 20.9 ± 18.1 * | 17.8 ± 17.4 * | 32.5 ± 15.5 | 22.2 ± 12.7 * | 16.2 ± 13.3 * | 24.0 ± 22.0 * |
| CB&M | 37.7 ± 24.2 | 47.2 ± 27.6 ** | 48.1 ± 24.8 ** | 48.8 ± 25.3 ** | 33.7 ± 23.0 | 39.0 ± 20.2 | 47.4 ± 25.9 | 49.7 ± 24.7 * |
| ABC | 74.1 ± 11.8 | - | - | 84.2 ± 15.1 * | 72.6 ± 15.1 | - | - | 76.1 ± 25.3 |
| CIQ | 12.3 ± 9.9 | - | - | 12.9 ± 4.4 * | 10.7 ± 3.3 | - | - | 10.5 ± 4.2 |
| F8WT Parameters | VR | CR | Main Effects | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T0 | T1 | T2 | T3 | |||
| ST | SF (steps/s) | 0.68 ± 0.16 | 0.78 ± 0.15 | 0.76 ± 0.12 | 0.81 ± 0.11 | 0.67 ± 0.18 | 0.70 ± 0.11 | 0.74 ± 0.13 | 0.75 ± 0.16 | 03 |
| WS (m/s) | 0.70 ± 0.37 | 0.83 ± 0.36 | 0.84 ± 0.35 | 1.00 ± 0.26 | 0.62 ± 0.30 | 0.70 ± 0.22 | 0.83 ± 0.26 | 0.82 ± 0.35 | 02, 03, 13 | |
| Stability | RMS-PML (adim) | 1.05 ± 0.31 | 1.01 ± 0.23 | 0.93 ± 0.18 | 0.86 ± 0.22 | 1.08 ± 0.34 | 0.92 ± 0.21 | 0.95 ± 0.21 | 0.95 ± 0.18 | 03 |
| RMS-TAP (adim) | 0.77 ± 0.36 | 0.67 ± 0.26 | 0.64 ± 0.26 | 0.55 ± 0.21 | 0.79 ± 0.34 | 0.71 ± 0.35 | 0.61 ± 0.23 | 0.67 ± 0.19 | 02, 03 | |
| RMS-TML (adim) | 1.04 ± 0.48 | 1.03 ± 0.50 | 1.06 ± 0.48 | 0.86 ± 0.26 | 0.97 ± 0.34 | 0.80 ± 0.21 | 0.75 ± 0.16 | 0.78 ± 0.17 | 02, 03 | |
| RMS-Tmag (adim) | 1.71 ± 0.48 | 1.63 ± 0.47 | 1.63 ± 0.45 | 1.46 ± 0.25 | 1.64 ± 0.37 | 1.50 ± 0.29 | 1.40 ± 0.19 | 1.45 ± 0.16 | 02, 03 | |
| RMS-HAP (adim) | 0.83 ± 0.38 | 0.74 ± 0.34 | 0.61 ± 0.21 | 0.58 ± 0.21 | 0.86 ± 0.44 | 0.78 ± 0.38 | 0.63 ± 0.17 | 0.71 ± 0.27 | 03 | |
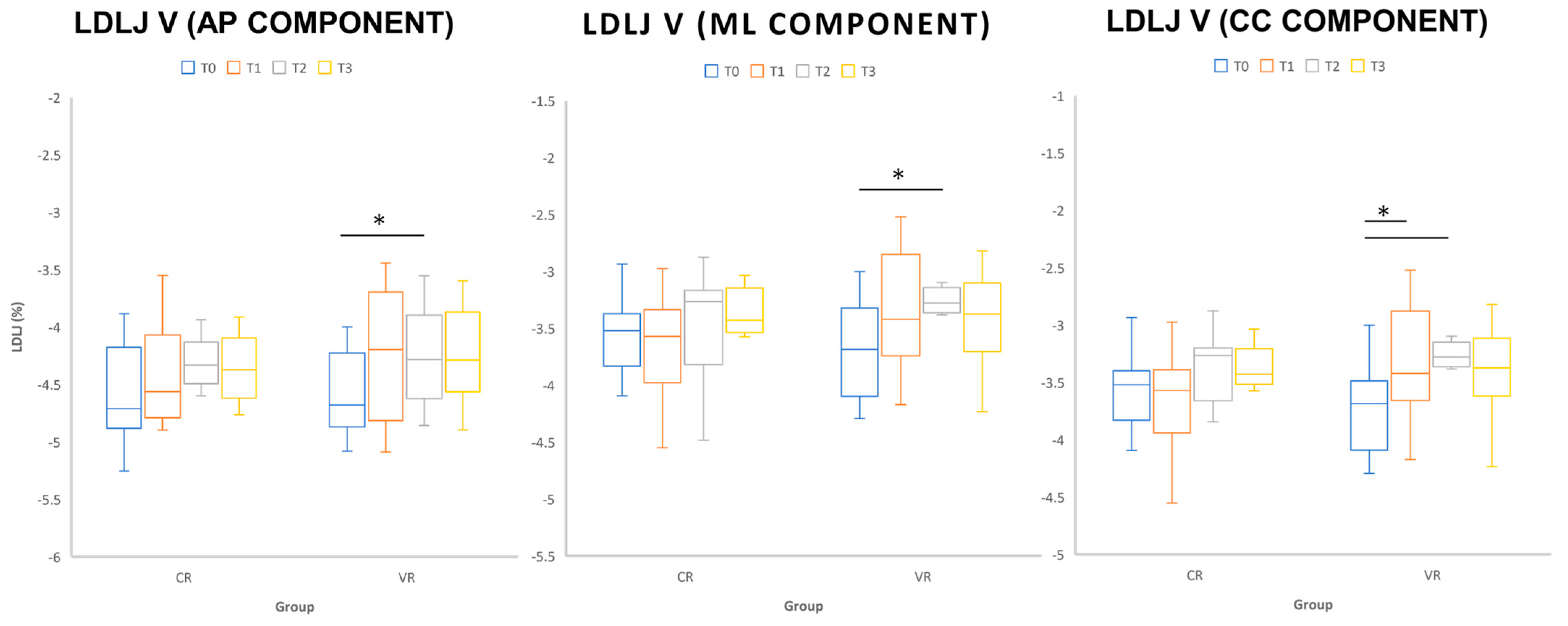
| Smooth | LDLJvAP (%) | −4.58 ± 0.38 | −4.25 ± 0.57 | −4.25 ± 0.43 | −4.25 ± 0.41 | −4.60 ± 0.43 | −4.43 ± 0.43 | −4.25 ± 0.33 | −4.37 ± 0.29 | 02, 03, 12 |
