Neuromorphic Tactile Edge Orientation Classification in an Unsupervised Spiking Neural Network
Abstract
1. Introduction
1.1. Neuromorphic Tactile Sensors
1.2. Tactile Sensing and Edge Detection
1.3. Spiking Neural Networks
2. Materials and Methods
2.1. Hardware—The NeuroTac Sensor
2.2. Experimental Setup and Procedure
2.3. Spiking Neural Network Architecture
2.3.1. Input Layer
2.3.2. Hidden Layer
2.3.3. Output Layer
2.4. Neuron Model
2.5. Unsupervised Learning
3. Results
3.1. Inspection of Data
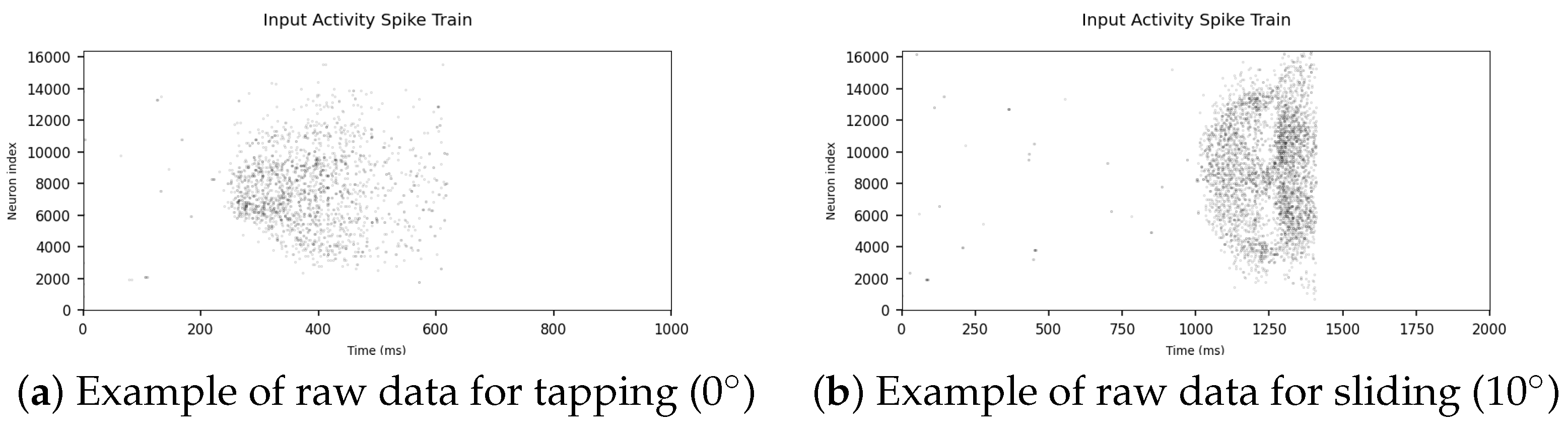
3.1.1. Raw Data
3.1.2. Data Clustering
