Spectral Fingerprint Investigation in the near Infra-Red to Distinguish Harmful Ethylene Glycol from Isopropanol in a Microchannel †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Optoelectronic Instrumental Configuration
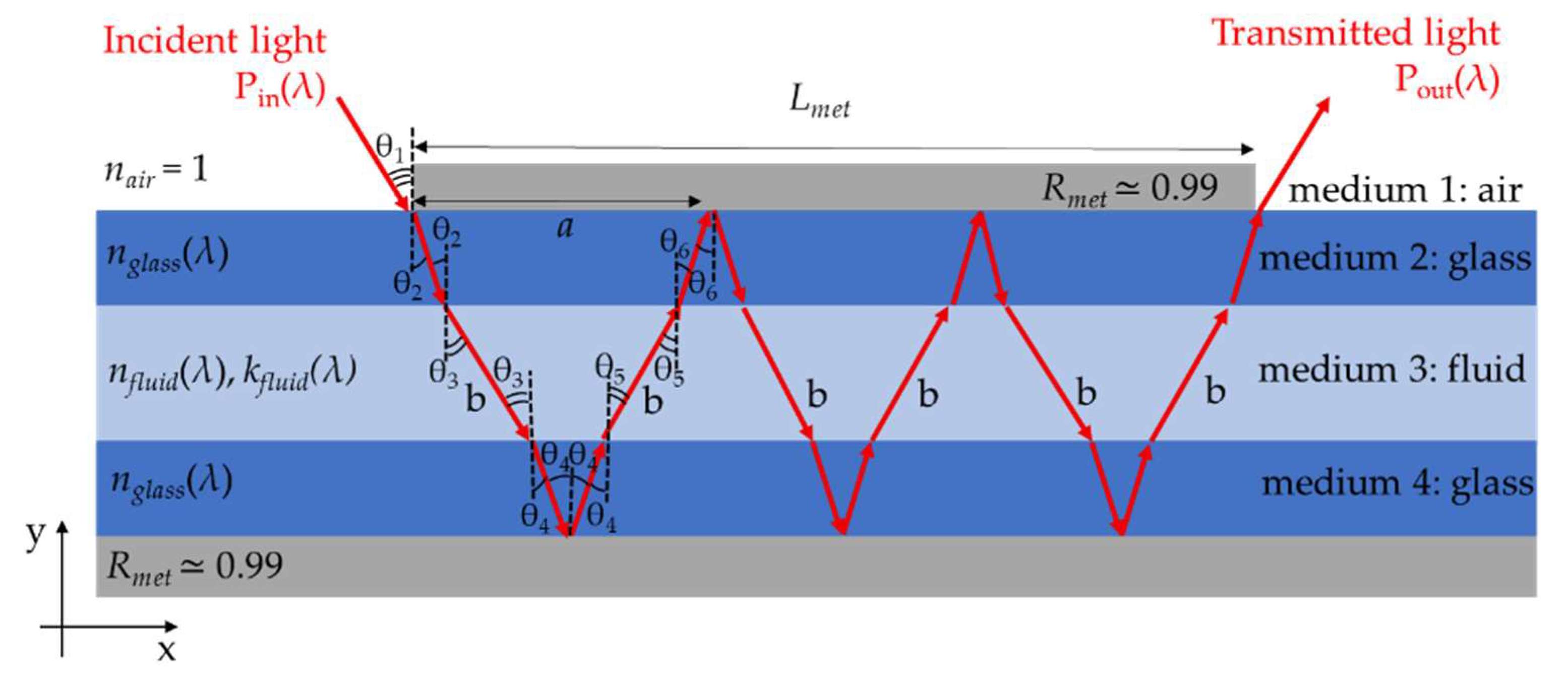
2.2. The Theoretical Model
3. Results and Discussion
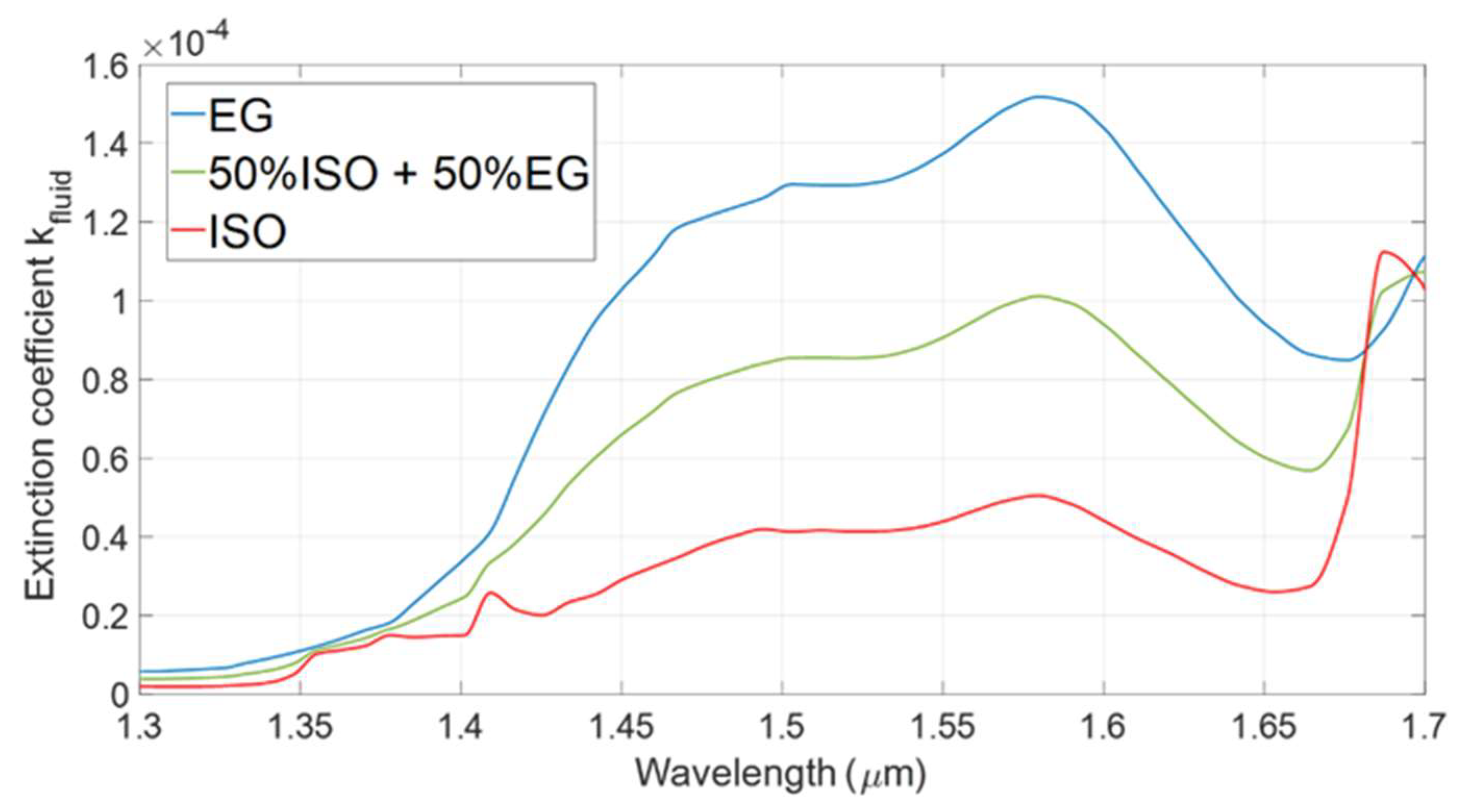
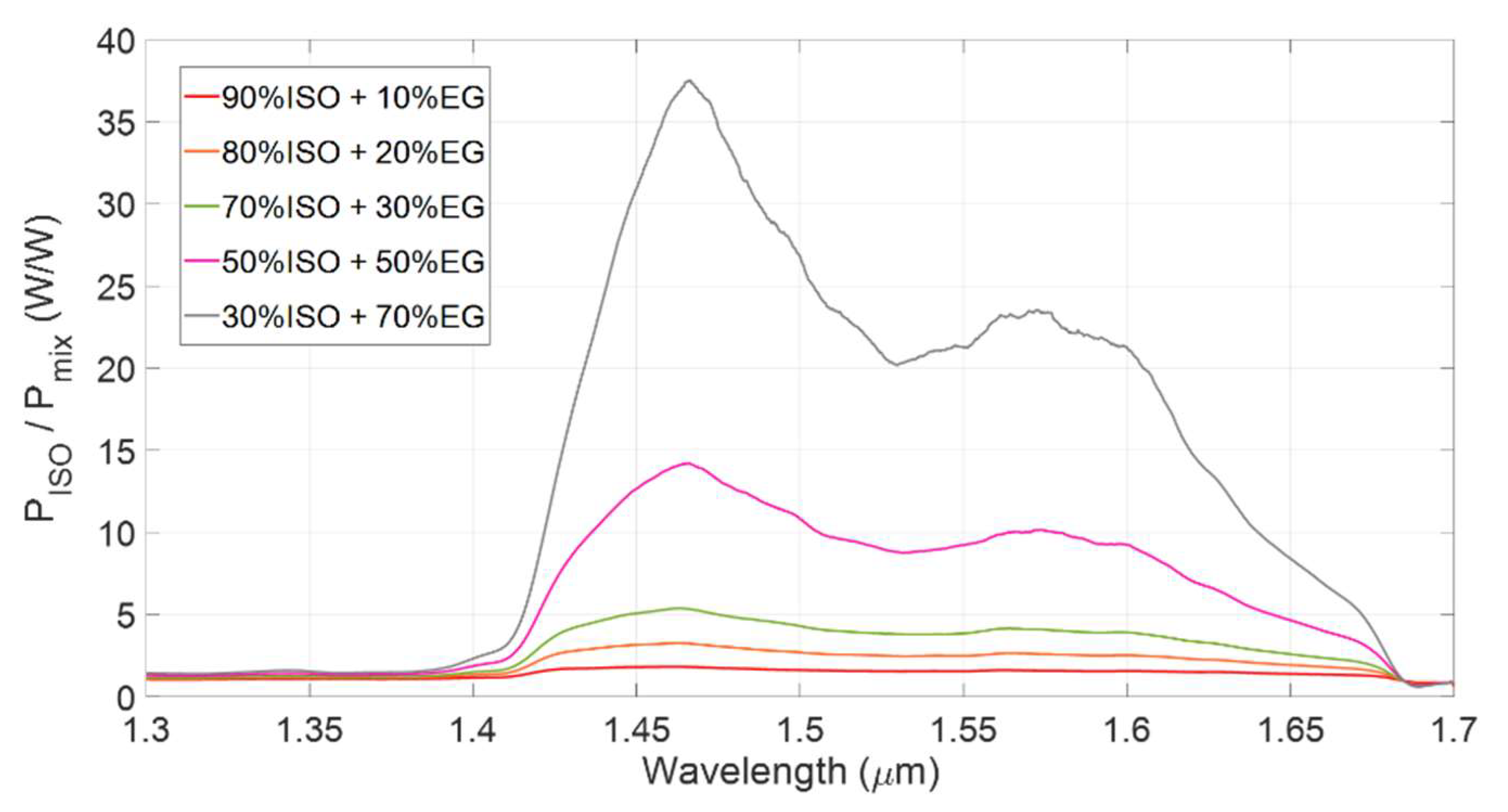
3.1. Spectral Analysis
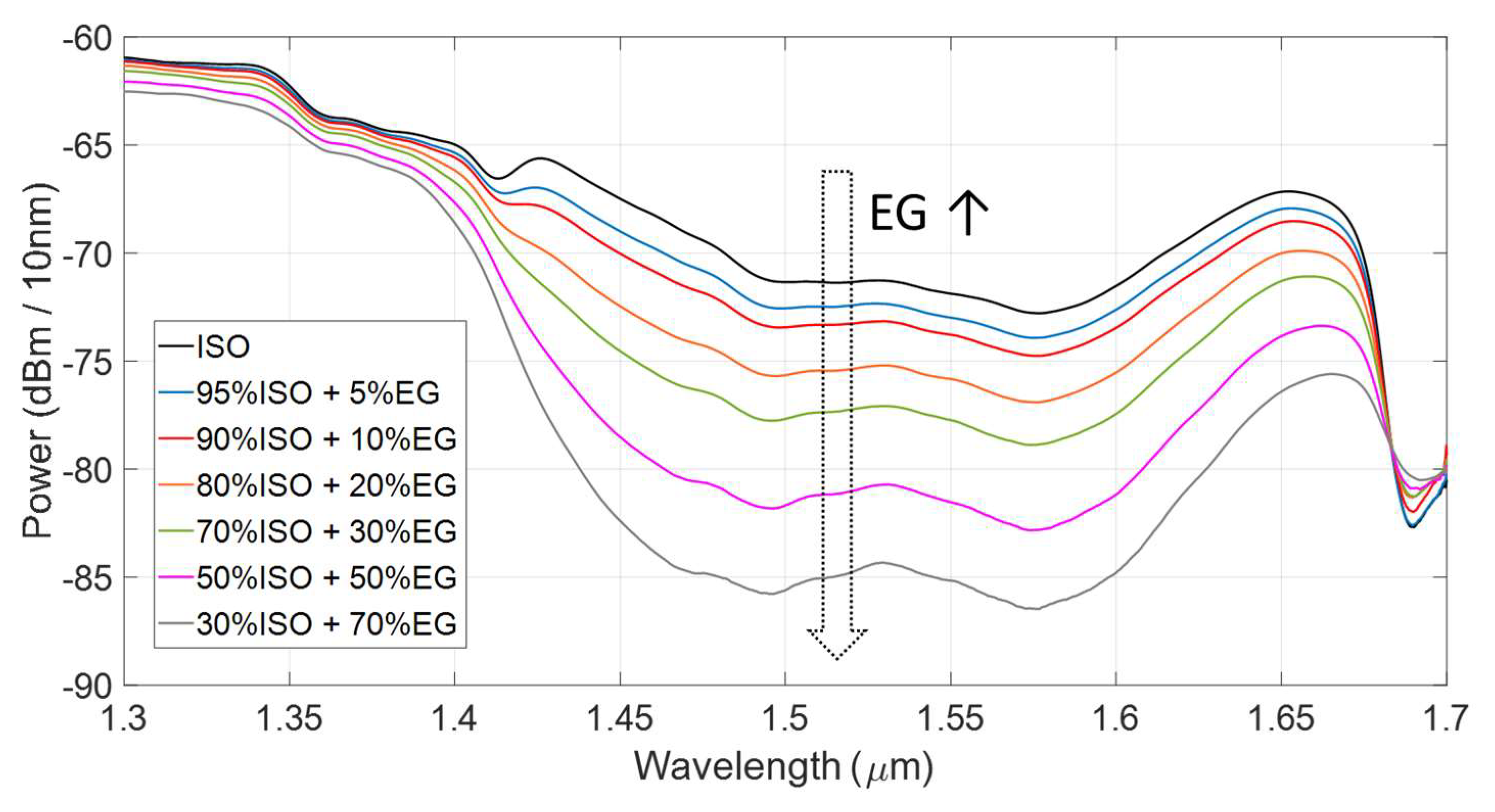
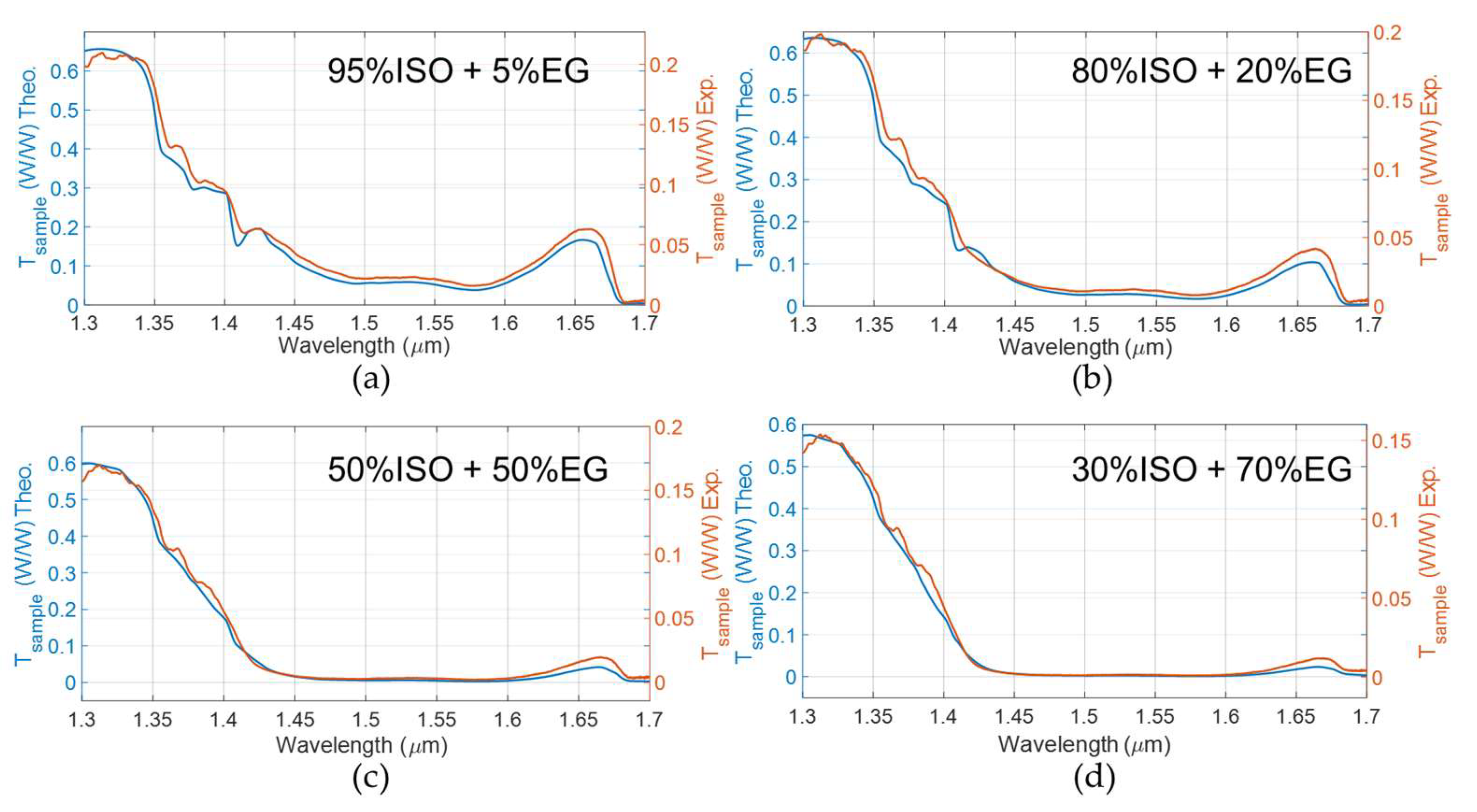
3.2. Responsivity and Sensitivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kraut, J.A.; Kurtz, I. Toxic Alcohol Ingestions: Clinical Features, Diagnosis, and Management. Clin. J. Am. Soc. Nephrol. 2008, 3, 208–225. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Glagola, J.J.; Nappe, T.M. Ethylene Glycol Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Gummin, D.D.; Mowry, J.B.; Beuhler, M.C.; Spyker, D.A.; Brooks, D.E.; Dibert, K.W.; Rivers, L.J.; Pham, N.P.T.; Ryan, M.L. 2019 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 37th Annual Report. Clin. Toxicol. 2020, 58, 1360–1541. