Measuring Kinematic Response to Perturbed Locomotion in Young Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
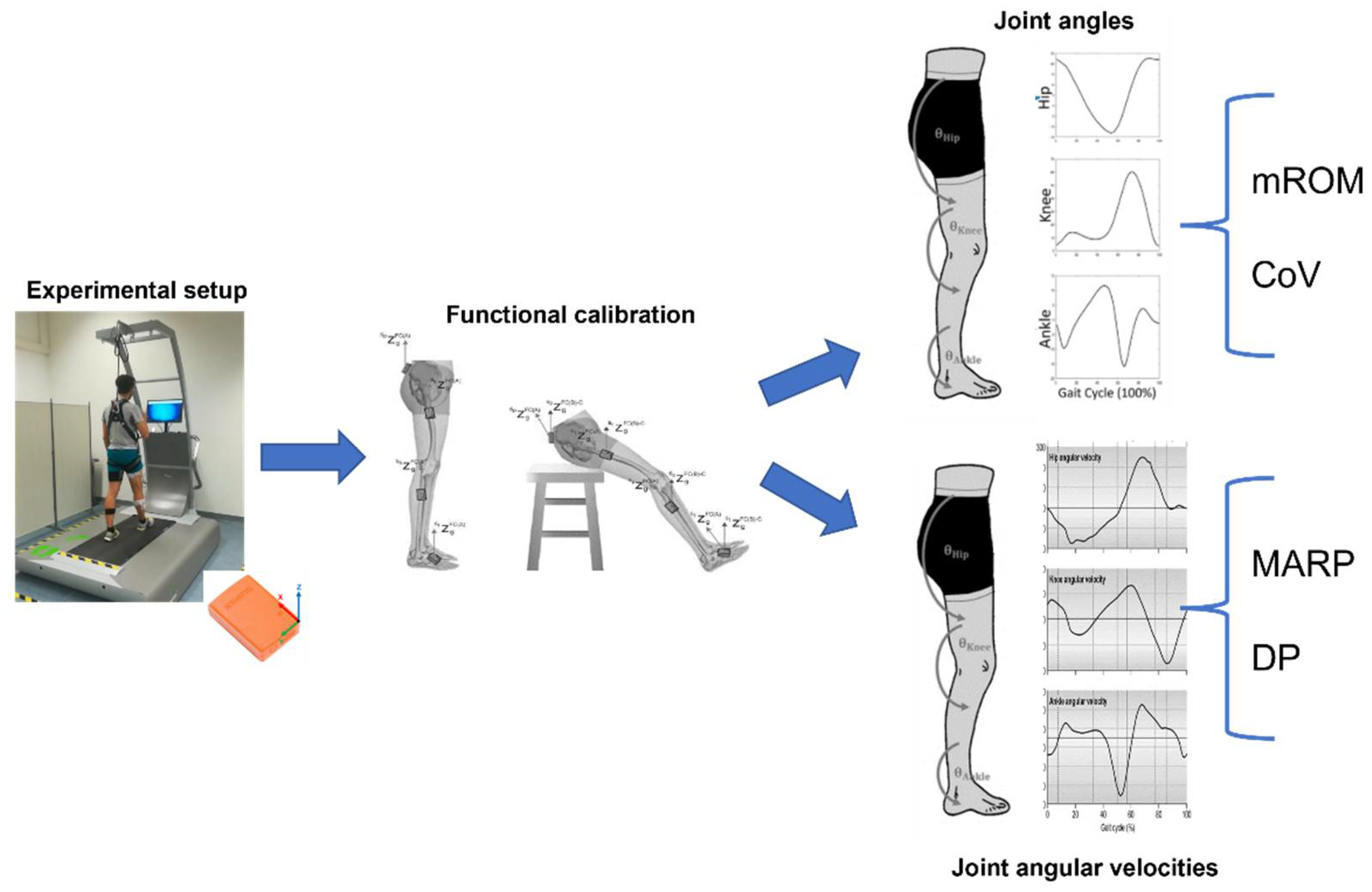
2.2. Instrumentation
2.3. Experimental Protocol
2.4. Data Processing and Analysis
2.5. Statistical Analysis
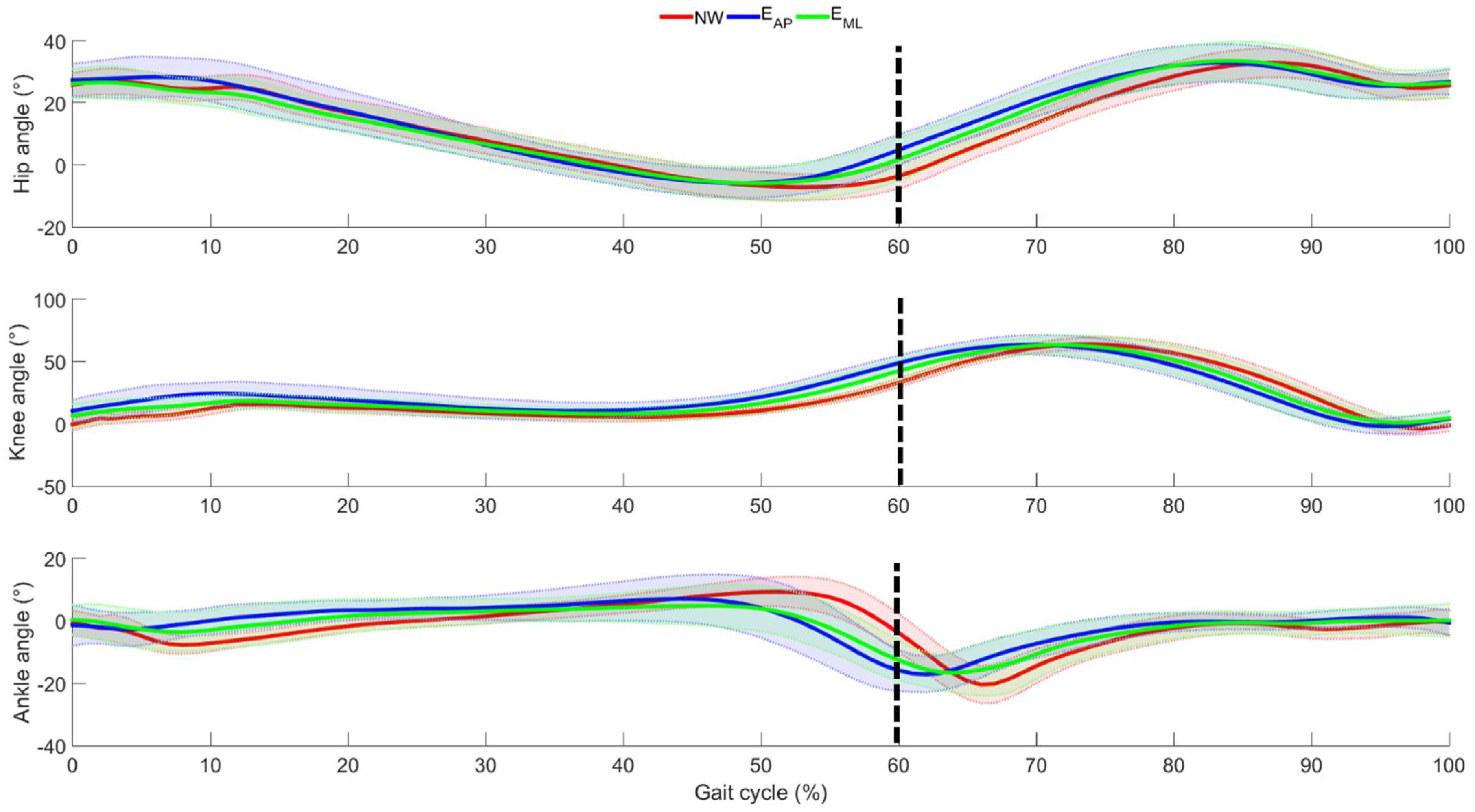
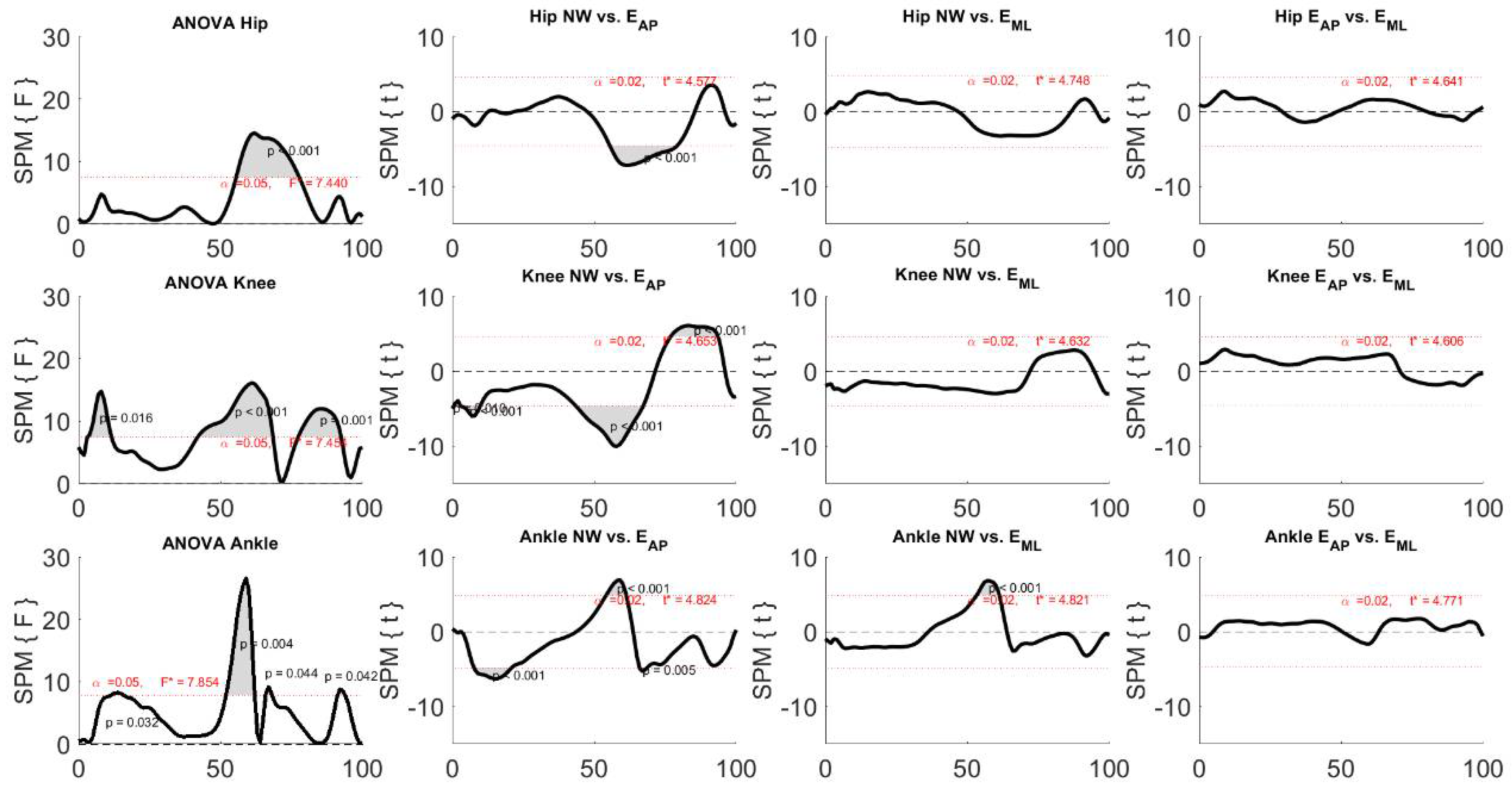
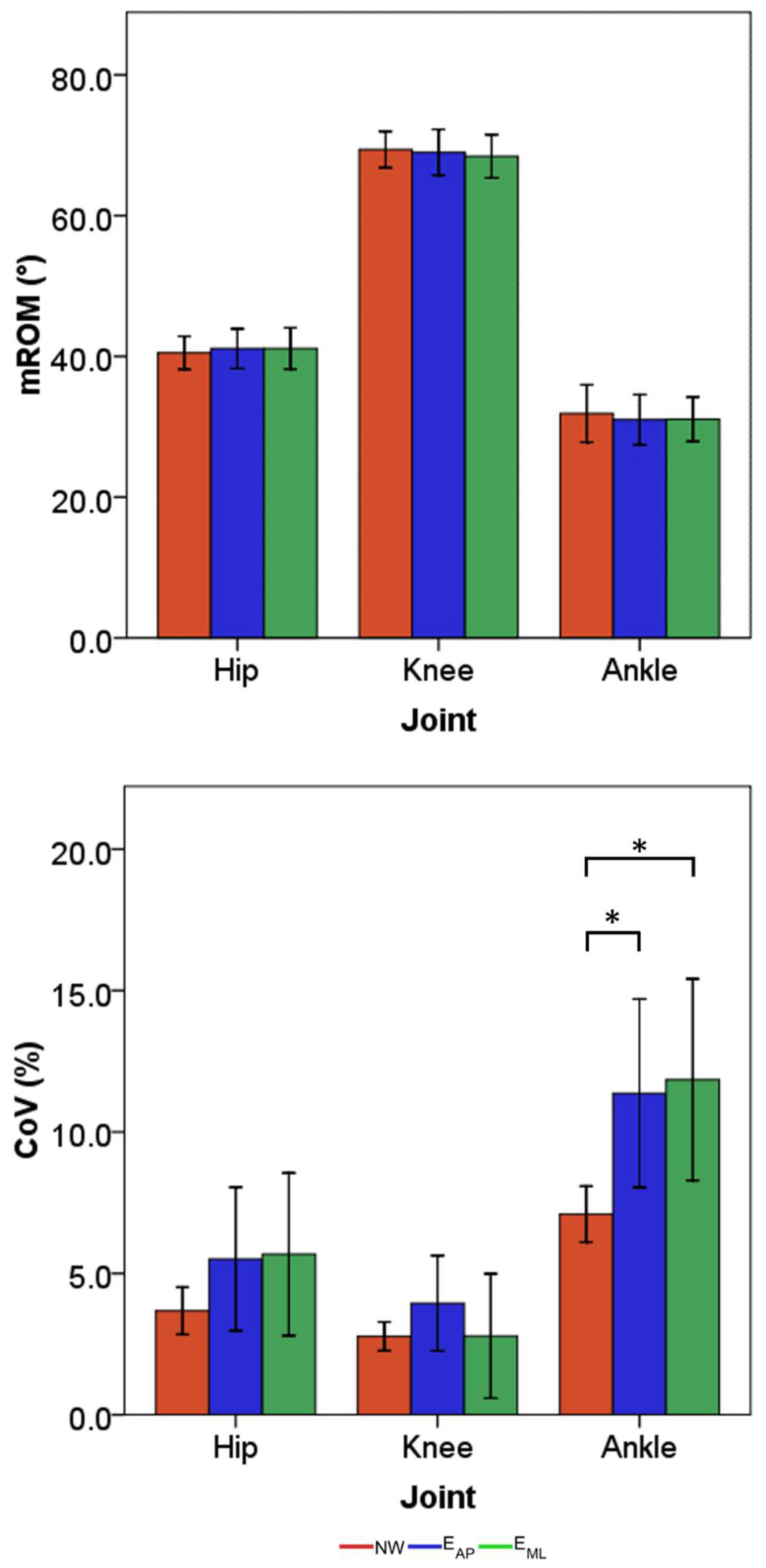
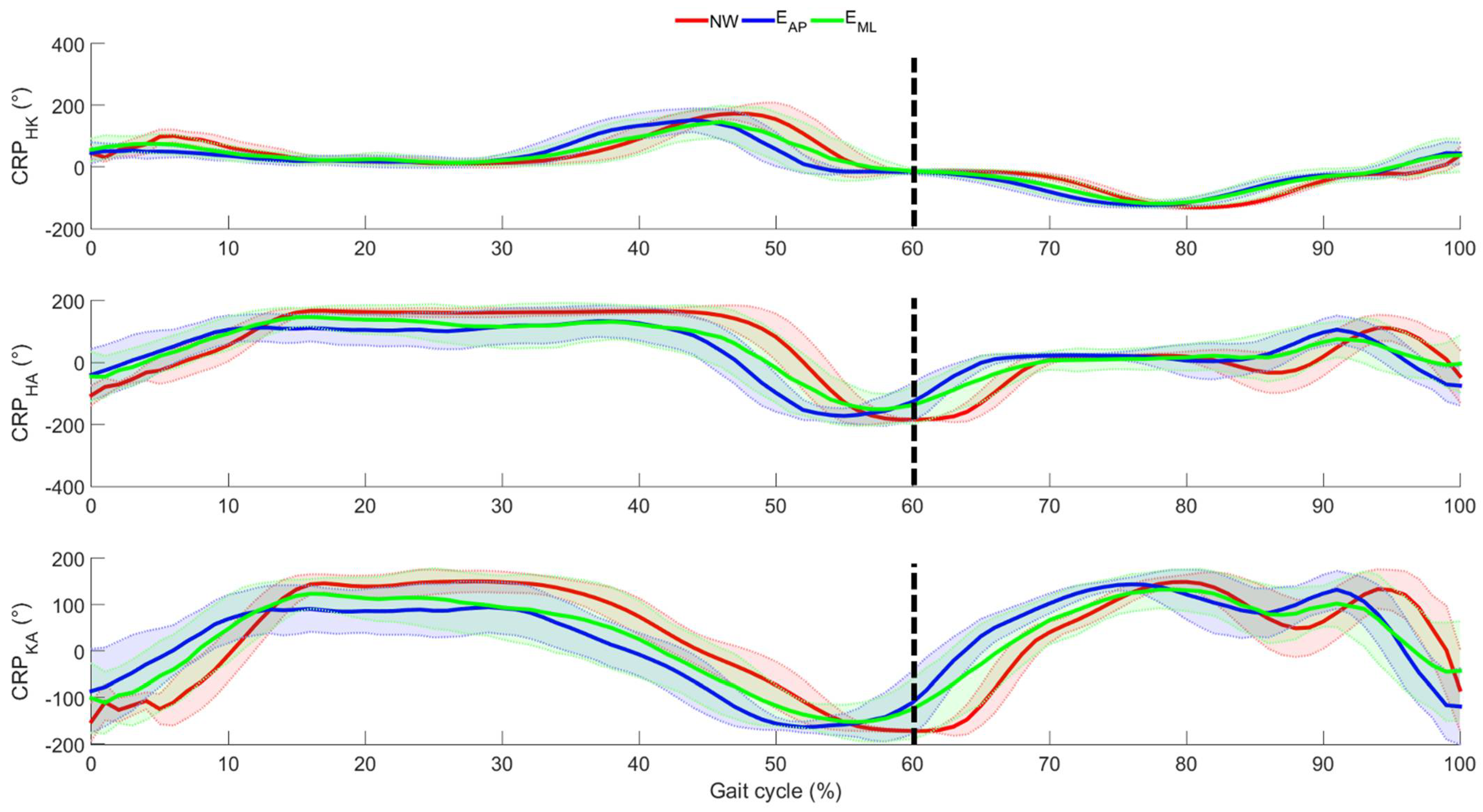
3. Results
4. Discussion
4.1. Effects of Perturbation on Lower Limb Joint Angles
4.2. Effects of Perturbation on Lower Limb Inter-Joint Coordination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marigold, D.S.; Patla, A.E. Strategies for dynamic stability during locomotion on a slippery surface: Effects of prior experience and knowledge. J. Neurophysiol. 2002, 88, 339–353. [Google Scholar] [CrossRef]
- Marigold, D.S.; Patla, A.E. Adapting locomotion to different surface compliances: Neuromuscular responses and changes in movement dynamics. J. Neurophysiol. 2005, 94, 1733–1750. [Google Scholar] [CrossRef]
- Ippersiel, P.; Robbins, S.M.; Dixon, P.C. Lower-limb coordination and variability during gait: The effects of age and walking surface. Gait Posture 2021, 85, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.; Peters, A.L.; Liu, B.A.; Maki, B.E. A perturbation-based balance training program for older adults: Study protocol for a randomised controlled trial. BMC Geriatr. 2007, 7, 1–14. [Google Scholar] [CrossRef]
- Van Dieën, J.H.; Pijnappels, M. Balance control in older adults. In Locomotion and Posture in Older Adults: The Role of Aging and Movement Disorders; Springer International Publishing: Cham, Switzerland, 2017; pp. 237–262. ISBN 9783319489803. [Google Scholar]