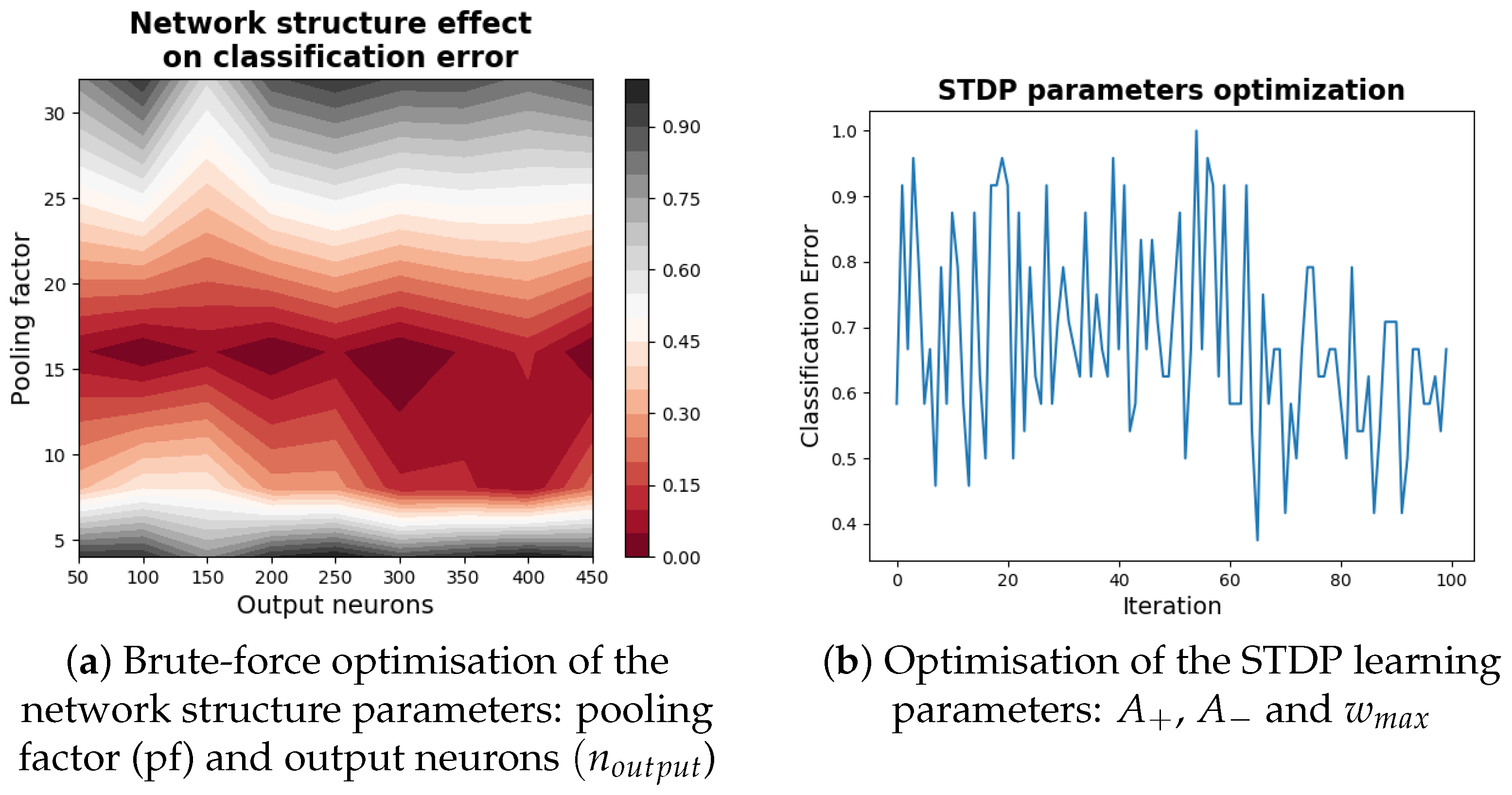
3.2. Network Optimisation
- Pooling factor (pf): this variable sets both the degree of reduction in network population size from the input to the hidden layer and the receptive field diameter of neurons in the hidden layer (we found from initial runs that setting these values equal led to the best performance). The range of values tested for the pF parameter are found in (Figure 6)
- Output neurons (): This parameter simply determines the number of neurons in the output layer. We vary it over the range 50–450 neurons in steps of 50.