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Zhao, Y.; Ma, X.; Gong, J. Ethylene Glycol: Properties, Synthesis, and Applications. Chem. Soc. Rev. 2012, 41, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Patočka, J.; Hon, Z. Ethylene Glycol, Hazardous Substance in the Household. Acta Med. 2010, 53, 19–23. [Google Scholar] [CrossRef]
- An, L.; Chen, R. Recent Progress in Alkaline Direct Ethylene Glycol Fuel Cells for Sustainable Energy Production. J. Power Sources 2016, 329, 484–501. [Google Scholar] [CrossRef]
- Varlet, V.; Farsalinos, K.; Augsburger, M.; Thomas, A.; Etter, J.-F. Toxicity Assessment of Refill Liquids for Electronic Cigarettes. Int. J. Environ. Res. Public Health 2015, 12, 4796–4815. [Google Scholar] [CrossRef] [Green Version]
- Greene, H.R.; Krasowski, M.D. Correlation of Osmolal Gap with Measured Concentrations of Acetone, Ethylene Glycol, Isopropanol, Methanol, and Propylene Glycol in Patients at an Academic Medical Center. Toxicol. Rep. 2020, 7, 81–88. [Google Scholar] [CrossRef]
- Lister, D.; Tierney, M.; Dickinson, G. Effectiveness of IV Ethanol Therapy Combined with Hemodialysis in the Treatment of Methanol and Ethylene Glycol Poisoning. Can. J. Hosp. Pharm. 2005, 58, 142–147. [Google Scholar] [CrossRef]