| LDLJvML (%) | −4.50 ± 0.34 | −4.09 ± 0.44 | −4.06 ± 0.23 | −4.11 ± 0.53 | −4.27 ± 0.42 | −4.15 ± 0.28 | −4.07 ± 0.34 | −4.13 ± 0.32 | 02 | |
| LDLJvCC (%) | −3.72 ± 0.42 | −3.35 ± 0.49 | −3.24 ± 0.28 | −3.39 ± 0.44 | −3.62 ± 0.45 | −3.63 ± 0.44 | −3.46 ± 0.43 | −3.42 ± 0.34 | 01, 02, 03 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tramontano, M.; Belluscio, V.; Bergamini, E.; Allevi, G.; De Angelis, S.; Verdecchia, G.; Formisano, R.; Vannozzi, G.; Buzzi, M.G. Vestibular Rehabilitation Improves Gait Quality and Activities of Daily Living in People with Severe Traumatic Brain Injury: A Randomized Clinical Trial. Sensors 2022, 22, 8553. https://doi.org/10.3390/s22218553
Tramontano M, Belluscio V, Bergamini E, Allevi G, De Angelis S, Verdecchia G, Formisano R, Vannozzi G, Buzzi MG. Vestibular Rehabilitation Improves Gait Quality and Activities of Daily Living in People with Severe Traumatic Brain Injury: A Randomized Clinical Trial. Sensors. 2022; 22(21):8553. https://doi.org/10.3390/s22218553
Chicago/Turabian StyleTramontano, Marco, Valeria Belluscio, Elena Bergamini, Giulia Allevi, Sara De Angelis, Giorgia Verdecchia, Rita Formisano, Giuseppe Vannozzi, and Maria Gabriella Buzzi. 2022. "Vestibular Rehabilitation Improves Gait Quality and Activities of Daily Living in People with Severe Traumatic Brain Injury: A Randomized Clinical Trial" Sensors 22, no. 21: 8553. https://doi.org/10.3390/s22218553
APA StyleTramontano, M., Belluscio, V., Bergamini, E., Allevi, G., De Angelis, S., Verdecchia, G., Formisano, R., Vannozzi, G., & Buzzi, M. G. (2022). Vestibular Rehabilitation Improves Gait Quality and Activities of Daily Living in People with Severe Traumatic Brain Injury: A Randomized Clinical Trial. Sensors, 22(21), 8553. https://doi.org/10.3390/s22218553