- , : these variables set the rate at which potentiation and depression of synaptic weights occur, respectively. We vary them both independently over the range [0.001–0.1]
- : This parameter is an upper boundary for synaptic weights. We optimise it over a range of [1–6]
3.3. Network Dynamics
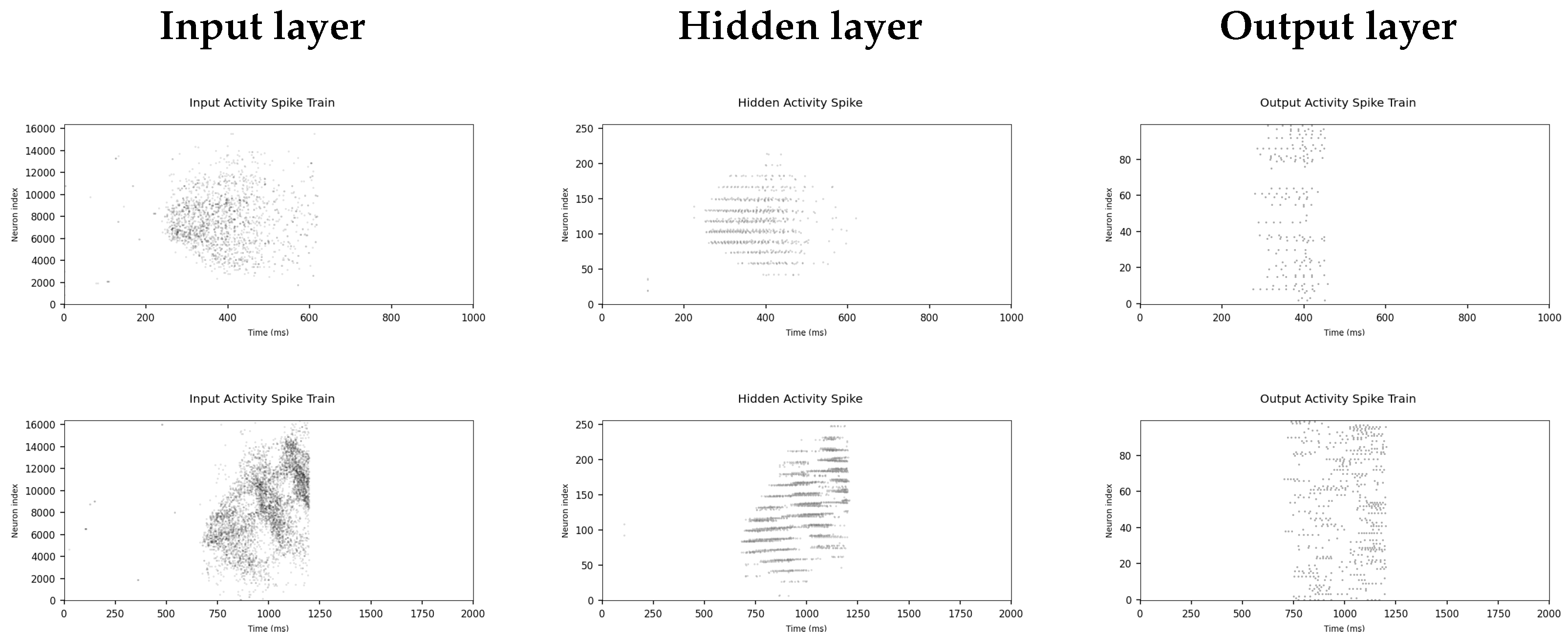
3.3.1. Spike Raster Plots
3.3.2. Weight Updates through STDP
Heatmaps
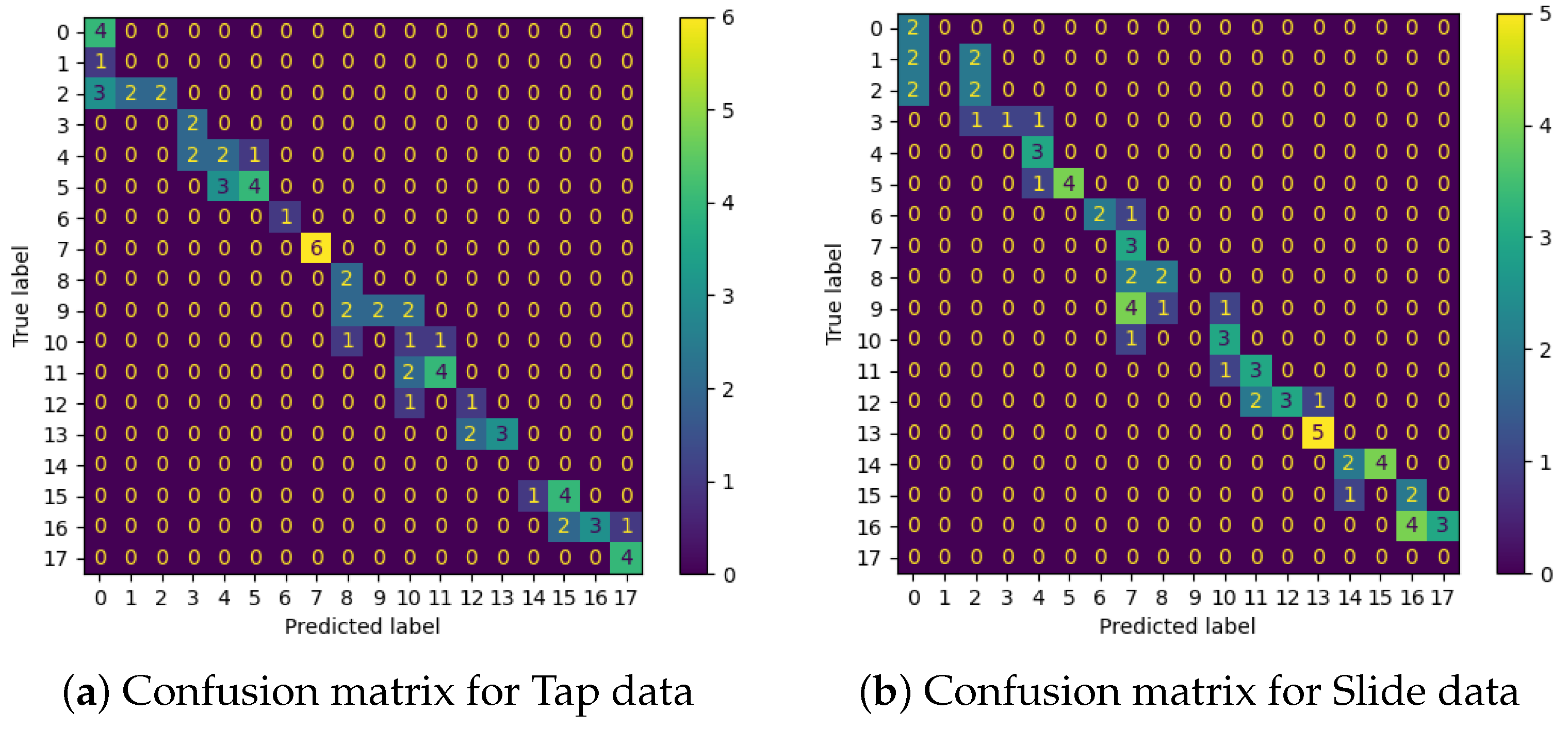
3.4. Edge Orientation Classification Performance
4. Discussion
4.1. Hardware
4.2. Network
4.3. Tactile Task
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| KNN | K-nearest Neighbours |
| SNN | Spiking Neural Network |
| STDP | Spike-Timing-Dependant Plasticity |
| t-SNE | t-distributed Stochastic Neighbour Embedding |
References
- Ward-Cherrier, B.; Pestell, N.; Cramphorn, L.; Winstone, B.; Giannaccini, M.E.; Rossiter, J.; Lepora, N.F. The TacTip Family: Soft Optical Tactile Sensors with 3D-Printed Biomimetic Morphologies. Soft Robot. 2018, 5, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, H.; Recchiuto, C.; Oddo, C.M.; Beccai, L.; Anthony, C.; Adams, M.; Carrozza, M.C.; Ward, M. A capacitive tactile sensor array for surface texture discrimination. Microelectron. Eng. 2011, 88, 1811–1813. [Google Scholar] [CrossRef]
- Goger, D.; Gorges, N.; Worn, H. Tactile sensing for an anthropomorphic robotic hand: Hardware and signal processing. In Proceedings of the 2009 IEEE International Conference on Robotics and Automation, Kobe, Japan, 12–17 May 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 895–901. [Google Scholar]
- Stassi, S.; Cauda, V.; Canavese, G.; Pirri, C.F. Flexible tactile sensing based on piezoresistive composites: A review. Sensors 2014, 14, 5296–5332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, H.; Jiang, L.; Fan, S.; Yang, J. Development of a flexible 3-D tactile sensor system for anthropomorphic artificial hand. IEEE Sens. J. 2012, 13, 510–518. [Google Scholar] [CrossRef]
- Yuan, W.; Dong, S.; Adelson, E.H. Gelsight: High-resolution robot tactile sensors for estimating geometry and force. Sensors 2017, 17, 2762. [Google Scholar] [CrossRef]
- Lepora, N.F.; Church, A.; De Kerckhove, C.; Hadsell, R.; Lloyd, J. From pixels to percepts: Highly robust edge perception and contour following using deep learning and an optical biomimetic tactile sensor. IEEE Robot. Autom. Lett. 2019, 4, 2101–2107. [Google Scholar] [CrossRef]
