Teaching Essential EMG Theory to Kinesiologists and Physical Therapists Using Analogies Visual Descriptions, and Qualitative Analysis of Biophysical Concepts
Abstract
1. Introduction
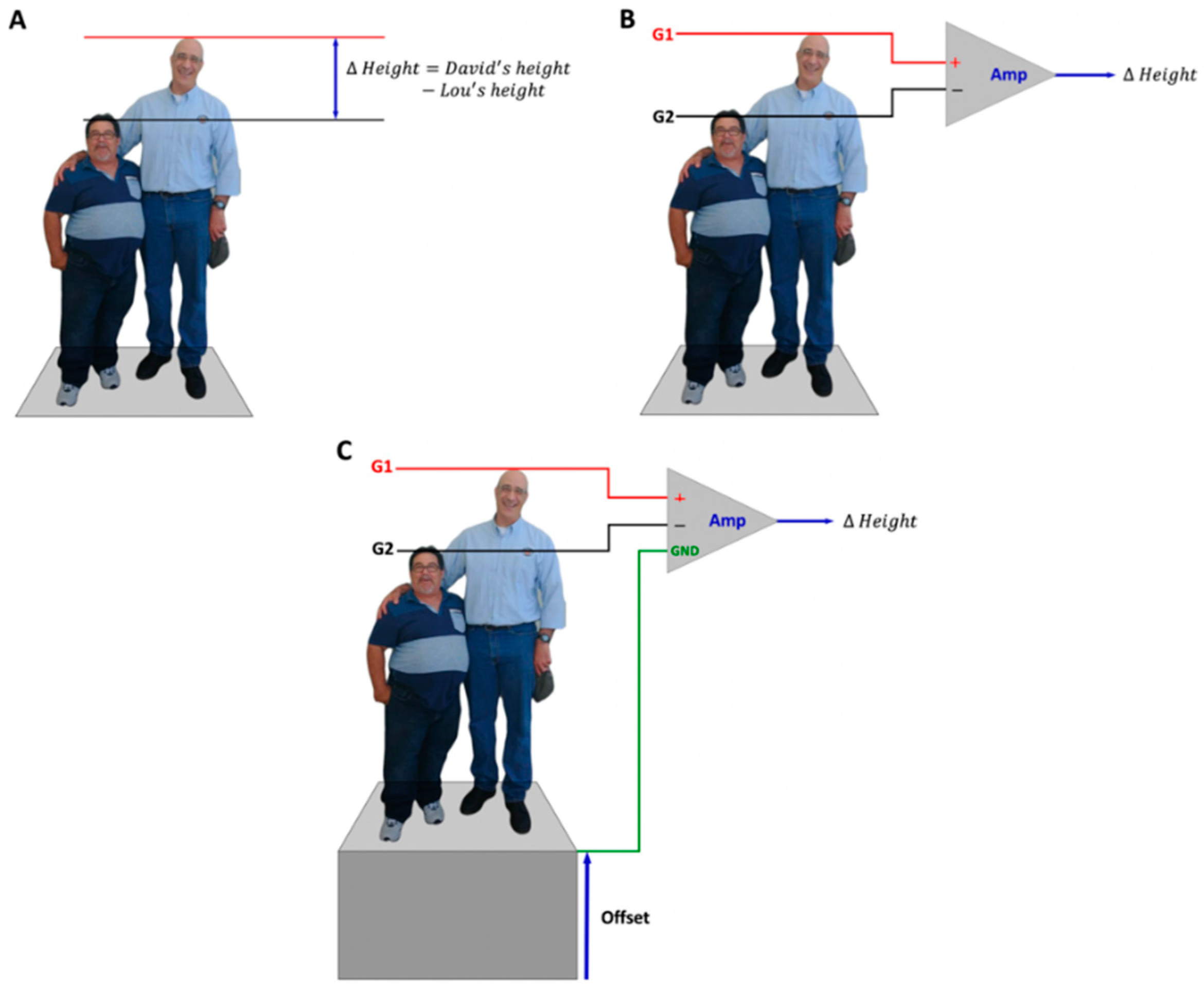
2. Differential Amplification
Comparing the Electric Potential at Two Different Points
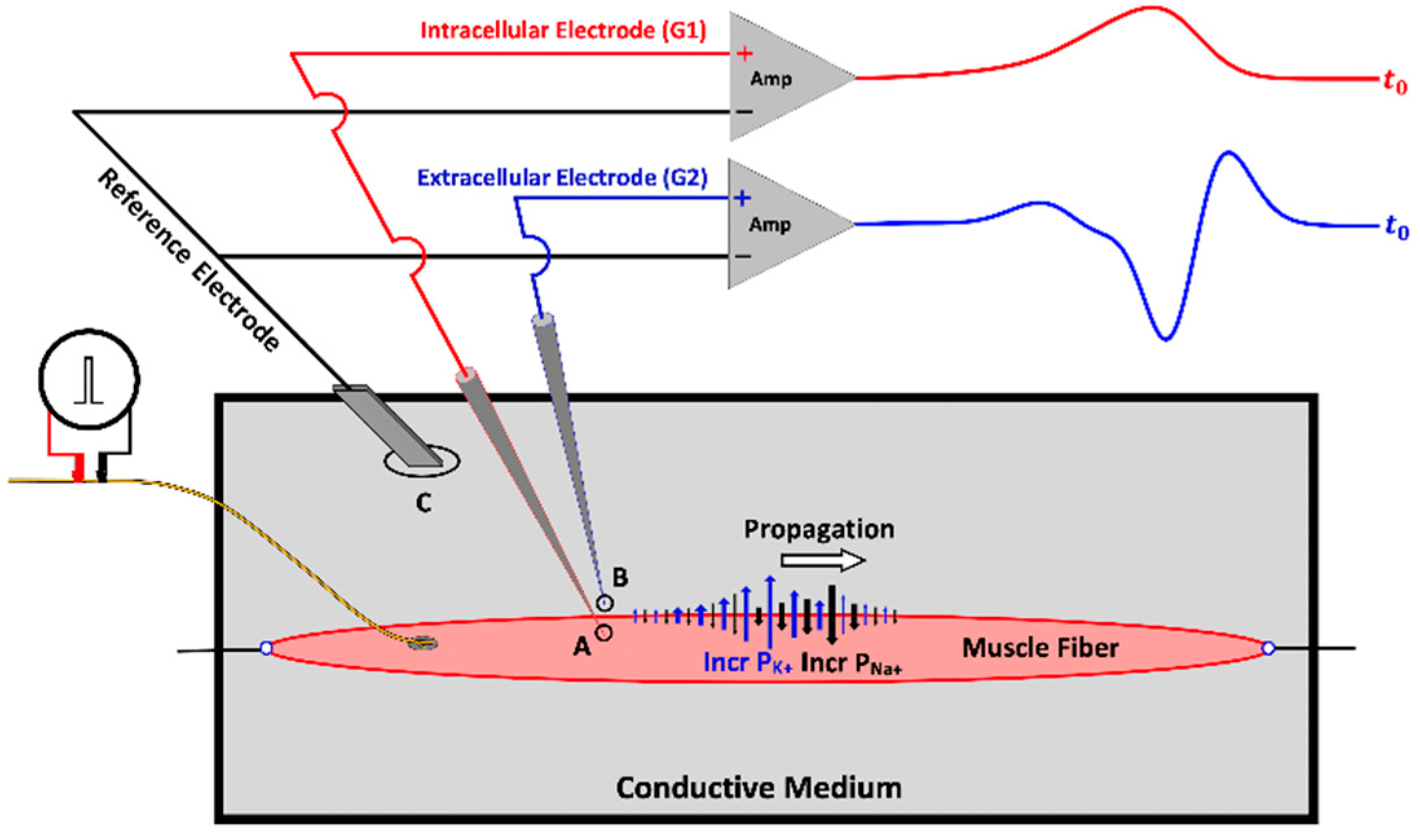
3. Muscle Fiber Action Potential
3.1. The Propagating Component of the MFAP
Muscle Fiber Conduction Velocity
3.2. The Non-Propagating Component on the MFAP
4. Motor Unit and Compound Muscle Action Potentials
4.1. Innervation Zone versus Motor Point
4.2. Factors That Affect Waveform Shape
4.2.1. Monopolar Recordings and Electrode Placement
4.2.2. Propagating and Non-Propagating Components and Crosstalk
5. Bipolar Recordings Represented as Two Time-Delayed Monopolar Signals
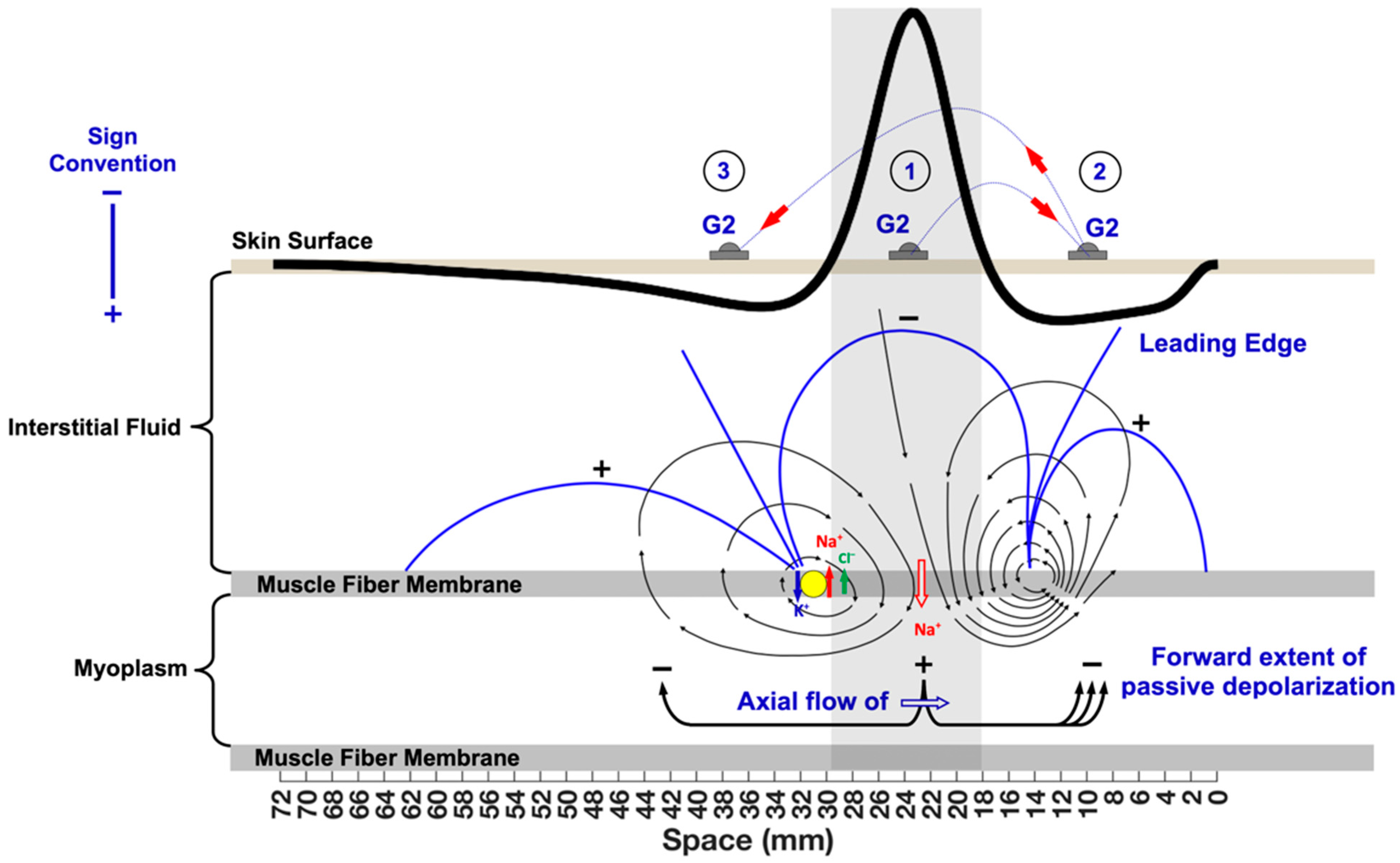
5.1. Understanding the G1 and G2 Sign Conventions
5.2. Laying the Foundations for Spatial Filtering
5.3. Qualitative Frequency Analysis of Bipolar MUAPs
6. Common Mode Interference
7. Volume Conduction
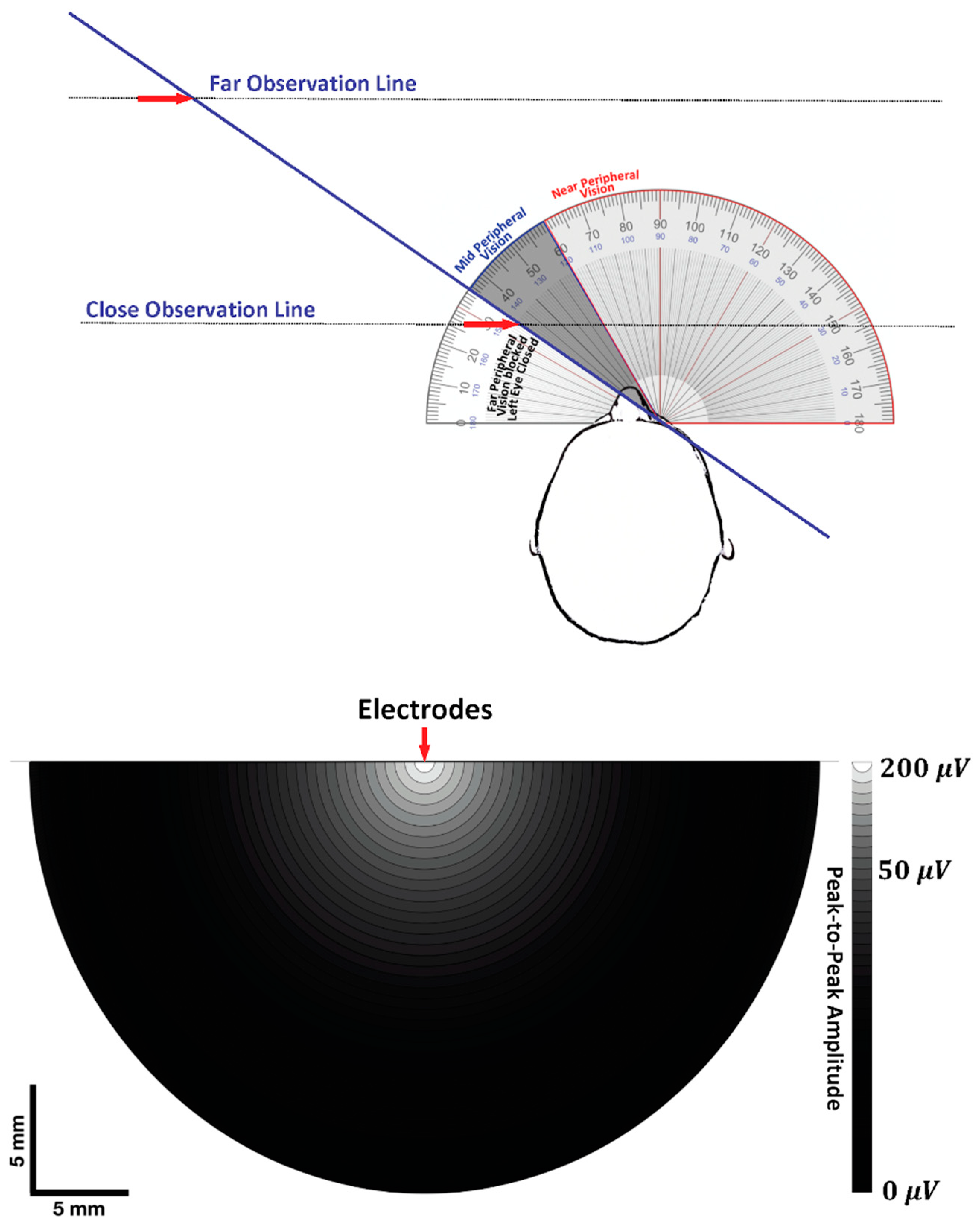
7.1. Visual Field and Electrode Pickup Volume
7.2. Trying to Cross the Street with One Eye Shut
7.2.1. Case 1: Near-Field Observation
7.2.2. Case 2: Far-Field Observation
7.2.3. The Geometry of Electrode-Source Detection
7.2.4. Dipole Field Lines and Apparent Distance between MFAP Peaks
7.2.5. Distance between MFAP Peaks, Interelectrode Distance, and Selectivity
8. Wavelength
9. Conduction Velocity and Wavelength
9.1. Addition
9.2. Attenuation
10. Factors Affecting the Analysis and Interpretation of the Surface EMG Signal
10.1. Global Surface EMG Measures
10.2. Surface Detected Motor Unit Activity
11. Summary and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EMG | Electromyogram/electromyography |
| sEMG | Surface electromyogram |
| HD-sEMG | High-density sEMG |
| IZ | Innervation zone of a motor unit or muscle |
| IP | Interference pattern |
| MU | Motor unit |
| MUAP | Motor unit action potential |
| NMJ | Neuromuscular junction |
| IED | Interelectrode distance |
| MFAP | Muscle fiber action potential |
| CMAP | Compound muscle action potential |
| M-wave | Motor wave |
| MP | Motor point |
| G1 | Grid Electrode 1 (E1) |
| G2 | Grid Electrode 2 (E2) |
| GND | Ground |
| EMF | Electromagnetic fields |
| ISEK | International Society of Electrophysiology and Kinesiology |
| CV | Conduction velocity |
| MFCV | Muscle fiber conduction velocity |
| NCV | Nerve conduction velocity |
| LD | Leading dipole |
| TD | Trailing dipole |
| SD | Single differential |
| DD | Double differential |
| VPP | Peak-to-Peak Voltage Amplitude |
| PLI | Power line interference |
| CMMR | Common mode rejection ratio |
References
- McManus, L.; De Vito, G.; Lowery, M.M. Analysis and Biophysics of Surface EMG for Physiotherapists and Kinesiologists: Toward a Common Language with Rehabilitation Engineers. Front. Neurol. 2020, 11, 576759. [Google Scholar] [CrossRef] [PubMed]
- Criswell, E.; Cram, J.R. Cram’s Introduction to Surface Electromyography; Jones and Bartlett: Sudbury, MA, USA, 2011; ISBN 978-0-7637-3274-5. [Google Scholar]
- Barbero, M.; Merletti, R.; Rainoldi, A. Atlas of Muscle Innervation Zones: Understanding Surface Electromyography and Its Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-88-470-2463-2. [Google Scholar]
- Kamen, G.; Gabriel, D.A. Essentials of Electromyography; Human Kinetics: Champaign, IL, USA, 2009; ISBN 978-1-4925-4047-2. [Google Scholar]
- Merletti, R.; Farina, D. (Eds.) Surface Electromyography: Physiology, Engineering and Applications; IEEE Press Series in Biomedical Engineering; IEEE Press-Wiley: Piscataway, NJ, USA; Hoboken, NJ, USA, 2016; ISBN 978-1-118-98702-5. [Google Scholar]