- McQuade, D.J.; Dargan, P.I.; Wood, D.M. Challenges in the Diagnosis of Ethylene Glycol Poisoning. Ann. Clin. Biochem. 2014, 51, 167–178. [Google Scholar] [CrossRef]
- Porter, W.H.; Rutter, P.W.; Yao, H.H. Simultaneous Determination of Ethylene Glycol and Glycolic Acid in Serum by Gas Chromatography Mass Spectrometry. J. Anal. Toxicol. 1999, 23, 591–597. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Li, Z.; He, J.; Chu, L. Design and Performance of a Composite Grating-Coupled Surface Plasmon Resonance Trace Liquid Concentration Sensor. Sensors 2019, 19, 5502. [Google Scholar] [CrossRef] [Green Version]
- Beć, K.B.; Grabska, J.; Huck, C.W. Principles and Applications of Miniaturized Near-Infrared (NIR) Spectrometers. Chemistry 2021, 27, 1514–1532. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Magazù, S. Ethylene Glycol–Polyethylene Glycol (EG-PEG) Mixtures: Infrared Spectra Wavelet Cross-Correlation Analysis. Appl. Spectrosc. 2017, 71, 401–409. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Yu, Q.; Peng, W. A Novel Visible Light-Excited Interference Behavior Occurred in Capillary Waveguide. IEEE Sens. J. 2020, 21, 4723–4730. [Google Scholar] [CrossRef]