- Aquilina, K.; Barton, D.A.; Lepora, N.F. Shear-invariant Sliding Contact Perception with a Soft Tactile Sensor. In Proceedings of the 2019 International Conference on Robotics and Automation (ICRA), Montreal, QC, Canada, 20–24 May 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 4283–4289. [Google Scholar]
- Zaghloul, K.A.; Boahen, K. A silicon retina that reproduces signals in the optic nerve. J. Neural Eng. 2006, 3, 257–267. [Google Scholar] [CrossRef]
- Lee, W.W.; Kukreja, S.L.; Thakor, N.V. Discrimination of dynamic tactile contact by temporally precise event sensing in spiking neuromorphic networks. Front. Neurosci. 2017, 11, 5. [Google Scholar] [CrossRef]
- Birkoben, T.; Winterfeld, H.; Fichtner, S.; Petraru, A.; Kohlstedt, H. A spiking and adapting tactile sensor for neuromorphic applications. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Oddo, C.M.; Controzzi, M.; Beccai, L.; Cipriani, C.; Carrozza, M.C. Roughness encoding for discrimination of surfaces in artificial active-touch. IEEE Trans. Robot. 2011, 27, 522–533. [Google Scholar] [CrossRef]
- Delbruck, T. Frame-free dynamic digital vision. In Proceedings of the International Symposium on Secure-Life Electronics, Tokyo, Japan, 6–7 March 2008; pp. 21–26. [Google Scholar] [CrossRef]
- Johansson, R.S.; Flanagan, J.R. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat. Rev. Neurosci. 2009, 10, 345–359. [Google Scholar] [CrossRef]
- Johnson, K.O. The roles and functions of cutaneous mechanoreceptors. Curr. Opin. Neurobiol. 2001, 11, 455–461. [Google Scholar] [CrossRef]
- Rongala, U.B.; Mazzoni, A.; Chiurazzi, M.; Camboni, D.; Milazzo, M.; Massari, L.; Ciuti, G.; Roccella, S.; Dario, P.; Oddo, C.M. Tactile decoding of edge orientation with artificial cuneate neurons in dynamic conditions. Front. Neurorobot. 2019, 13, 44. [Google Scholar] [CrossRef]
- Friedl, K.E.; Voelker, A.R.; Peer, A.; Eliasmith, C. Human-Inspired Neurorobotic System for Classifying Surface Textures by Touch. IEEE Robot. Autom. Lett. 2016, 1, 516–523. [Google Scholar] [CrossRef]
- Yuan, W.; Li, R.; Srinivasan, M.A.; Adelson, E.H. Measurement of shear and slip with a GelSight tactile sensor. In Proceedings of the 2015 IEEE International Conference on Robotics and Automation (ICRA), Seattle, WA, USA, 26–30 May 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 304–311. [Google Scholar]