- Kumar, S.; Mital, A. Electromyography in Ergonomics; CRC Press: Boca Raton, FL, USA, 1996; ISBN 978-0-7484-0130-7. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Merletti, R.; Muceli, S. Tutorial. Surface EMG Detection in Space and Time: Best Practices. J. Electromyogr. Kinesiol. 2019, 49, 102363. [Google Scholar] [CrossRef] [PubMed]
- Merletti, R.; Cerone, G.L. Tutorial. Surface EMG Detection, Conditioning and Pre-Processing: Best Practices. J. Electromyogr. Kinesiol. 2020, 54, 102440. [Google Scholar] [CrossRef] [PubMed]
- Besomi, M.; Hodges, P.W.; Van Dieën, J.; Carson, R.G.; Clancy, E.A.; Disselhorst-Klug, C.; Holobar, A.; Hug, F.; Kiernan, M.C.; Lowery, M.; et al. Consensus for Experimental Design in Electromyography (CEDE) Project: Electrode Selection Matrix. J. Electromyogr. Kinesiol. 2019, 48, 128–144. [Google Scholar] [CrossRef]
- Besomi, M.; Hodges, P.W.; Clancy, E.A.; Van Dieën, J.; Hug, F.; Lowery, M.; Merletti, R.; Søgaard, K.; Wrigley, T.; Besier, T.; et al. Consensus for Experimental Design in Electromyography (CEDE) Project: Amplitude Normalization Matrix. J. Electromyogr. Kinesiol. 2020, 53, 102438. [Google Scholar] [CrossRef]
- Gallina, A.; Disselhorst-Klug, C.; Farina, D.; Merletti, R.; Besomi, M.; Holobar, A.; Enoka, R.M.; Hug, F.; Falla, D.; Søgaard, K.; et al. Consensus for Experimental Design in Electromyography (CEDE) Project: High-Density Surface Electromyography Matrix. J. Electromyogr. Kinesiol. 2022, 64, 102656. [Google Scholar] [CrossRef]
- McManus, L.; Lowery, M.; Merletti, R.; Søgaard, K.; Besomi, M.; Clancy, E.A.; van Dieën, J.H.; Hug, F.; Wrigley, T.; Besier, T.; et al. Consensus for Experimental Design in Electromyography (CEDE) Project: Terminology Matrix. J. Electromyogr. Kinesiol. 2021, 59, 102565. [Google Scholar] [CrossRef]
- Tankisi, H.; Burke, D.; Cui, L.; de Carvalho, M.; Kuwabara, S.; Nandedkar, S.D.; Rutkove, S.; Stålberg, E.; van Putten, M.J.A.M.; Fuglsang-Frederiksen, A. Standards of Instrumentation of EMG. Clin. Neurophysiol. 2020, 131, 243–258. [Google Scholar] [CrossRef]
- Loeb, G.E.; Gans, C. Electromyography for Experimentalists; University of Chicago Press: Chicago, IL, USA, 1986; ISBN 0-226-49015-7. [Google Scholar]
- Rodriguez-Falces, J.; Navallas, J.; Gila, L.; Malanda, A.; Dimitrova, N.A. Influence of the Shape of Intracellular Potentials on the Morphology of Single-Fiber Extracellular Potentials in Human Muscle Fibers. Med. Biol. Eng. Comput. 2012, 50, 447–460. [Google Scholar] [CrossRef]
- Rodríguez, J.; Navallas, J.; Gila, L.; Dimitrova, N.A.; Malanda, A. Estimating the Duration of Intracellular Action Potentials in Muscle Fibres from Single-Fibre Extracellular Potentials. J. Neurosci. Methods 2011, 197, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Rosenfalck, P. Intra- and Extracellular Potential Fields of Active Nerve and Muscle Fibres. A Physico-Mathematical Analysis of Different Models. Acta Physiol. Scand. Suppl. 1969, 321, 1–168. [Google Scholar] [PubMed]
- Leffler, C.T.; Gozani, S.N.; Nguyen, Z.Q.; Cros, D. An Automated Electrodiagnostic Technique for Detection of Carpal Tunnel Syndrome. Neurol. Clin. Neurophysiol. 2000, 2000, 2–9. [Google Scholar] [CrossRef]
- Ruff, R.L. Sodium Channel Slow Inactivation and the Distribution of Sodium Channels on Skeletal Muscle Fibres Enable the Performance Properties of Different Skeletal Muscle Fibre Types. Acta Physiol. Scand. 1996, 156, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Fortune, E.; Lowery, M.M. Effect of Membrane Properties on Skeletal Muscle Fiber Excitability: A Sensitivity Analysis. Med. Biol. Eng. Comput. 2012, 50, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Plonsey, R.; Barr, R.C. Bioelectricity: A Quantitative Approach; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; ISBN 978-0-387-48865-3. [Google Scholar]
