The Effect of Active Stretching Training in Patients with Chronic Venous Insufficiency Monitored by Raster-Stereography
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
- -
- Age > 18 years
- -
- Bilateral chronic venous insufficiency CEAP clinical classification [3]: C2-3EpAsPr.
- -
- Previous lower limbs trauma
- -
- Heart/respiratory/renal insufficiency
- -
- Use of graduated compression stockings
- -
- Competitive physical activity
- -
- Physical activity in the aquatic environment
- -
- Body mass index > 35
- -
- Inability to perform any form of physical activity
2.3. Randomization
2.4. Intervention
2.5. Outcome Measurements
2.6. Lower Limb Volumetry
2.7. Air Plethysmography (APG)
2.8. Ankle Range of Motion (ROM)
2.9. Quality of Life (QoL)
2.10. Optoelectronic Body Posture Machine
- -
- Lordotic angle ITL-ILS max°: the maximum lordotic angle measured between the tangents to the surface of the thoraco-lumbar inflexion point (ITL) and the lower lumbo-sacral inflexion point (ILS).
- -
- Pelvic tilt in degrees (°): The angle between the line connecting left dimple (DL) and right dimple (DR) and the horizontal.
- -
- Pelvic tilt (mm): pelvic tilt refers to the difference in height of the sacral dimples (DL-DR).
2.11. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Lower Limb Volume
3.3. Air Plethysmography (APG)
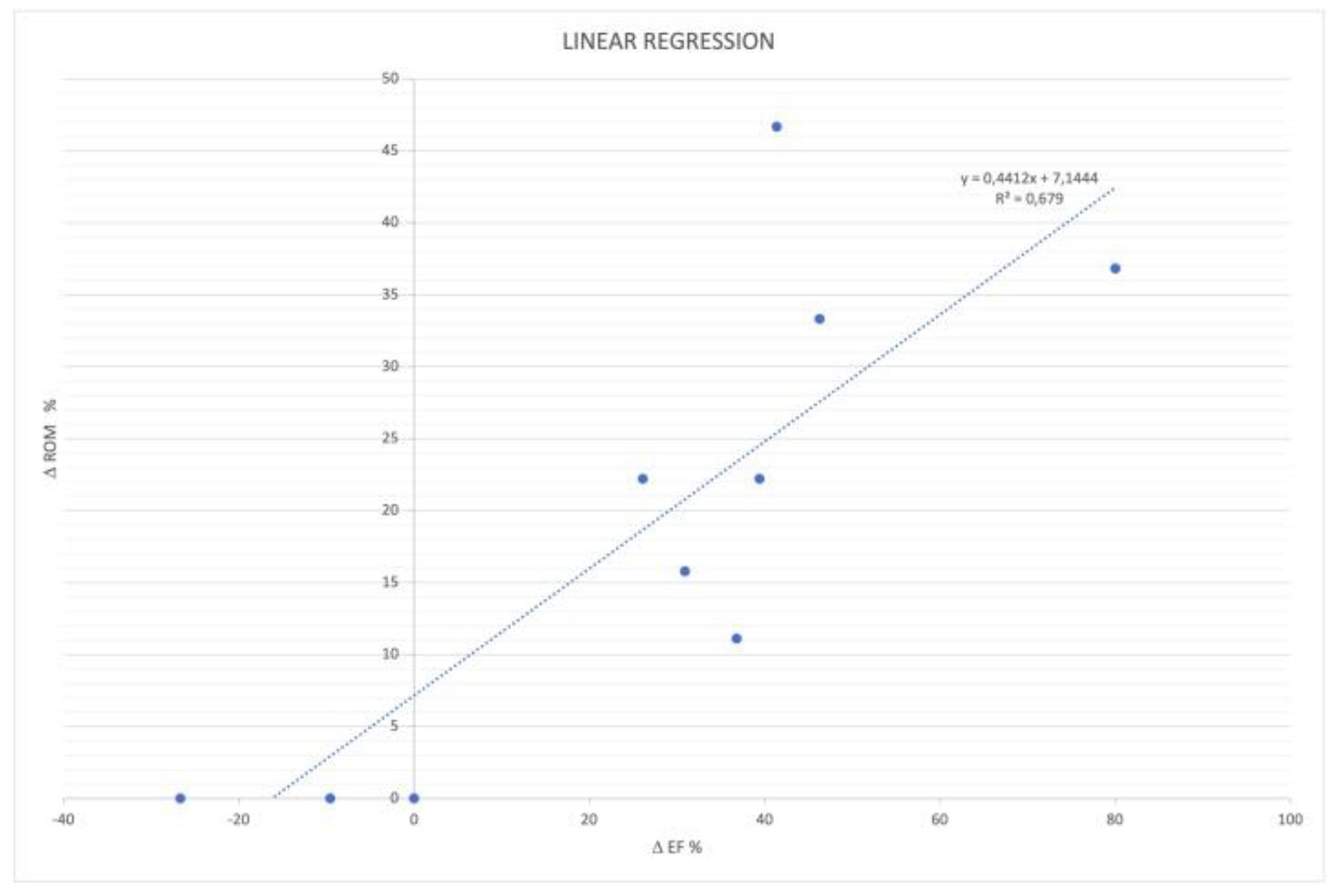
3.4. Ankle ROM
3.5. Quality of Life
3.6. Body Posture Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Callam, M.J. Epidemiology of varicose veins. Br. J. Surg. 1994, 81, 167–173. [Google Scholar] [CrossRef]
- Beebe-Dimmer, J.L.; Pfeifer, J.R.; Engle, J.S.; Schottenfeld, D. The epidemiology of chronic venous insufficiency and varicose veins. Ann. Epidemiol. 2005, 15, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Lurie, F.; Passman, M.; Meisner, M.; Dalsing, M.; Masuda, E.; Welch, H.; Bush, R.L.; Blebea, J.; Carpentier, P.H.; De Maeseneer, M.; et al. The 2020 update of the CEAP classification system and reporting standards. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Jawien, A. The influence of environmental factors in chronic venous insufficiency. Angiology 2003, 54, S19–S31. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.J.; Lee, J. Chronic venous insufficiency and varicose veins of the lower extremities. Korean J. Intern. Med. 2019, 34, 269–283. [Google Scholar] [CrossRef]
- Vlajinac, H.D.; Radak, D.J.; Marinkovic, J.M.; Maksimovic, M.Z. Risk factors for chronic venous disease. Phlebology 2012, 27, 416–422. [Google Scholar] [CrossRef]
- Elamrawy, S.; Darwish, I.; Moustafa, S.; Elshaer, N.; Ahmed, N. Epidemiological, lifestyle, and occupational factors associated with lower limb varicose veins: A case control study. J. Egypt Public Heal. Assoc. 2021, 96, 19. [Google Scholar] [CrossRef]
- Łastowiecka-Moras, E. Standing and sitting postures at work and symptoms of ve nous insufficiency results from questionnaires and a Doppler ultrasound study. Int. J. Occup. Saf. Ergon. 2021, 27, 963–969. [Google Scholar] [CrossRef]