- James, J.W.; Pestell, N.; Lepora, N.F. Slip detection with a biomimetic tactile sensor. IEEE Robot. Autom. Lett. 2018, 3, 3340–3346. [Google Scholar] [CrossRef]
- Luo, S.; Mou, W.; Li, M.; Althoefer, K.; Liu, H. Rotation and translation invariant object recognition with a tactile sensor. In Proceedings of the SENSORS, 2014 IEEE, Valencia, Spain, 2–5 November 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 1030–1033. [Google Scholar]
- Lederman, S.J.; Klatzky, R.L. Relative Availability of Surface and Object Properties during Early Haptic Processing. J. Exp. Psychol. Hum. Percept. Perform. 1997, 23, 1680–1707. [Google Scholar] [CrossRef]
- Ponce Wong, R.D.; Hellman, R.B.; Santos, V.J. Spatial asymmetry in tactile sensor skindeformation aids perception of edgeorientation during haptic exploration. IEEE Trans. Haptics 2014, 7, 191–202. [Google Scholar] [CrossRef]
- Kumar, D.; Ghosh, R.; Nakagawa-Silva, A.; Soares, A.B.; Thakor, N.V. Neuromorphic approach to tactile edge orientation estimation using spatiotemporal similarity. Neurocomputing 2020, 407, 246–258. [Google Scholar] [CrossRef]
- Parvizi-Fard, A.; Amiri, M.; Kumar, D.; Iskarous, M.M.; Thakor, N.V. A functional spiking neuronal network for tactile sensing pathway to process edge orientation. Sci. Rep. 2021, 11, 1320. [Google Scholar] [CrossRef]
- Pruszynski, J.A.; Johansson, R.S. Edge-orientation processing in first-order tactile neurons. Nat. Neurosci. 2014, 17, 1404–1409. [Google Scholar] [CrossRef]
- Thorpe, S.; Delorme, A.; Van Rullen, R. Spike-based strategies for rapid processing. Neural Netw. 2021, 14, 715–725. [Google Scholar] [CrossRef]
- Bing, Z.; Meschede, C.; Röhrbein, F.; Huang, K.; Knoll, A.C. A survey of robotics control based on learning-inspired spiking neural networks. Front. Neurorobot. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Nichols, E.; McDaid, L.; Siddique, N. Case study on a self-organizing spiking neural network for robot navigation. Int. J. Neural Syst. 2010, 20, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Kheradpisheh, S.R.; Ganjtabesh, M.; Thorpe, S.J.; Masquelier, T. STDP-based spiking deep convolutional neural networks for object recognition. Neural Netw. 2018, 99, 56–67. [Google Scholar] [CrossRef]