- Salim, A.; Lim, S. Review of Recent Metamaterial Microfluidic Sensors. Sensors 2018, 18, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borecki, M.; Korwin-Pawlowski, M.L.; Beblowska, M.; Szmidt, J.; Jakubowski, A. Optoelectronic Capillary Sensors in Microfluidic and Point-of-Care Instrumentation. Sensors 2010, 10, 3771–3797. [Google Scholar] [CrossRef]
- Alberti, S.; Datta, A.; Jágerská, J. Integrated Nanophotonic Waveguide-Based Devices for IR and Raman Gas Spectroscopy. Sensors 2021, 21, 7224. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sahay, P. Breath Analysis Using Laser Spectroscopic Techniques: Breath Biomarkers, Spectral Fingerprints, and Detection Limits. Sensors 2009, 9, 8230–8262. [Google Scholar] [CrossRef]
- Bello, V.; Bodo, E. A NIR-Spectroscopy-Based Approach for Detection of Fluids in Rectangular Glass Micro-Capillaries. Eng. Proc. 2020, 2, 43. [Google Scholar] [CrossRef]
- Bodo, E.; Bello, V. Microfluidic Devices with Selectable Optical Pathlength for Quality Control of Alcoholic Solutions. In Proceedings of the 8th International Electronic Conference on Sensors and Application, Online, 1–15 November 2021. [Google Scholar]