- Uhl, J.F.; Chahim, M.; Allaert, F.A. Static foot disorders: A major risk factor for chronic venous disease? Phlebology 2012, 27, 13–18. [Google Scholar] [CrossRef]
- Thibert, A.; Briche, N.; Vernizeau, B.D.; Mougin-Guillaume, F.; Béliard, S. Therapeutic Patient Education Working Group of the French Society of Vascular Medicine. Systematic review of adapted physical activity and therapeutic education of patients with chronic venous disease. J. Vasc. Surg. Venous Lymphat. Disord. 2022, 10, 1385–1400. [Google Scholar] [CrossRef]
- Mancini, S.; Piccinetti, A.; Nappi, G.; Mancini, S.; Caniato, A.; Coccheri, S. Clinical, functional and quality of life changes after balneokinesis with sulphurous water in patients with varicose veins. Vasa 2003, 32, 26–30. [Google Scholar] [PubMed]
- Forestier, R.J.; Briancon, G.; Francon, A.; Erol, F.B.; Mollard, J.M. Balneohydrotherapy in the treatment of chronic venous insufficiency. Vasa 2014, 43, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, P.H.; Satger, B. Randomized trial of balneotherapy associated with patient education in patients with advanced chronicvenous. insufficiency. J. Vasc. Surg. 2009, 49, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, P.H.; Blaise, S.; Satger, B.; Genty, C.; Rolland, C.; Roques, C.; Bosson, J.-L. A multicenter randomized controlled trial evaluating balneotherapy in patients with advanced chronic venous insufficiency. J. Vasc. Surg. 2014, 59, 447–454.e1. [Google Scholar] [CrossRef]
- Padberg, F.T.; Johnston, M.V.; Sisto, S.A.; Burnand, K.G.; Wakefield, T.W.; Perkowski, P. Structured exercise improves calf muscle pump function in chronic venous insufficiency: A randomized trial. J. Vasc. Surg. 2004, 39, 79–87. [Google Scholar] [CrossRef]
- Ercan, S.; Çetin, C.; Yavuz, T.; Demir, H.M.; Atalay, Y.B. Effects of isokinetic calf muscle exercise program on muscle strength and venous function in patients with chronic venous insufficiency. Phlebol. J. Venous Dis. 2017, 33, 261–266. [Google Scholar] [CrossRef]
- Klonizakis, M.D.; Tew, G.A.; Gumber, A.; Crank, H.; King, B.; Middleton, G.; Michaels, J.A. Supervised exercise training as an adjunct therapy for venous leg ulcers: A randomized controlled feasibility trial. Br. J. Dermatol. 2018, 178, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Menegatti, E.; Masiero, S.; Zamboni, P.; Avruscio, G.; Tessari, M.; Pagani, A.; Gianesini, S. Randomized controlled trial on Dryland and Thermal Aquatic standardized exercise protocol for chronic venous disease (DATA study). J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 1226–1234.e2, (da spostare prima era al 25). [Google Scholar] [CrossRef]
- Guürdal Karakelle, S.; Ipek, Y.; Tulin, O.; Alpagut, I.U. The efficiency of exercise training in patients with venous insufficiency: A double blinded, randomized controlled trial. Phlebology 2021, 36, 440–449. [Google Scholar] [CrossRef]
