1. Introduction
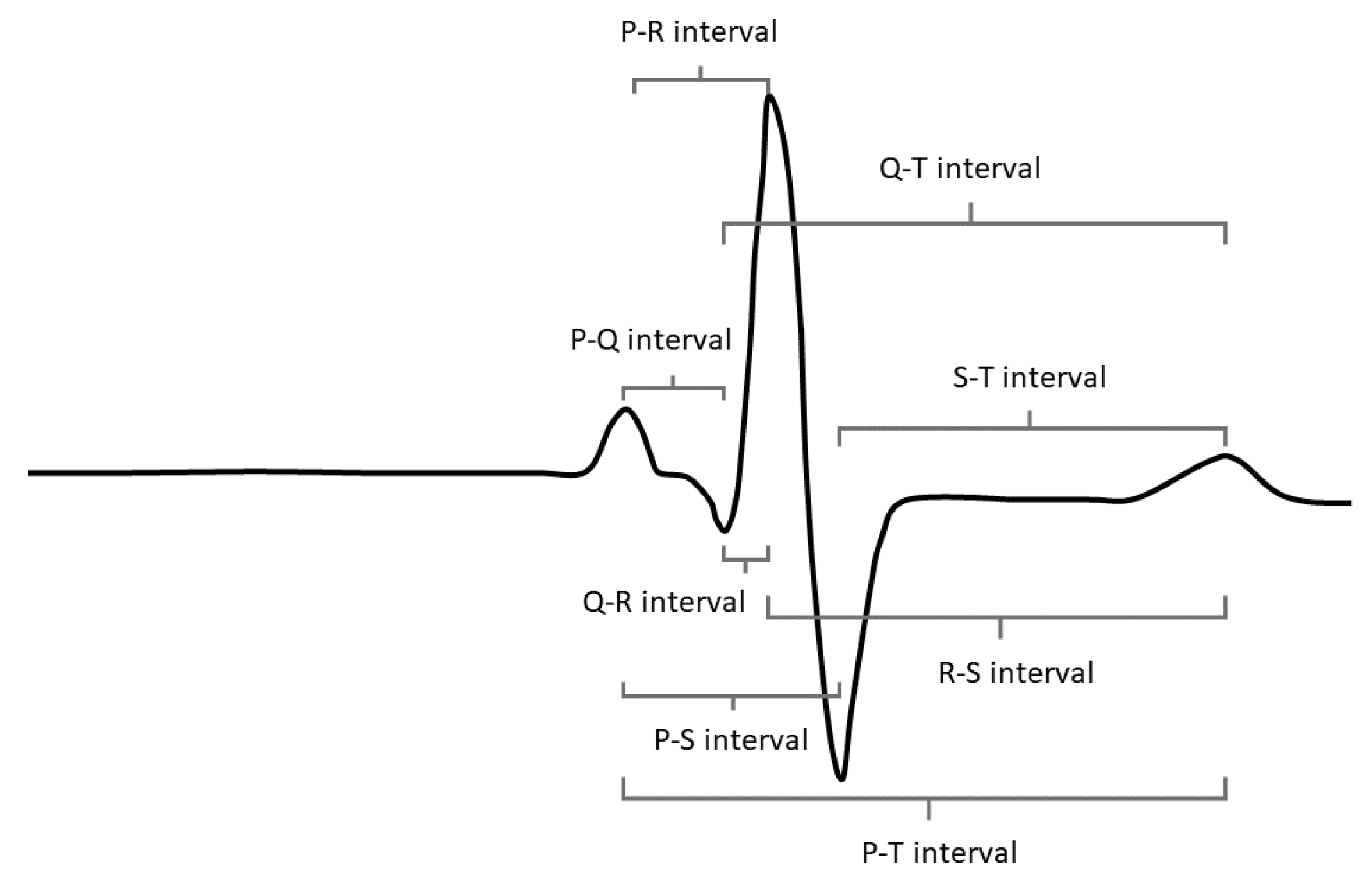
Psychological stress causes changes in the autonomic nervous system that can be observed in the electrocardiogram (ECG) signal. The R peak—the most prominent feature in the ECG signal, shown in
Figure 1—is most commonly used to derive features in the time domain for the classification of psychological stress because it is relatively easy to detect under noisy conditions and requires less infrastructure to collect (e.g., the R peak can be detected with fewer electrodes and in lower signal-to-noise ratio signals than the smaller peaks in the waveform). Features in the time domain also tend to provide more reliable metrics over short-duration monitoring periods than features extracted from the frequency domain. This presents the question of whether there are more features available within the time domain of the ECG signal beyond traditional heart rate variability (HRV) features derived only from the R peak and whether these novel features (derived from the P, Q, S, and T peaks) can be leveraged to improve stress classification algorithms. This exploration is supported by the advancement of many wearable ECG detection systems that allow the collection of the full ECG waveform, driving the possibility of the operational utility of these novel features and a clear paradigm for their implementation.