- Hussain, Z. Electrophysiology of Membrane Potentials: Mathematical Phsyiology and Mathematical Medicine. Int. J. Biol. Biotech. 2022, 19, 161–170. [Google Scholar]
- Carp, J.S.; Tennissen, A.M.; Wolpaw, J.R. Conduction Velocity Is Inversely Related to Action Potential Threshold in Rat Motoneuron Axons. Exp. Brain Res. 2003, 150, 497–505. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Negro, F.; Felici, F.; Farina, D. Distribution of Muscle Fibre Conduction Velocity for Representative Samples of Motor Units in the Full Recruitment Range of the Tibialis Anterior Muscle. Acta Physiol. 2018, 222, e12930. [Google Scholar] [CrossRef]
- Houtman, C.J.; Stegeman, D.F.; Van Dijk, J.P.; Zwarts, M.J. Changes in Muscle Fiber Conduction Velocity Indicate Recruitment of Distinct Motor Unit Populations. J. Appl. Physiol. 2003, 95, 1045–1054. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Negro, F.; Falla, D.; Bazzucchi, I.; Farina, D.; Felici, F. Higher Muscle Fiber Conduction Velocity and Early Rate of Torque Development in Chronically Strength-Trained Individuals. J. Appl. Physiol. 2018, 125, 1218–1226. [Google Scholar] [CrossRef]
- Rodriguez-Falces, J.; Place, N. Muscle Fibre Conduction Velocity Varies in Opposite Directions after Short- vs. Long-Duration Muscle Contractions. Eur. J. Appl. Physiol. 2021, 121, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Zwarts, M.J.; Van Weerden, T.W.; Haenen, H.T. Relationship between Average Muscle Fibre Conduction Velocity and EMG Power Spectra during Isometric Contraction, Recovery and Applied Ischemia. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 212–216. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.C.D.; Gabriel, D.A. Reliability of a Simple Method for Determining Muscle Fiber Conduction Velocity. Muscle Nerve 2012, 45, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Gallina, A.; Ritzel, C.H.; Merletti, R.; Vieira, T.M.M. Do Surface Electromyograms Provide Physiological Estimates of Conduction Velocity from the Medial Gastrocnemius Muscle? J. Electromyogr. Kinesiol. 2013, 23, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Rutkove, S.B. Effects of Temperature on Neuromuscular Electrophysiology. Muscle Nerve 2001, 24, 867–882. [Google Scholar] [CrossRef]
- Farina, D.; Falla, D. Effect of Muscle-Fiber Velocity Recovery Function on Motor Unit Action Potential Properties in Voluntary Contractions. Muscle Nerve 2008, 37, 650–658. [Google Scholar] [CrossRef]
- Farina, D.; Ferguson, R.A.; Macaluso, A.; De Vito, G. Correlation of Average Muscle Fiber Conduction Velocity Measured during Cycling Exercise with Myosin Heavy Chain Composition, Lactate Threshold, and VO2max. J. Electromyogr. Kinesiol. 2007, 17, 393–400. [Google Scholar] [CrossRef]
- Rodriguez-Falces, J.; Place, N. Sarcolemmal Excitability, M-Wave Changes, and Conduction Velocity During a Sustained Low-Force Contraction. Front. Physiol. 2021, 12, 732624. [Google Scholar] [CrossRef]
- Merlo, E.; Pozzo, M.; Antonutto, G.; di Prampero, P.E.; Merletti, R.; Farina, D. Time–Frequency Analysis and Estimation of Muscle Fiber Conduction Velocity from Surface EMG Signals during Explosive Dynamic Contractions. J. Neurosci. Methods 2005, 142, 267–274. [Google Scholar] [CrossRef]
- Quinzi, F.; Bianchetti, A.; Felici, F.; Sbriccoli, P. Higher Torque and Muscle Fibre Conduction Velocity of the Biceps Brachii in Karate Practitioners during Isokinetic Contractions. J. Electromyogr. Kinesiol. 2018, 40, 81–87. [Google Scholar] [CrossRef]