- Franceschi, C.; Zamboni, P. Principles of Venous Haemodynamics; Nova Science: New York, NY, USA, 2008; p. 44. [Google Scholar]
- Hall, G.; Laddu, D.R.; Phillips, S.A.; Lavie, C.J.; Arena, R. A tale of two pandemics: How will COVID-19 and global trends in physical inactivity and sedentary behavior affect one another? Prog. Cardiovasc. Dis. 2021, 64, 108–110. [Google Scholar] [CrossRef]
- Smirmaul, B.P.C.; Arena, R. The Urgent Need to Sit Less and Move More During the COVID-19 Pandemic. J. Cardiopulm. Rehabil. Prev. 2020, 40, 287–289. [Google Scholar] [CrossRef]
- Amorese, A.J.; Ryan, A.S. Home-Based Tele-Exercise in Musculoskeletal Conditions and Chronic Disease: A Literature Review. Front. Rehabil. Sci. 2022, 3, 811465. [Google Scholar] [CrossRef] [PubMed]
- Kaulesar Sukul, D.M.; den Hoed, P.T.; Johannes, E.J.; van Dolder, R.; Benda, E. Direct and indirect methods for the quantification of leg volume: Comparison between water displacement volumetry, the disk model method and the frustum sign model method, using the correlation coefficient and the limits of agreement. J. Biomed. Eng. 1993, 15, 477–480. [Google Scholar] [CrossRef]
- Christopoulos, D.G.; Nicolaides, A.N.; Szendro, G.; Irvine, A.T.; Bull, M.L.; Eastcott, H.H.G. Air-plethysmography and the effect of elastic compression on venous hemodynamics of the leg. J. Vasc. Surg. 1987, 5, 148–159. [Google Scholar] [CrossRef]
- Alawna, M.A.; Unver, B.H.; Yuksel, E.O. The Reliability of a Smartphone Goniometer Application Compared with a Traditional Goniometer for Measuring Ankle Joint Range of Motion. J. Am. Podiatr. Med. Assoc. 2019, 109, 22–29. [Google Scholar] [CrossRef]
- Brosseau, L.; Tousignant, M.; Budd, J.; Chartier, N.; Duciaume, L.; Plamondon, S.; O’Sullivan, J.P.; O’Donoghue, S.; Balmer, S. Intratester and intertester reliability and criterion validity of the parallelogram and universal goniometers for active knee flexion in healthy subjects. Physiother. Res. Int. 1997, 2, 150–166. [Google Scholar] [CrossRef]
- Paty, J.; Turner-Bowker, D.; Elash, C.A.; Wright, D. The VVSymQ® instrument: Use of a new patient-reported outcome measure for assessment of varicose vein symptoms. Phlebology 2016, 31, 481–488. [Google Scholar] [CrossRef]
- Degenhardt, B.F.; Starks, Z.; Bhatia, S.; Franklin, G.-A. Appraisal of the DIERS method for calculating postural measurements: An observational study. Scoliosis Spinal Disord. 2017, 12, 28. [Google Scholar] [CrossRef]
- Degenhardt, B.F.; Starks, Z.; Bhatia, S. Reliability of the DIERS Formetric 4D Spine Shape Parameters in Adults without Postural Deformities. Biomed. Res. Int. 2020, 1796247. [Google Scholar]
- Wilczyński, J.; Pedrycz, A.; Mucha, D.; Ambroży, T.; Mucha, D. Body Posture, Postural Stability, and Metabolic Age in Patients with Parkinson’s Disease. Biomed. Res. Int. 2017, 3975417. [Google Scholar] [CrossRef]