- Taborri, J.; Mileti, I.; Del Prete, Z.; Rossi, S.; Palermo, E. Yaw Postural Perturbation Through Robotic Platform: Aging Effects on Muscle Synergies. In Proceedings of the 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob), Enschede, The Netherlands, 26–29 August 2018; pp. 916–921. [Google Scholar]
- Nordin, E.; Moe-Nilssen, R.; Ramnemark, A.; Lundin-Olsson, L. Changes in step-width during dual-task walking predicts falls. Gait Posture 2010, 32, 92–97. [Google Scholar] [CrossRef]
- Plummer-D’Amato, P.; Altmann, L.J.P.; Saracino, D.; Fox, E.; Behrman, A.L.; Marsiske, M. Interactions between cognitive tasks and gait after stroke: A dual task study. Gait Posture 2008, 27, 683–688. [Google Scholar] [CrossRef] [PubMed]
- McFadyen, B.J.; Cantin, J.F.; Swaine, B.; Duchesneau, G.; Doyon, J.; Dumas, D.; Fait, P. Modality-Specific, Multitask Locomotor Deficits Persist Despite Good Recovery After a Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2009, 90, 1596–1606. [Google Scholar] [CrossRef]
- Szturm, T.; Maharjan, P.; Marotta, J.J.; Shay, B.; Shrestha, S.; Sakhalkar, V. The interacting effect of cognitive and motor task demands on performance of gait, balance and cognition in young adults. Gait Posture 2013, 38, 596–602. [Google Scholar] [CrossRef]
- O’Connor, S.M.; Donelan, J.M. Fast visual prediction and slow optimization of preferred walking speed. J. Neurophysiol. 2012, 107, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Santuz, A.; Ekizos, A.; Eckardt, N.; Kibele, A.; Arampatzis, A. Challenging human locomotion: Stability and modular organisation in unsteady conditions. Sci. Rep. 2018, 8, 1–13. [Google Scholar]