Many studies have explored the utility of the ECG signal for psychological stress detection and have shown that traditional R-peak-based HRV features can reliably indicate stressed state [
1,
2]. To the best of our knowledge, these studies have not explored the efficacy of small peak features, largely due to the fact that the small peaks have not been easily detectable without research-grade ECG recording equipment. Other studies have pursued a multimodal approach to both physiological and psychological stress classification with the inclusion of other signal streams—electrodermal activity (EDA) being the most common—in their feature sets, because each additional signal stream provides some reduction in collinearity due to the difference in physiological processes being measured [
3,
4,
5,
6,
7]. In this investigation, we strive to understand whether the incorporation of small peak features can bring the same sort of reduction in collinearity to stress classification because of the subtle physiological differences between the production of the R peak and the smaller peaks within the heart. These parts of the heart beat cycle are less well-studied in psychophysiology, so in this work we hope to add to our understanding of their psychophysiological importance and their potential utility for stress classification. The full set of potential small peak intervals available to our classification algorithm before a feature down-select are shown in
Figure 1.
This more scientific exploration of small peak utility must be accompanied by an understanding of the operational utility of these features. As previously mentioned, the development of wearable ECG-monitoring systems is increasing rapidly, but wearable systems are plagued with noise due to poor electrode contact and motion artifacts [
8]. For this reason, we also aimed to understand the effects of noise on automated peak detection for each of the five peaks within the waveform to identify which small peaks would be lost first under noisy conditions.
The research questions we address in this work are threefold: (1) Do features derived from the intervals between the P, Q, S, and T waves within the ECG waveform augment binary stress classification? (2) Can we characterize the correlations between the novel and traditional features to improve our understanding of the influence of the novel features? (3) What is the impact of noise on the detectability of these smaller parts of the ECG waveform? Our goal is to understand both the efficacy of adding these features to stress detection algorithms and the practicality of their use in operational environments where noise is ubiquitous.
The paper is organized as follows:
Section 2 describes the methods of model development, pre-existing data set used for the analysis, model comparison, and peak detection performance,
Section 3 presents the features included in the traditional feature model (TFM) and novel feature model (NFM), the comparisons between the two, and the results of the peak detection performance analysis. Lastly, in
Section 4, we situate our results within the gaps in understanding in this area and cover the limitations of this study.
2. Model Development Methods
Each of the stress classification models was developed by down-selecting time-domain ECG features that were extracted from a pre-existing data set, assessing validity coefficients for each feature, inspecting correlations between the features, and ultimately assessing model performance with three widely used classification algorithms. Outside of model development, an assessment of automated peak detection performance was also conducted to understand the potential operational utility of small peak features. The TFM and NFM were both developed using the set of traditional features, but the NFM was also given the full set of potential novel features before the model down-selection, while the TFM was not. The NFM was not based purely on small peak features but instead on a combination of traditional and novel features. The following sections step through these development procedures in more detail for both the TFM and NFM and the methods used to evaluate their performance.
2.1. Data Set
The data set used for this study is a data set publicly available on PhysioNet, a database of physiological signal data sets managed by the National Institutes of Health [
9]. This data set was created as part of an investigation of exposure therapy as a method of mitigating arachnophobia in spider-fearful individuals and was downloaded for this study in July 2020. The data set contains ECG recordings from 57 participants (aged 18–40) with reported fear of spiders while they were exposed to 16 different video clips of spiders in the wild with short rest periods in between [
10]. We acknowledge here that fear and stress are not the same psychological construct, but their physiological manifestations are similar, and we will revisit the impact of this limitation in
Section 4. ECG data collection was conducted using a wearable BITalino biosignal measurement device, and the study was approved by the Ethical Committee of the Faculty of Human Sciences of Saarland University, as stated by the authors of the original study. Each ECG recording is accompanied by a time-stamp log of video delivery, and we used these stamps to label each time stamp as unstressed —0—or stressed —1— and used these labels as the true values for classification. This approach was taken due to the lack of the subjective self-assessments of participants’ responses to the video stimuli. Therefore, we cannot state that these classifications are absolutely true, but they provide a valuable proxy for stressed state. In this analytic structure, there are 16 ten-second samples each for stressed and nonstressed states from each of the 57 participants. This totals 1824 samples for model training and testing, but only those samples where both the R peak and small peak features could be reliably detected were used, resulting in 960 samples left for model training and testing. All data processing was performed using the publicly available Python package neurokit2 [
11].