- Uhl, J.F.; Gillot, C. Anatomy of the veno-muscular pumps of the lower limb. Phlebology 2015, 30, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.; Knepper, J.; May, C.; Knight, A.; Pace, N.; Jayaraj, A. Ambulatory venous pressure, air plethysmography, and the role of calf venous pump in chronic venous disease. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Simka, M. Calf muscle pump impairment and delayed healing of venous leg ulcers: Air plethysmographic findings. J. Dermatol. 2007, 34, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, C.R.; Franceschi, C.; Kalodiki, E. Optimizing calf muscle pump function. Phlebology 2018, 33, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Araujo, D.N.; Ribeiro, C.T.; Maciel, A.C.; Bruno, S.S.; Fregonezi, G.A.; Dias, F.A.L. Physical exercise for the treatment of non-ulcerated chronic venous insufficiency. Cochrane Database Syst. Rev. 2016, 12, CD010637. [Google Scholar] [CrossRef]
- Dixy, F.; Brooke, R.; McCollum, C. Venous disease is associated with an impaired range of ankle movement. Eur. J. Vasc. Endovasc. Surg. 2003, 25, 556–561. [Google Scholar] [CrossRef]
- Ohlendorf, D.; Adjami, F.; Scharnweber, B.; Schulze, J.; Ackermann, H.; Oremek, G.; Kopp, S.; Groneberg, D. Standard values of the upper body posture in male adults. Adv. Clin. Exp. Med. 2018, 27, 1521–1528. [Google Scholar] [CrossRef]
- Son, J.-H.; Park, G.D.; Park, H.S. The effect of sacroiliac joint mobilization on pelvic deformation and the static balance ability of female university students with si joint dysfunction. J. Phys. Ther. Sci. 2014, 26, 845–848. [Google Scholar] [CrossRef][Green Version]
- Awick, E.A.; Ehlers, D.K.; Aguiñaga, S.; Daugherty, A.M.; Kramer, A.F.; McAuley, E. Effects of a randomized exercise trial on physical activity, psychological distress and quality of life in older adults. Gen. Hosp. Psychiatry 2017, 49, 44–50. [Google Scholar] [CrossRef]



| PHASE 1: RESPIRATORY EXERCISES | |||
| Exercise Type | Starting Position | Exercise Performance | Exercise Duration |
| Upper thoracic respiration (Figure 1A) | Supine position with legs bent and hands resting on the high chest | Breath-in to expand the upper chest ribs towards the hands. Breath-out completely emptying the chest | 1′ |
| Diaphragmatic breathing (Figure 1B) | Supine position with legs bent and hands resting at the level of the navel | Deep breath-in, expanding the abdomen and bringing the navel as high as possible. Breath-out slowly, trying to deflate the abdomen as much as possible. | 1′ |
| PHASE 2: VERTEBRAL COLUMN AND PELVIS MOBILIZATION | |||
| Exercise Type | Starting Position | Exercise Performance | Exercise Duration |
| Antero-retropulsion of the cervical column tract (Figure 1C,D) | Supine position with legs bent and arms to the sides with palms up. | Deep inspiration bringing the chin forward and upwards. Expiration pushing the chin down, trying to flatten the cervical column tract. | 1′ |