- Madehkhaksar, F.; Klenk, J.; Sczuka, K.; Gordt, K.; Melzer, I.; Schwenk, M. The effects of unexpected mechanical perturbations during treadmill walking on spatiotemporal gait parameters, and the dynamic stability measures by which to quantify postural response. PLoS ONE 2018, 13, e0195902. [Google Scholar] [CrossRef]
- Rabago, C.A.; Dingwell, J.B.; Wilken, J.M. Reliability and minimum detectable change of temporal-spatial, kinematic, and dynamic stability measures during perturbed gait. PLoS ONE 2015, 10, e0142083. [Google Scholar] [CrossRef] [PubMed]
- Roeles, S.; Rowe, P.J.; Bruijn, S.M.; Childs, C.R.; Tarfali, G.D.; Steenbrink, F.; Pijnappels, M. Gait stability in response to platform, belt, and sensory perturbations in young and older adults. Med. Biol. Eng. Comput. 2018, 56, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Inkol, K.A.; Huntley, A.H.; Vallis, L.A. Repeated Exposure to Forward Support-Surface Perturbation During Overground Walking Alters Upper-Body Kinematics and Step Parameters. J. Mot. Behav. 2019, 51, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Santuz, A.; Brüll, L.; Ekizos, A.; Schroll, A.; Eckardt, N.; Kibele, A.; Schwenk, M.; Arampatzis, A. Neuromotor Dynamics of Human Locomotion in Challenging Settings. iScience 2020, 23, 100796. [Google Scholar] [CrossRef] [PubMed]