- Zwarts, M.J.; Stegeman, D.F. Multichannel Surface EMG: Basic Aspects and Clinical Utility. Muscle Nerve 2003, 28, 1–17. [Google Scholar] [CrossRef]
- Kumagai, K.; Yamada, M. The Clinical Use of Multichannel Surface Electromyography. Pediatrics Int. 1991, 33, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Butugan, M.K.; Sartor, C.D.; Watari, R.; Martins, M.C.S.; Ortega, N.R.S.; Vigneron, V.A.M.; Sacco, I.C.N. Multichannel EMG-Based Estimation of Fiber Conduction Velocity during Isometric Contraction of Patients with Different Stages of Diabetic Neuropathy. J. Electromyogr. Kinesiol. 2014, 24, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Blijham, P.J.; Hengstman, G.J.D.; Ter Laak, H.J.; Van Engelen, B.G.M.; Zwarts, M.J. Muscle-Fiber Conduction Velocity and Electromyography as Diagnostic Tools in Patients with Suspected Inflammatory Myopathy: A Prospective Study. Muscle Nerve 2004, 29, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Blijham, P.J.; van Engelen, B.G.M.; Drost, G.; Stegeman, D.F.; Schelhaas, H.J.; Zwarts, M.J. Diagnostic Yield of Muscle Fibre Conduction Velocity in Myopathies. J. Neurol. Sci. 2011, 309, 40–44. [Google Scholar] [CrossRef]
- Dumitru, D. Physiologic Basis of Potentials Recorded in Electromyography. Muscle Nerve 2000, 23, 1667–1685. [Google Scholar] [CrossRef]
- Lateva, Z.C.; McGill, K.C. Estimating Motor-Unit Architectural Properties by Analyzing Motor-Unit Action Potential Morphology. Clin. Neurophysiol. 2001, 112, 127–135. [Google Scholar] [CrossRef]
- Roeleveld, K.; Blok, J.H.; Stegeman, D.F.; Van Oosterom, A. Volume Conduction Models for Surface EMG.; Confrontation with Measurements. J. Electromyogr. Kinesiol. 1997, 7, 221–232. [Google Scholar] [CrossRef]
- Mesin, L. Crosstalk in Surface Electromyogram: Literature Review and Some Insights. Phys. Eng. Sci. Med. 2020, 43, 481–492. [Google Scholar] [CrossRef]
- Calder, K.M.; Hall, L.-A.; Lester, S.M.; Inglis, J.G.; Gabriel, D.A. Reliability of the Biceps Brachii M-Wave. J. Neuroeng. Rehabil. 2005, 2, 33. [Google Scholar] [CrossRef]
- Bowden, J.L.; McNulty, P.A. Mapping the Motor Point in the Human Tibialis Anterior Muscle. Clin. Neurophysiol. 2012, 123, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-Y.; Kim, J.-S.; Lee, W.I. Anatomic Localization of Motor Points of Hip Adductors. Am. J. Phys. Med. Rehabil. 2009, 88, 336–341. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, B.-N.; An, X.; Chung, R.-H.; Han, S.-H. Location of the Motor Entry Point and Intramuscular Motor Point of the Tibialis Posterior Muscle: For Effective Motor Point Block. Clin. Anat. 2011, 24, 91–96. [Google Scholar] [CrossRef] [PubMed]
- An, X.C.; Lee, J.H.; Im, S.; Lee, M.S.; Hwang, K.; Kim, H.W.; Han, S.-H. Anatomic Localization of Motor Entry Points and Intramuscular Nerve Endings in the Hamstring Muscles. Surg. Radiol. Anat. 2010, 32, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Narita, H.; Yoshida, H.; Chiba, S.; Oda, A.; Ishikawa, T.; Takahashi, N.; Tani, T.; Watanabe, S.; Tonosaki, Y. Does the Location of the Motor Point Identified with Electrical Stimulation Correspond to That Identified with the Gross Anatomical Method? J. Phys. Ther. Sci 2011, 23, 737–739. [Google Scholar] [CrossRef]
- Merletti, R.; Farina, D.; Gazzoni, M. The Linear Electrode Array: A Useful Tool with Many Applications. J. Electromyogr. Kinesiol. 2003, 13, 37–47. [Google Scholar] [CrossRef]
- Jahanmiri-Nezhad, F.; Barkhaus, P.E.; Rymer, W.Z.; Zhou, P. Innervation Zones of Fasciculating Motor Units: Observations by a Linear Electrode Array. Front. Hum. Neurosci. 2015, 9, 329. [Google Scholar] [CrossRef]