- Bello, V.; Bodo, E.; Merlo, S. Micro-Opto-Fluidic Platform for Spectroscopic Identification of Water-Based Fluids. In Optical Sensors 2021, Proceedings of the SPIE Optics + Optoelectronics, Online, 19–23 April 2021; SPIE: Bellingham, WA, USA, 2021; Volume 11772, p. 117720C. [Google Scholar] [CrossRef]
- Kedenburg, S.; Vieweg, M.; Gissibl, T.; Giessen, H. Linear Refractive Index and Absorption Measurement of Nonlinear Optical Liquids in the Visible and Near-Infrared Spectral Region. Opt. Mater. Express 2012, 2, 1588–1611. [Google Scholar] [CrossRef]
- Curcio, J.; Petty, C. The Near Infrared Absorption Spectrum of Liquid Water. J. Opt. Soc. Am. 1951, 41, 302–304. [Google Scholar] [CrossRef]
- Bello, V.; Bodo, E.; Merlo, S. Quality Control of Ethanol-Based Hand Sanitizer Gels in Micro-Opto-Fluidic Devices. In Proceedings of the CLEO: Applications and Technology 2021, San Jose, CA, USA, 9–14 May 2021; p. JTh3A-87. [Google Scholar] [CrossRef]
- Sani, E.; Dell’Oro, A. Optical Constants of Ethylene Glycol Over an Extremely Wide Spectral Range. Opt. Mater. 2014, 37, 36–41. [Google Scholar] [CrossRef]
- Sani, E.; Dell’Oro, A. Spectral Optical Constants of Ethanol and Isopropanol from Ultraviolet to Far Infrared. Opt. Mater. 2016, 60, 137–141. [Google Scholar] [CrossRef]