- Rum, L.; Vannozzi, G.; Macaluso, A.; Laudani, L. Neuromechanical response of the upper body to unexpected perturbations during gait initiation in young and older adults. Aging Clin. Exp. Res. 2020, 33, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Zubair, H.N.; Stout, E.E.; Dounskaia, N.; Beloozerova, I.N. The role of intersegmental dynamics in coordination of the forelimb joints during unperturbed and perturbed skilled locomotion. J. Neurophysiol. 2018, 120, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Hafer, J.F.; Boyer, K.A. Age related differences in segment coordination and its variability during gait. Gait Posture 2018, 62, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Runge, C.F.; Shupert, C.L.; Horak, F.B.; Zajac, F.E. Ankle and hip postural strategies defined by joint torques. Gait Posture 1999, 10, 161–170. [Google Scholar] [CrossRef]
- Nestico, J.; Novak, A.; Perry, S.D.; Mansfield, A. Does increased gait variability improve stability when faced with an expected balance perturbation during treadmill walking? Gait Posture 2021, 86, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Germanotta, M.; Taborri, J.; Rossi, S.; Frascarelli, F.; Palermo, E.; Cappa, P.; Castelli, E.; Petrarca, M. Spasticity Measurement Based on Tonic Stretch Reflex Threshold in Children with Cerebral Palsy Using the PediAnklebot. Front. Hum. Neurosci. 2017, 11, 277. [Google Scholar] [CrossRef]
- Hamdi, M.M.; Awad, M.I.; Abdelhameed, M.M.; Tolbah, F.A. Lower limb motion tracking using IMU sensor network. In Proceedings of the 7th Cairo International Biomedical Engineering Conference, CIBEC 2014, Giza, Egypt, 11–13 December 2014; pp. 28–33. [Google Scholar]
- Seel, T.; Raisch, J.; Schauer, T. IMU-based joint angle measurement for gait analysis. Sensors 2014, 14, 6891–6909. [Google Scholar] [CrossRef] [PubMed]