- Franz, A.; Klaas, J.; Schumann, M.; Frankewitsch, T.; Filler, T.J.; Behringer, M. Anatomical versus Functional Motor Points of Selected Upper Body Muscles. Muscle Nerve 2018, 57, 460–465. [Google Scholar] [CrossRef]
- Gobbo, M.; Maffiuletti, N.A.; Orizio, C.; Minetto, M.A. Muscle Motor Point Identification Is Essential for Optimizing Neuromuscular Electrical Stimulation Use. J. Neuroeng. Rehabil. 2014, 11, 17. [Google Scholar] [CrossRef]
- Christie, A.D.; Inglis, J.G.; Boucher, J.P.; Gabriel, D.A. Reliability of the FCR H-Reflex. J. Clin. Neurophysiol. 2005, 22, 204–209. [Google Scholar]
- Christie, A.; Lester, S.; LaPierre, D.; Gabriel, D.A. Reliability of a New Measure of H-Reflex Excitability. Clin. Neurophysiol. 2004, 115, 116–123. [Google Scholar] [CrossRef]
- Guzmán-Venegas, R.A.; Araneda, O.F.; Silvestre, R.A. Differences between Motor Point and Innervation Zone Locations in the Biceps Brachii. An Exploratory Consideration for the Treatment of Spasticity with Botulinum Toxin. J. Electromyogr. Kinesiol. 2014, 24, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Saitou, K.; Masuda, T.; Michikami, D.; Kojima, R.; Okada, M. Innervation Zones of the Upper and Lower Limb Muscles Estimated by Using Multichannel Surface EMG. J. Hum. Ergol. 2000, 29, 35–52. [Google Scholar]
- Beretta Piccoli, M.; Rainoldi, A.; Heitz, C.; Wüthrich, M.; Boccia, G.; Tomasoni, E.; Spirolazzi, C.; Egloff, M.; Barbero, M. Innervation Zone Locations in 43 Superficial Muscles: Toward a Standardization of Electrode Positioning. Muscle Nerve 2014, 49, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Botter, A.; Oprandi, G.; Lanfranco, F.; Allasia, S.; Maffiuletti, N.A.; Minetto, M.A. Atlas of the Muscle Motor Points for the Lower Limb: Implications for Electrical Stimulation Procedures and Electrode Positioning. Eur. J. Appl. Physiol. 2011, 111, 2461. [Google Scholar] [CrossRef]
- Delagi, E.F. Anatomical Guide for the Electromyographer: The Limbs and Trunk; Charles C Thomas Pub Ltd.: Springfield, IL, USA, 2011; ISBN 978-0-398-08649-7. [Google Scholar]
- Lee, H.J.; DeLisa, J.A. Manual of Nerve Conduction Study and Surface Anatomy for Needle Electromyography; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; ISBN 978-0-7817-5821-5. [Google Scholar]
- Warfel, J.H. The Extremities: Muscles and Motor Points, 6th ed.; Lea & Febiger: Philadelphia, PA, USA, 1993; ISBN 978-0-8121-1582-6. [Google Scholar]
- Bromberg, M.B.; Spiegelberg, T. The Influence of Active Electrode Placement on CMAP Amplitude. Electroencephalogr. Clin. Neurophysiol. 1997, 105, 385–389. [Google Scholar] [CrossRef]
- Rainoldi, A.; Nazzaro, M.; Merletti, R.; Farina, D.; Caruso, I.; Gaudenti, S. Geometrical Factors in Surface EMG of the Vastus Medialis and Lateralis Muscles. J. Electromyogr. Kinesiol. 2000, 10, 327–336. [Google Scholar] [CrossRef]
- Beck, T.W.; Housh, T.J.; Cramer, J.T.; Weir, J.P. The Effects of Interelectrode Distance over the Innervation Zone and Normalization on the Electromyographic Amplitude and Mean Power Frequency versus Concentric, Eccentric, and Isometric Torque Relationships for the Vastus Lateralis Muscle. J. Electromyogr. Kinesiol. 2009, 19, 219–231. [Google Scholar] [CrossRef]
- Phongsamart, G.; Wertsch, J.J.; Ferdjallah, M.; King, J.C.; Foster, D.T. Effect of Reference Electrode Position on the Compound Muscle Action Potential (CMAP) Onset Latency. Muscle Nerve 2002, 25, 816–821. [Google Scholar] [CrossRef]
- Boyd, D.C.; Lawrence, P.D.; Bratty, P.J. On Modeling the Single Motor Unit Action Potential. IEEE Trans. Biomed. Eng. 1978, BME-25, 236–243. [Google Scholar] [CrossRef]