- John Wiley & Sons, Inc. SpectraBase; SpectraBase Compound ID=8Ro8UyUNHr6 SpectraBase Spectrum ID=5rDQoxoqFzV. Available online: https://spectrabase.com/spectrum/5rDQoxoqFzV (accessed on 29 December 2021).
- John Wiley & Sons, Inc. SpectraBase; SpectraBase Compound ID=9cBA9IZSWuJ SpectraBase Spectrum ID=LHQElUGVPJq. Available online: https://spectrabase.com/spectrum/LHQElUGVPJq (accessed on 29 December 2021).
- Myers, T.L.; Tonkyn, R.G.; Danby, T.O.; Taubman, M.S.; Bernacki, B.E.; Birnbaum, J.C.; Sharpe, S.W.; Johnson, T.J. Accurate Measurement of the Optical Constants n and k for a Series of 57 Inorganic and Organic Liquids for Optical Modeling and Detection. Appl. Spectrosc. 2018, 72, 535–550. [Google Scholar] [CrossRef]
- Abo Riziq, A.; Erlick, C.; Dinar, E.; Rudich, Y. Optical Properties of Absorbing and Non-Absorbing Aerosols Retrieved by Cavity Ring Down (CRD) Spectroscopy. Atmos. Chem. Phys. 2007, 7, 1523–1536. [Google Scholar] [CrossRef] [Green Version]
- Flores, J.M.; Trainic, M.; Borrmann, S.; Rudich, Y. Effective Broadband Refractive Index Retrieval by a White Light Optical Particle Counter. Phys. Chem. Chem. Phys. 2009, 11, 7943–7950. [Google Scholar] [CrossRef]