- Palermo, E.; Rossi, S.; Marini, F.; Patanè, F.; Cappa, P. Experimental evaluation of accuracy and repeatability of a novel body-to-sensor calibration procedure for inertial sensor-based gait analysis. Measurement 2014, 52, 145–155. [Google Scholar] [CrossRef]
- Montufar, J.; Arango, J.; Porter, M.; Nakagawa, S. Pedestrians’ Normal Walking Speed and Speed When Crossing a Street. Transp. Res. Rec. J. Transp. Res. Board 2007, 2002, 90–97. [Google Scholar] [CrossRef]
- Dietz, V.; Fouad, K.; Bastiaanse, C.M. Neuronal coordination of arm and leg movements during human locomotion. Eur. J. Neurosci. 2001, 14, 1906–1914. [Google Scholar] [CrossRef]
- Mazzaro, N.; Grey, M.J.; Sinkjær, T. Contribution of afferent feedback to the soleus muscle activity during human locomotion. J. Neurophysiol. 2005, 93, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Salarian, A.; Russmann, H.; Vingerhoets, F.J.G.; Dehollain, C.; Blanc, Y.; Burkhard, P.R.; Aminian, K. Gait assessment in Parkinson’s disease: Toward an ambulatory system for long-term monitoring. IEEE Trans. Biomed. Eng. 2004, 51, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.C.; Sartor, C.D.; Souza, F.T.; Sacco, I.C.N. Intralimb Coordination Patterns in Absent, Mild, and Severe Stages of Diabetic Neuropathy: Looking Beyond Kinematic Analysis of Gait Cycle. PLoS ONE 2016, 11, e0147300. [Google Scholar] [CrossRef]
- Mileti, I.; Taborri, J.; Rossi, S.; Del Prete, Z.; Paoloni, M.; Suppa, A.; Palermo, E. Reactive Postural Responses to Continuous Yaw Perturbations in Healthy Humans: The Effect of Aging. Sensors 