- Dimitrova, N.A.; Dimitrov, G.V.; Nikitin, O.A. Neither High-Pass Filtering nor Mathematical Differentiation of the EMG Signals Can Considerably Reduce Cross-Talk. J. Electromyogr. Kinesiol. 2002, 12, 235–246. [Google Scholar] [CrossRef]
- Winter, D.A.; Fuglevand, A.J.; Archer, S.E. Crosstalk in Surface Electromyography: Theoretical and Practical Estimates. J. Electromyogr. Kinesiol. 1994, 4, 15–26. [Google Scholar] [CrossRef]
- De Luca, C.J.; Merletti, R. Surface Myoelectric Signal Cross-Talk among Muscles of the Leg. Electroencephalogr. Clin. Neurophysiol. 1988, 69, 568–575. [Google Scholar] [CrossRef]
- Solomonow, M.; Baratta, R.; Bernardi, M.; Zhou, B.; Lu, Y.; Zhu, M.; Acierno, S. Surface and Wire EMG Crosstalk in Neighbouring Muscles. J. Electromyogr. Kinesiol. 1994, 4, 131–142. [Google Scholar] [CrossRef]
- Koh, T.J.; Grabiner, M.D. Evaluation of Methods to Minimize Cross Talk in Surface Electromyography. J. Biomech. 1993, 26, 151–157. [Google Scholar] [CrossRef]
- De Luca, C.J. The Use of Surface Electromyography in Biomechanics. J. Appl. Biomech. 1997, 13, 135–163. [Google Scholar] [CrossRef]
- Dimitrov, G.V.; Disselhorst-Klug, C.; Dimitrova, N.A.; Schulte, E.; Rau, G. Simulation Analysis of the Ability of Different Types of Multi-Electrodes to Increase Selectivity of Detection and to Reduce Cross-Talk. J. Electromyogr. Kinesiol. 2003, 13, 125–138. [Google Scholar] [CrossRef]
- Rainoldi, A.; Melchiorri, G.; Caruso, I. A Method for Positioning Electrodes during Surface EMG Recordings in Lower Limb Muscles. J. Neurosci. Methods 2004, 134, 37–43. [Google Scholar] [CrossRef]
- Mesin, L.; Merletti, R.; Rainoldi, A. Surface EMG: The Issue of Electrode Location. J. Electromyogr. Kinesiol. 2009, 19, 719–726. [Google Scholar] [CrossRef]
- Basmajian, J.V.; DeLuca, C.J. Apparatus, Detection, and Recording Techniques. In Muscles Alive, Their Functions Revealed by Electromyography; Lippincott Williams and Wilkins: Baltimore, MD, USA, 1985; pp. 19–64. ISBN O-683-00414-X. [Google Scholar]
- Wood, D.E.; Ewins, D.J.; Balachandran, W. Comparative Analysis of Power-Line Interference between Two- or Three-Electrode Biopotential Amplifiers. Med. Biol. Eng. Comput. 1995, 33, 63–68. [Google Scholar] [CrossRef]
- Winter, B.B.; Webster, J.G. Reductionl of Interference Due to Common Mode Voltage in Biopotential Amplifiers. IEEE Trans. Biomed. Eng. 1983, BME-30, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Clancy, E.A.; Morin, E.L.; Merletti, R. Sampling, Noise-Reduction and Amplitude Estimation Issues in Surface Electromyography. J. Electromyogr. Kinesiol. 2002, 12, 16. [Google Scholar] [CrossRef]
- Goldman, R.I.; Stern, J.M.; Engel, J.; Cohen, M.S. Acquiring Simultaneous EEG and Functional MRI. Clin. Neurophysiol. 2000, 111, 1974–1980. [Google Scholar] [CrossRef]
- Perreault, E.J.; Hunter, I.W.; Kearney, R.E. Quantitative Analysis of Four EMG Amplifiers. J. Biomed. Eng. 1993, 15, 413–419. [Google Scholar] [CrossRef]
- Gabriel, D.A.; Lester, S.M.; Lenhardt, S.A.; Cambridge, E.D.J. Analysis of Surface EMG Spike Shape across Different Levels of Isometric Force. J. Neurosci. Methods 2007, 159, 146–152. [Google Scholar] [CrossRef]
- Inglis, J.G.; Gabriel, D.A. Sex Differences in Motor Unit Discharge Rates at Maximal and Submaximal Levels of Force Output. Appl. Physiol. Nutr. Metab. 2020, 45, 1197–1207. [Google Scholar] [CrossRef]
- Hary, D.; Bekey, G.A.; Antonelli, D.J. Circuit Models and Simulation Analysis of Electromyographic Signal Sources-I: The Impedance of EMG Electrodes. IEEE Trans. Biomed. Eng. 1987, BME-34, 91–97. [Google Scholar] [CrossRef]
- Panero, J.; Zelnik, M. Human Dimension & Interior Space: A Source Book of Design Reference Standards; Whitney Library of Design: New York, NY, USA, 1979; ISBN 978-0-8230-7271-2. [Google Scholar]