- Bain, A.; Rafferty, A.; Preston, T.C. The Wavelength-Dependent Complex Refractive Index of Hygroscopic Aerosol Particles and Other Aqueous Media: An Effective Oscillator Model. Geophys. Res. Lett. 2019, 46, 10636–10645. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodo, E.; Merlo, S.; Bello, V. Spectral Fingerprint Investigation in the near Infra-Red to Distinguish Harmful Ethylene Glycol from Isopropanol in a Microchannel. Sensors 2022, 22, 459. https://doi.org/10.3390/s22020459
Bodo E, Merlo S, Bello V. Spectral Fingerprint Investigation in the near Infra-Red to Distinguish Harmful Ethylene Glycol from Isopropanol in a Microchannel. Sensors. 2022; 22(2):459. https://doi.org/10.3390/s22020459
Chicago/Turabian StyleBodo, Elisabetta, Sabina Merlo, and Valentina Bello. 2022. "Spectral Fingerprint Investigation in the near Infra-Red to Distinguish Harmful Ethylene Glycol from Isopropanol in a Microchannel" Sensors 22, no. 2: 459. https://doi.org/10.3390/s22020459
APA StyleBodo, E., Merlo, S., & Bello, V. (2022). Spectral Fingerprint Investigation in the near Infra-Red to Distinguish Harmful Ethylene Glycol from Isopropanol in a Microchannel. Sensors, 22(2), 459. https://doi.org/10.3390/s22020459