2019, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Ranavolo, A.; Donini, L.M.; Mari, S.; Serrao, M.; Silvetti, A.; Iavicoli, S.; Cava, E.; Asprino, R.; Pinto, A.; Draicchio, F. Lower-Limb Joint Coordination Pattern in Obese Subjects. Biomed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, N.; Jensen, J.L.; Bates, B.T.; Scholten, S.D.; Tzetzis, G. A dynamical systems investigation of lower extremity coordination during running over obstacles. Clin. Biomech. 2001, 16, 213–221. [Google Scholar] [CrossRef]
- Friston, K.J. Statistical Parametric Mapping. In Neuroscience Databases; Springer: Boston, MA, USA, 2003. [Google Scholar]
- Aprigliano, X.F.; Martelli, D.; Tropea, P.; Pasquini, G.; Micera, S.; Carlo, D.; Foundation, G.; Foundation, B. Control of Movement Aging does not affect the intralimb coordination elicited by slip-like perturbation of different intensities. J. Neurophysiol. 2017, 118, 1739–1748. [Google Scholar] [CrossRef]
- Matjacic, Z.; Zadravec, M.; Olen, A. Influence of Treadmill Speed and Perturbation Intensity on Selection of Balancing Strategies during Slow Walking Perturbed in the Frontal Plane. Appl. Bionics Biomec. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Eng, J.J.; Winter, D.A.; Patla, A.E. Strategies for recovery from a trip in early and late swing during human walking. Exp. Brain Res. 1994, 102, 339–349. [Google Scholar] [CrossRef]
- Belanger, M.; Patla, A.E. Phase-Dependent Compensatory Responses to Perturbation Applied during Walking i. J. Mot. Behav. 1987, 19, 37–41. [Google Scholar] [CrossRef]
- Miyake, T.; Aprigliano, F.; Sugano, S.; Micera, S. Human Movement Science Repeated exposure to tripping like perturbations elicits more precise control and lower toe clearance of the swinging foot during steady walking. Hum. Mov. Sci. 2021, 76, 102775. [Google Scholar] [CrossRef]