- Kenneth Walker, H.; Dallas Hall, W.; Willis Hurst, J. Spector, Robert 116 Visial Fields. In Clinical Methods: The History, Physical, and Laboratory Examinations; Butterworths: Boston, MA, USA, 1990; pp. 565–572. [Google Scholar]
- Lynn, P.A.; Bettles, N.D.; Hughes, A.D.; Johnson, S.W. Influence of Electrode Geometry on Bipolar Recordings of the Surface Electromyogram. Med. Biol. Eng. Comput. 1978, 16, 651–660. [Google Scholar] [CrossRef]
- Roeleveld, K.; Stegeman, D.F.; Vingerhoets, H.M.; Oosterom, A.V. Motor Unit Potential Contribution to Surface Electromyography. Acta Physiol. Scand. 1997, 160, 175–183. [Google Scholar] [CrossRef]
- Ohashi, J. Difference in Changes of Surface EMG during Low-Level Static Contraction between Monopolar and Bipolar Lead. Appl. Hum. Sci. 1995, 14, 79–88. [Google Scholar] [CrossRef]
- Barkhaus, P.E.; Nandedkar, S.D. Recording Characteristics of the Surface EMG Electrodes. Muscle Nerve 1994, 17, 1317–1323. [Google Scholar] [CrossRef]
- Botelho, D.P.; Curran, K.; Lowery, M.M. Anatomically Accurate Model of EMG during Index Finger Flexion and Abduction Derived from Diffusion Tensor Imaging. PLoS Comput. Biol. 2019, 15, e1007267. [Google Scholar] [CrossRef]
- Fuglevand, A.J.; Winter, D.A.; Patla, A.E.; Stashuk, D. Detection of Motor Unit Action Potentials with Surface Electrodes: Influence of Electrode Size and Spacing. Biol. Cybern. 1992, 67, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, B.K.; Mast, E.; Busschers, R.; Verloop, A.J.; Wallinga, W.; Rutten, W.L.C.; Gerrits, P.O.; Boom, H.K. Single Fibre Action Potentials in Skeletal Muscle Related to Recording Distances. J. Electromyogr. Kinesiol. 1994, 4, 37–46. [Google Scholar] [CrossRef][Green Version]
- De Lisa, J.A.; Brozovich, F.V. Volume Conduction in Electromyography: Experimental and Theoretical Review. Electromyogr. Clin. Neurophysiol. 1983, 23, 651–667. [Google Scholar]
- Dimitrova, N.A.; Dimitrov, G.V. Electromyography (EMG) Modeling. In Wiley Encyclopedia of Biomedical Engineering; Akay, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; ISBN 978-0-471-74036-0. [Google Scholar]
- Fridén, J.; Lovering, R.M.; Lieber, R.L. Fiber Length Variability within the Flexor Carpi Ulnaris and Flexor Carpi Radialis Muscles: Implications for Surgical Tendon Transfer. J. Hand Surg. 2004, 29, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Cooney, W.P.; Linscheid, R.L.; An, K.-N. Opposition of the Thumb: An Anatomic and Biomechanical Study of Tendon Transfers. J. Hand Surg. 1984, 9, 777–786. [Google Scholar] [CrossRef]
- Murray, W.M.; Buchanan, T.S.; Delp, S.L. The Isometric Functional Capacity of Muscles That Cross the Elbow. J. Biomech. 2000, 33, 943–952. [Google Scholar] [CrossRef]
- Reucher, H.; Rau, G.; Silny, J. Spatial Filtering of Noninvasive Multielectrode EMG: Part I-Introduction to Measuring Technique and Applications. IEEE Trans. Biomed. Eng. 1987, BME-34, 98–105. [Google Scholar] [CrossRef]
- Disselhorst-Klug, C.; Silny, J.; Rau, G. Improvement of Spatial Resolution in Surface-EMG: A Theoretical and Experimental Comparison of Different Spatial Filters. IEEE Trans. Biomed. Eng. 1997, 44, 567–574. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Holobar, A.; Falla, D.; Felici, F.; Enoka, R.M.; Farina, D. Tutorial: Analysis of Motor Unit Discharge Characteristics from High-Density Surface EMG Signals. J. Electromyogr. Kinesiol. 2020, 53, 102426. [Google Scholar] [CrossRef] [PubMed]
- Zipp, P. Effect of Electrode Parameters on the Bandwidth of the Surface Emg Power-Density Spectrum. Med. Biol. Eng. Comput. 1978, 16, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Kadefors, R. Myo-Electric Signal Processing as an Estimation Problem. In New Concepts of the Motor Unit, Neuromuscular Disorders, Electromyographic Kinesiology; Karger Publishers: Basel, Switzerland, 1973; Volume 1, pp. 519–532. ISBN 978-3-8055-1451-4. [Google Scholar]