2.2. Signal Denoising and Feature Extraction
Each ECG recording was filtered using a 5
th- order Butterworth filter with a normalized cutoff frequency of 0.3 and subsequently split into 10 s samples, one from each stressed and nonstressed period in the data set. We selected sample durations of 10 s to accommodate the shortest intervals between video clips. These short time intervals were also relevant for psychological stress detection in real time. Peak detection was conducted using the “findpeaks” and “delineate” functions based on the Pan–Tompkins algorithm for peak detection available in the neurokit2 Python package [
12,
13]. Novel features consist of the mean and standard deviation of time intervals between the P, Q, R, S, and T peaks and were extracted by subtracting the time stamps between the selected peaks. After feature extraction, each feature was converted to a z-score to scale and center the data, calculated across participants. Only time domain features were used for this study because the 10 s sample lengths were too short to derive reliable and meaningful frequency domain features.
Explanation of Features
This section provides an explanation of each of the features included in both models. There were a total of 24 R-peak-based features and a total of 12 small-peak-based features for each of the models to down-select from. In this paper, we have only included the features that were ultimately selected by the two models in this explanation. Each feature is described in
Table 1.
2.3. Model Development Procedure
The same method of feature selection was used for both the TFM and the NFM. We split the 960 samples into a training set of 67% and a testing set of 33%. The full set of available features for both models was reduced using a forward and backward stepwise feature selection algorithm with the Akaike Information Criterion (AIC) as the evaluation metric. The smaller the AIC value, the less information loss in the model, so at each step the feature with the highest AIC value was removed from the set.
Validity coefficients were computed for each feature by calculating the point-biserial correlation, , between the criterion (i.e., stress or no stress) and the feature values as an additional check of the results of the stepwise feature selection procedure. In addition to these validity coefficients, feature distributions were examined in a histogram visualization to observe the differences between the feature distributions for stressed and nonstressed samples through visual inspection. After the feature down-selection for both models, Akaike Information Criterion (AIC) scores were compared between the two to measure the difference in information loss between the models.
2.4. Comparison of Multiple Classification Algorithms
We evaluated three different classification methods with both the TFM and NFM. These three methods were linear discriminant analysis (LDA), logistic regression (LR), and a support vector classification (SVC). These formulations are different in their approaches, and the goal with this investigation was to see how well the two feature sets performed under different, but commonly used, classification algorithms. LDA is based on least squares regression, whereas LR is based on maximum likelihood estimation. LDA assumes normality and homoscedasticity in the data set under the two conditions (i.e., stressed and not stressed), while LR does not. SVC is a maximum margin classifier that maximizes the distance between each feature and the hyperplane decision boundary. Explainability is highest in the LDA and LR models and lowest in the SVC model. We compared the performance of these three classification algorithms in precision, recall, F1, AUC, and accuracy with both the TFM and NFM. Each of these metrics is presented as an average value from 10-fold cross-validation conducted on the reserved test data.