- Dingwell, J.B.; Marin, L.C. Kinematic variability and local dynamic stability of upper body motions when walking at different speeds. J. Biomech. 2006, 39, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Tschiesche, K.; Blickhan, R. Kinetic and kinematic adjustments during perturbed walking across visible and camou fl aged drops in ground level. J. Biomech. 2014, 47, 2286–2291. [Google Scholar] [CrossRef] [PubMed]
- Afschrift, M.; Van Deursen, R.; De Groote, F.; Jonkers, I. Increased use of stepping strategy in response to medio-lateral perturbations in the elderly relates to altered reactive tibialis anterior activity. Gait Posture 2019, 68, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Afschrift, M.; De Groote, F.; Jonkers, I. Similar sensorimotor transformations control balance during standing and walking. PLoS Comput. Biol. 2021, 17, e1008369. [Google Scholar] [CrossRef]
- Hof, A.L.; Vermerris, S.M.; Gjaltema, W.A. Balance responses to lateral perturbations in human treadmill walking. J. Exp. Biol. 2010, 213, 2655–2664. [Google Scholar] [CrossRef]
- Gomes, A.A.; Onodera, A.N.; Otuzi, M.E.I.; Pripas, D.; Mezzarane, R.A.; Sacco, I.C.N. Electromyography and kinematic changes of gait cycle at different cadences in diabetic neuropathic individuals. Muscle Nerve 2011, 44, 258–268. [Google Scholar] [CrossRef]
- Van Emmerik, R.E.A.; Hamill, J.; McDermott, W.J. Variability and Coordinative Function in Human Gait. Quest 2012, 57, 102–123. [Google Scholar] [CrossRef]