| Antero and retroversion of the lumbar tract (Figure 1E,F) | Supine position with legs bent and arms to the sides with palms up. | Deep inspiration bringing the pelvis forward against gravity and expiration pushing the pelvis and lumbar tract toward the floor. | 1′ |
| PHASE 3: GLOBAL STRETCHING AND CALF PUMP ACTIVATION | |||
| Exercise Type | Starting Position | Exercise Performance | Exercise Duration |
| Posterior kinetic chain (Figure 1G,H) | Supine position with legs bent and arms to the sides with palms up. | Raise the arms above the head and bring the right knee to the chest. Coordinate inspiration and expiration phase, detaching the shoulders from the floor and pushing the knee to the chest. Repeat with contralateral leg. | 3′ |
| Posterior kinetic chain (Figure 1I,J) | Supine position with legs bent and arms to the sides with palms up. | Bring the knees to the chest. From this position lift the legs up, alternating feet dorsiflexion and plantarflexion. | 5′ |
| Posterior kinetic chain (Figure 1K,L) | Supine position with legs lying on the ground and arms to the sides with palms up. | Bring the right knee to the chest grabbing it with the hands. Coordinate inspiration and expiration phase pushing the knee to the chest. Repeat with contralateral leg. | 3′ |
| Posterior kinetic chain (Figure 1M) | Supine position with legs bent and arms to the sides with palms up. | Bring the knees to the chest grabbing them with the hands. Coordinate inspiration and expiration phase pushing the knee to the chest and alternating feet dorsiflexion and plantarflexion. | 5′ |
| Posterior kinetic chain (Figure 1N,O) | Supine position, lower limbs raised from the ground, flexed leaning against the wall, abducted arms at 120° | Extend right limb with the foot in dorsiflexion, sliding with the heel to the wall upwards. Coordinate inspiration and expiration phase pushing the knee to the chest and alternating feet dorsiflexion and plantarflexion. Repeat with contralateral leg. | 5′ |
| PHASE 4: “CORE” AND LOWER LIMBS STRENGTHENING | |||
| Exercise Type | Starting Position | Exercise Performance | Exercise Duration |
| Core (Figure 1P) | Quadruped supine position | Extend right leg and bring up the contralateral arm during expiration. Stay in this position for three complete respirations. Repeat with contralateral | 3′ |
| Core (Figure 1Q,R) | Sitting position keeping the feet flat on the floor. Abducted arms. | Breath-in and breath-out bringing the right knee close to the chest. Stay in this position for three complete respirations. Repeat with contralateral | 3′ |
| Lower limbs strength (Figure 1S,T) | Lateral decubitus legs bent and 1 arm extended | Breath-out lifting the upper leg. Stay in this position for three complete respirations. Repeat with contralateral | 3′ |
| Lower limbs strength (Figure 1U,V) | Lateral decubitus with legs extended. | Breath-out lifting the superior leg. Stay in this position for three complete respirations. Repeat with contralateral | 3′ |