- Li, J.; Liu, I.B.; Gao, W.; Huang, X. Spiking neural network with synaptic plasticity for recognition. In Proceedings of the 2018 IEEE 3rd Advanced Information Technology, Electronic and Automation Control Conference, IAEAC 2018, Chongqing, China, 12–14 October 2018; pp. 1728–1732. [Google Scholar] [CrossRef]
- Davies, M.; Wild, A.; Orchard, G.; Sandamirskaya, Y.; Guerra, G.A.F.; Joshi, P.; Plank, P.; Risbud, S.R. Advancing neuromorphic computing with loihi: A survey of results and outlook. Proc. IEEE 2021, 109, 911–934. [Google Scholar] [CrossRef]
- Ponulak, F. Supervised learning in spiking neural networks with ReSuMe method. Phd Pozn. Univ. Technol. 2006, 46, 47. [Google Scholar]
- Bohte, S.M.; Kok, J.N.; La Poutré, J.A. SpikeProp: Backpropagation for networks of spiking neurons. In Proceedings of the European Symposium on Artificial Neural Networks, Computational Intelligence and Machine Learning, Bruges, Belgium, 2–4 October 2000; Volume 48, pp. 419–424. [Google Scholar]
- Shrestha, S.B.; Orchard, G. Slayer: Spike layer error reassignment in time. Adv. Neural Inf. Process. Syst. 2018, 31. [Google Scholar]
- Markram, H.; Gerstner, W.; Sjöström, P.J. Spike-timing-dependent plasticity: A comprehensive overview. Front. Synaptic Neurosci. 2012, 4, 2. [Google Scholar] [CrossRef]
- Ward-Cherrier, B.; Pestell, N.; Lepora, N. NeuroTac: A Neuromorphic Optical Tactile Sensor applied to Texture Recognition. In Proceedings of the 2020 IEEE International Conference on Robotics and Automation (ICRA), Paris, France, 31 May–31 August 2020. [Google Scholar]
- Müller, G.R.; Conradt, J. A miniature low-power sensor system for real time 2D visual tracking of LED markers. In Proceedings of the 2011 IEEE International Conference on Robotics and Biomimetics, ROBIO 2011, Phuket, Thailand, 7–11 December 2011; pp. 2429–2434. [Google Scholar] [CrossRef]
- Brette, R.; Gerstner, W. Adaptive exponential integrate-and-fire model as an effective description of neuronal activity. J. Neurophysiol. 2005, 94, 3637–3642. [Google Scholar] [CrossRef]
- Davison, A.P.; Brüderle, D.; Eppler, J.; Kremkow, J.; Muller, E.; Pecevski, D.; Perrinet, L.; Yger, P. PyNN: A common interface for neuronal network simulators. Front. Neuroinform. 2009, 2. [Google Scholar] [CrossRef]
- Fardet, T.; Vennemo, S.B.; Mitchell, J.; Mørk, H.; Graber, S.; Hahne, J.; Spreizer, S.; Deepu, R.; Trensch, G.; Weidel, P.; et al. NEST 2.20.0. 2020. Available online: https://zenodo.org/record/3605514#.YyQcQbRBxPY (accessed on 30 July 2022). [CrossRef]