- Stergiou, N.; Harbourne, R.T.; Cavanaugh, J.T. Optimal movement variability: A new theoretical perspective for neurologic physical therapy. J. Neurol. Phys. Ther. 2006, 30, 120–129. [Google Scholar] [CrossRef]
- Chiu, S.-L.; Chou, L.-S. Variability in inter-joint coordination during walking of elderly adults and its association with clinical balance measures. Clin. Biomech. 2013, 28, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.; Chen, H.; Liu, M.; Liu, H.; Lu, T. Age effects on the inter-joint coordination during obstacle-crossing. J. Biomech. 2009, 42, 2501–2506. [Google Scholar] [CrossRef] [PubMed]
- Ogaya, S.; Iwata, A.; Higuchi, Y.; Fuchioka, S. The association between intersegmental coordination in the lower limb and gait speed in elderly females. Gait Posture 2016, 48, 1–5. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taborri, J.; Santuz, A.; Brüll, L.; Arampatzis, A.; Rossi, S. Measuring Kinematic Response to Perturbed Locomotion in Young Adults. Sensors 2022, 22, 672. https://doi.org/10.3390/s22020672
Taborri J, Santuz A, Brüll L, Arampatzis A, Rossi S. Measuring Kinematic Response to Perturbed Locomotion in Young Adults. Sensors. 2022; 22(2):672. https://doi.org/10.3390/s22020672
Chicago/Turabian StyleTaborri, Juri, Alessandro Santuz, Leon Brüll, Adamantios Arampatzis, and Stefano Rossi. 2022. "Measuring Kinematic Response to Perturbed Locomotion in Young Adults" Sensors 22, no. 2: 672. https://doi.org/10.3390/s22020672
APA StyleTaborri, J., Santuz, A., Brüll, L., Arampatzis, A., & Rossi, S. (2022). Measuring Kinematic Response to Perturbed Locomotion in Young Adults. Sensors, 22(2), 672. https://doi.org/10.3390/s22020672