| PHASE 5: RELAXATION | |||
| Exercise Type | Starting Position | Exercise Performance | Exercise Duration |
| Cervical column tract (Figure 1W) | Sitting position | Neck semi-circling | 1′ |
| Shoulder (Figure 1X) | Sitting position | Shoulder semi-circling and circling | 2′ |
| AGS Exercise Group (10 Patients) | Control Group (10 Patients) | p | |
|---|---|---|---|
| Age (years) | 62.9 ± 9.7 | 54.5 ± 15.5 | 0.1632 |
| Gender (number of males vs. female) | 0 out of 10 | 2 out of 8 | 0.9999 |
| BMI | 23.3 ± 1.7 | 24.8 ± 1.7 | 0.2190 |
| LEG VOLUME (mL) | 2340 ± 239 | 2419 ± 335 | 0.5496 |
| CEAP | 2.7 ± 0.5 | 2.8 ± 0.4 | 0.7481 |
| MAIN SYMTOMPS | |||
| Heaviness (number of patients out of total) | 10 out of 10 | 10 out of 10 | 0.9999 |
| Pain (number of patients out of total) | 10 out of 10 | 10 out of 10 | 0.9999 |
| Swelling (number of patients out of total) | 10 out of 10 | 10 out of 10 | 0.9999 |
| Throbbing (number of patients out of total) | 7 out of 10 | 8 out of 10 | 0.9999 |
| Itching (number of patients out of total) | 6 out of 10 | 7 out of 10 | 0.9999 |
| AS Exercise Group | C Group | Reference Value Reported in Literature [16] | |||||
|---|---|---|---|---|---|---|---|
| T0 | T3 | p | T0 | T3 | p | ||
| Lordotic angle ITL-ILS max° | 51.7 ± 10.5 | 46.6 ± 9.2 | <0.01 | 39.3 ± 14.8 | 39.6 ± 14.9 | 0.6043 | 35.45 ± 7.55 |
| Pelvic Torsion (DL-DR°) | 4.4 ± 2.2 | 2.5 ± 1.9 | <0.01 | 2.1 ± 2.0 | 2.0 ± 2.2 | 0.3434 | −0.07 ± 2.95 |
| Pelvic Tilting (DL-DR mm) | 5.3 ± 3.6 | 3.6 ± 2.8 | <0.04 | 6.3 ± 5.1 | 6.1 ± 5.6 | 0.3434 | −0.12 ± 5.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menegatti, E.; Mandini, S.; Pagani, A.; Mandini, B.; Zerbini, V.; Piva, T.; Raisi, A.; Fabbri, M.; Fogli, M.; Mazzoni, G.; et al. The Effect of Active Stretching Training in Patients with Chronic Venous Insufficiency Monitored by Raster-Stereography. Sensors 2022, 22, 8509. https://doi.org/10.3390/s22218509
Menegatti E, Mandini S, Pagani A, Mandini B, Zerbini V, Piva T, Raisi A, Fabbri M, Fogli M, Mazzoni G, et al. The Effect of Active Stretching Training in Patients with Chronic Venous Insufficiency Monitored by Raster-Stereography. Sensors. 2022; 22(21):8509. https://doi.org/10.3390/s22218509
Chicago/Turabian StyleMenegatti, Erica, Simona Mandini, Anselmo Pagani, Beatrice Mandini, Valentina Zerbini, Tommaso Piva, Andrea Raisi, Marinella Fabbri, Marco Fogli, Gianni Mazzoni, and et al. 2022. "The Effect of Active Stretching Training in Patients with Chronic Venous Insufficiency Monitored by Raster-Stereography" Sensors 22, no. 21: 8509. https://doi.org/10.3390/s22218509
APA StyleMenegatti, E., Mandini, S., Pagani, A., Mandini, B., Zerbini, V., Piva, T., Raisi, A., Fabbri, M., Fogli, M., Mazzoni, G., Zamboni, P., & Gianesini, S. (2022). The Effect of Active Stretching Training in Patients with Chronic Venous Insufficiency Monitored by Raster-Stereography. Sensors, 22(21), 8509. https://doi.org/10.3390/s22218509