2.5. Peak Detection Algorithm Performance Analysis
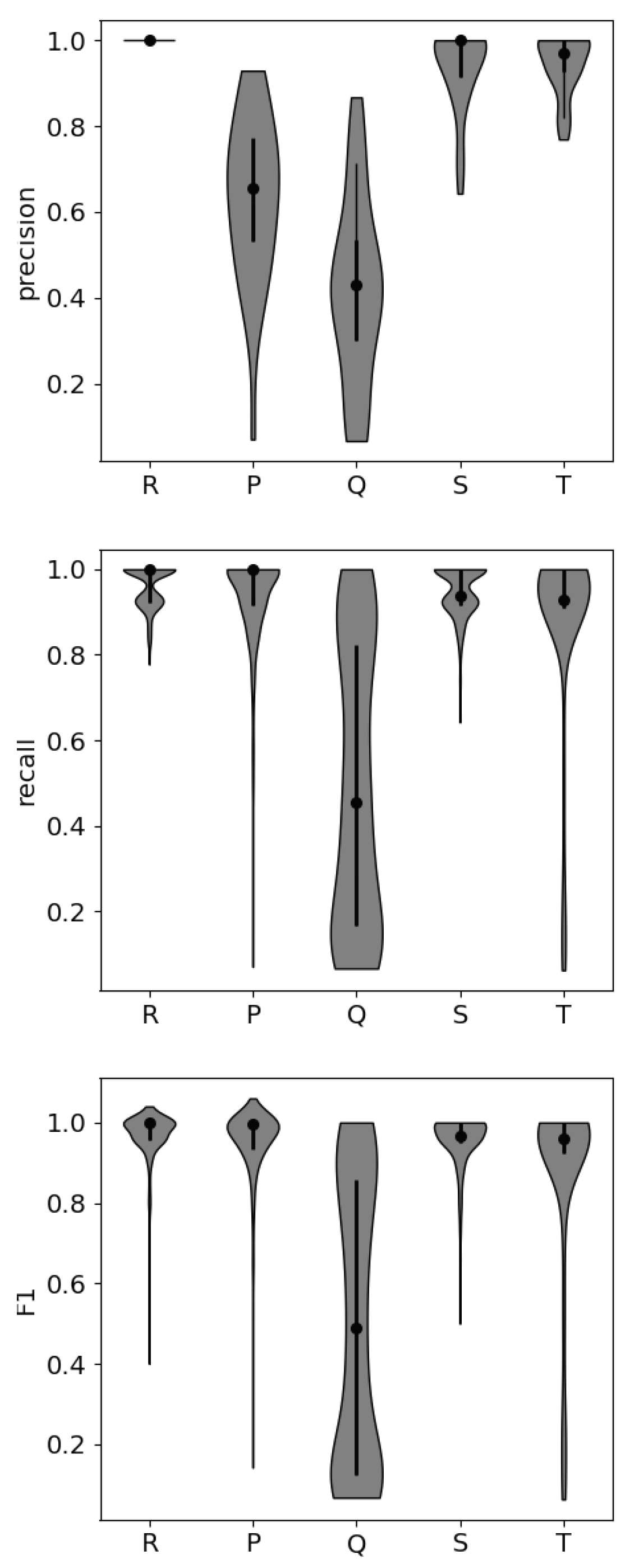
If the small peak features show promise for stress detection, the capabilities of automated peak detection algorithms must be evaluated to ensure operational feasibility. It is time-consuming to identify features through visual inspection, so the smaller parts of the ECG waveform must be detectable by unsupervised peak detection algorithms in order to be effectively incorporated into stress detection algorithms that function without constant supervision. To assess the detectability of each feature, we randomly selected 10 of the 57 participants’ data sets to assess precision, recall, and F1 scores for the neurokit2 peak detection function when compared with peak detection through visual inspection. The peak detection algorithm begins by calculating the gradient of the ECG signal to identify the location of the QRS complexes within the signal and identifies each of the peaks by calculating local maxima within that region.
4. Discussion
The results of this study suggest that the inclusion of small peak features in stress classification algorithms can reduce information loss, potentially increasing the predictive power of these algorithms, even with short ECG samples. We can see this result in the comparison of the TFM with only R-peak-based features and the NFM with both R-peak- and small-peak-based features.
The NFM performs slightly better than the TFM in the commonly used precision, recall, F1, AUC, and accuracy metrics, but they are not statistically significantly improved, given that the differences are within the standard deviations of each value. The differences between the two models in these metrics are largest with LR classification, and they perform almost identically with the SVC. These results do not show conclusively that the inclusion of small peak features augments the binary classification in this case, but they do suggest that these features can be used effectively in conjunction with traditional HRV features. However, in the AIC metric comparison, the NFM does perform better than the TFM. The NFM has a substantially better AIC score than the TFM, showing that the NFM is
times more probable to minimize the information loss than the TFM. This difference could be due to the reduction in multicollinearity brought about by the incorporation of small peak features or the higher validity coefficients between the small peak features and stress. The AIC is based on the Kullback–Liebler divergence between the predicted value distribution and the true value distribution, so it measures information loss (i.e., the balance between the model’s predictive power and its simplicity) [
15]. This differs from the other comparison metrics shown in
Table 3 because they measure only predictive power. This could explain the difference in the AIC scores between the models.