- Gütig, R.; Aharonov, R.; Rotter, S.; Sompolinsky, H. Learning Input Correlations through Nonlinear Temporally Asymmetric Hebbian Plasticity. J. Neurosci. 2003, 23, 3697–3714. [Google Scholar] [CrossRef]
- Van der Maaten, L.; Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Johansson, R.S.; Vallbo, A.B. Tactile sensibility in the human hand: Relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J. Physiol. 1979, 286, 283–300. [Google Scholar] [CrossRef]
- Furber, S.; Bogdan, P. SpiNNaker: A Spiking Neural Network Architecture; Now Publishers: Boston, MA, USA, 2020. [Google Scholar] [CrossRef]
- Legenstein, R.; Naeger, C.; Maass, W. What can a neuron learn with spike-timing-dependent plasticity? Neural Comput. 2005, 17, 2337–2382. [Google Scholar] [CrossRef]
- Xue, F.; Hou, Z.; Li, X. Computational capability of liquid state machines with spike-timing-dependent plasticity. Neurocomputing 2013, 122, 324–329. [Google Scholar] [CrossRef]






| Parameter | Value |
|---|---|
| Resting membrane potential | −60 mV |
| Membrane capacity | 1.0 nF |
| Membrane time constant | 10 ms |
| Decay time of synaptic current | 5 ms |
| Reset potential | −60 mV |
| Spike Threshold | −50 mv |
| Refractory period | 5 ms |
| Parameter | Search Space | Optimised Value |
|---|---|---|
| pf | 4, 8, 16, 32 | 16 |
| 50–450 in steps of 50 | 100 | |
| 0.001–0.1 | 0.036 | |
| 0.001–0.1 | 0.073 | |
| 1–6 | 4.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macdonald, F.L.A.; Lepora, N.F.; Conradt, J.; Ward-Cherrier, B. Neuromorphic Tactile Edge Orientation Classification in an Unsupervised Spiking Neural Network. Sensors 2022, 22, 6998. https://doi.org/10.3390/s22186998
Macdonald FLA, Lepora NF, Conradt J, Ward-Cherrier B. Neuromorphic Tactile Edge Orientation Classification in an Unsupervised Spiking Neural Network. Sensors. 2022; 22(18):6998. https://doi.org/10.3390/s22186998
Chicago/Turabian StyleMacdonald, Fraser L. A., Nathan F. Lepora, Jörg Conradt, and Benjamin Ward-Cherrier. 2022. "Neuromorphic Tactile Edge Orientation Classification in an Unsupervised Spiking Neural Network" Sensors 22, no. 18: 6998. https://doi.org/10.3390/s22186998
APA StyleMacdonald, F. L. A., Lepora, N. F., Conradt, J., & Ward-Cherrier, B. (2022). Neuromorphic Tactile Edge Orientation Classification in an Unsupervised Spiking Neural Network. Sensors, 22(18), 6998. https://doi.org/10.3390/s22186998