Mayya et al., 2015, explored the efficacy of time domain, frequency domain, and nonlinear heart rate variability (HRV) features over 60 s test intervals and found that RMSSD was the most influential feature for determining stressed state when model performance was measured using classification accuracy [
16]. In our investigation, the TFM includes this feature, but the RMSSD feature is not included in the NFM. This suggests that the importance of this feature is eclipsed by the presence of the small peak features that are more strongly correlated with stressed state over the short sample length (10 s) used in this study.
The
coefficients of the small peak features are all higher than those of the traditional features.
Table 2 shows their values ranging from changes of 1.78 to 6.78 standard deviations in the response variable with a 1 standard deviation change in each feature. This indicates that over this short time-scale (10 s), the small peak features have far more predictive power for psychological stress detection than their traditional counterparts.
This, the difference in information loss between the two models, the reduction in multicollinearity, higher validity coefficients, and the inclusion of small peak features over other commonly included features, suggest that the small peak intervals could be indicative of somewhat independent processes within the heart that reflect the reaction of the autonomic nervous system to stress as seen in the ECG signal.
The novel features included in the NFM were all derived from the P, S, and T peaks. These peaks reflect known physiological processes, but they have not been studied deeply as psychophysiological correlates of stress. The duration of the P-R interval is dependent upon signal conduction through the atria, atrioventricular node, His bundle, and Purkinje fibers [
17]. This conduction velocity determines the length of time allowed for the passage of blood from the atria to the ventricles. While we do not know the relationship between this difference and stress, our results show that the P-R interval mean and standard deviation are both negatively correlated with stress (e.g., shorter intervals and a tighter distribution of intervals are correlated with stress). The S-T interval falls within the period of ventricular repolarization, the relaxation and preparation for the next heartbeat [
18]. Some studies have shown that the S-T interval is correlated with blood pressure; in our study, the shorter S-T interval mean and smaller standard deviation were found to be correlated with stress, and we hypothesize that this may be due to a correlation between blood pressure and stress [
19]. Work by Pollak and Obrist suggests that pulse transit time (PTT) may be measurable through time intervals within the ECG signal [
20]. PTT is usually measured with the ECG signal and a secondary pulse measurement, so measuring this within the ECG signal itself could reduce the number of sensors required for this psychophysiologically relevant signal to be collected, simplifying operational monitoring systems. While these are all potential explanations for the importance of these features in the NFM, we cannot make any definitive statements from this study. Further testing with multimodal sensor systems should be conducted to understand this relationship fully.
From an operational standpoint, the S and T peaks are most reliably detected with an automated detection algorithm, making them the most reliable for inclusion in stress classification models. The Q peak is least reliably detected, due to its smaller magnitude in comparison with the other peaks. The intervals associated with the Q peak were not included in the NFM, so this result does not negatively impact the potential for operational use of the NFM.
In this investigation, we made the assumption that exposing spider-fearful individuals to videos of spiders would create a psychophysiological response shown in the ECG signal similar to responses indicative of psychological stress. In the study conducted by Ihmig et al., HRV metrics were extracted for their correlation with “disturbance and emotional arousal”, so we believe that our assumption is valid [
21]. Additionally, it has also been shown in other studies that both fear and stress are related to arousal and increases in heart rate [
22].
Future work may include the validation of the TFM and NFM on other data sets. Testing with other data sets involving other types of psychological stress (e.g., social stress) could add to our depth of understanding in the utility of small peak features in this paradigm. Additionally, we plan to investigate the utility of small peaks for nonbinary stress classification, determining whether their inclusion augments the detection of multiple levels of stress. Another important area of future work is the validation of the NFM with different types of stress (e.g., workload stress, emotional stress, etc.) and the investigation of whether small peaks contribute to the classification of different types of stress.
In parallel with further assessments of the utility of the NFM in different experimental scenarios with nonbinary stress classification, an investigation of novel peak detection algorithms could expand the potential for use in the NFM. There are several groups working to develop peak detection algorithms that amplify the small peaks within the ECG waveform, and leveraging their work in this area is an important next step. In the future, it may be valuable to conduct a study of the efficacy of adding small peaks in conjunction with the use of such an algorithm.