- Sinderby, C.; Friberg, S.; Comtois, N.; Grassino, A. Chest Wall Muscle Cross Talk in Canine Costal Diaphragm Electromyogram. J. Appl. Physiol. 1996, 81, 2312–2327. [Google Scholar] [CrossRef] [PubMed]
- Campanini, I.; Merlo, A.; Disselhorst-Klug, C.; Mesin, L.; Muceli, S.; Merletti, R. Fundamental Concepts of Bipolar and High-Density Surface EMG Understanding and Teaching for Clinical, Occupational, and Sport Applications: Origin, Detection, and Main Errors. Sensors 2022, 22, 4150. [Google Scholar] [CrossRef]
- Hassan, T.; McIntosh, K.C.D.; Gabriel, D.A.; Clancy, E.A. Estimation of Impulse Response between Electromyogram Signals for Use in Conduction Delay Distribution Estimation. Med. Biol. Eng. Comput. 2013, 51, 757–768. [Google Scholar] [CrossRef]
- Hug, F. Can Muscle Coordination Be Precisely Studied by Surface Electromyography? J. Electromyogr. Kinesiol. 2011, 21, 1–12. [Google Scholar] [CrossRef]
- Farina, D. The Extraction of Neural Strategies from the Surface EMG. J. Appl. Physiol. 2004, 96, 1486–1495. [Google Scholar] [CrossRef]
- Farina, D.; Merletti, R.; Enoka, R.M. The Extraction of Neural Strategies from the Surface EMG: An Update. J. Appl. Physiol. 2014, 117, 1215–1230. [Google Scholar] [CrossRef]
- Mallette, M.M.; Cheung, S.S.; Kumar, R.I.; Hodges, G.J.; Holmes, M.W.R.; Gabriel, D.A. The Effects of Local Forearm Heating and Cooling on Motor Unit Properties during Submaximal Contractions. Exp. Physiol. 2021, 106, 200–211. [Google Scholar] [CrossRef]
- Mallette, M.M.; Green, L.A.; Gabriel, D.A.; Cheung, S.S. The Effects of Local Forearm Muscle Cooling on Motor Unit Properties. Eur. J. Appl. Physiol. 2018, 118, 401–410. [Google Scholar] [CrossRef]
- Martinez-Valdes, E.; Negro, F.; Falla, D.; De Nunzio, A.M.; Farina, D. Surface Electromyographic Amplitude Does Not Identify Differences in Neural Drive to Synergistic Muscles. J. Appl. Physiol. 2018, 124, 1071–1079. [Google Scholar] [CrossRef]
- Gabriel, D.A.; Kamen, G. Experimental and Modeling Investigation of Spectral Compression of Biceps Brachii SEMG Activity with Increasing Force Levels. J. Electromyogr. Kinesiol. 2009, 19, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Keenan, K.G.; Farina, D.; Maluf, K.S.; Merletti, R.; Enoka, R.M. Influence of Amplitude Cancellation on the Simulated Surface Electromyogram. J. Appl. Physiol. 2005, 98, 120–131. [Google Scholar] [PubMed]
- Keenan, K.G.; Valero-Cuevas, F.J. Epoch Length to Accurately Estimate the Amplitude of Interference EMG Is Likely the Result of Unavoidable Amplitude Cancellation. Biomed. Signal Process. Control 2008, 3, 154–162. [Google Scholar] [CrossRef]
- Škarabot, J.; Balshaw, T.G.; Maeo, S.; Massey, G.J.; Lanza, M.B.; Maden-Wilkinson, T.M.; Folland, J.P. Neural Adaptations to Long-Term Resistance Training: Evidence for the Confounding Effect of Muscle Size on the Interpretation of Surface Electromyography. J. Appl. Physiol. 2021, 131, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Green, L.A.; McGuire, J.; Gabriel, D.A. Flexor Carpi Radialis Surface Electromyography Electrode Placement for Evoked and Voluntary Measures. Muscle Nerve 2015, 52, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Inglis, J.G.; McIntosh, K.; Gabriel, D.A. Neural, Biomechanical, and Physiological Factors Involved in Sex-Related Differences in the Maximal Rate of Isometric Torque Development. Eur. J. Appl. Physiol. 2017, 117, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Christie, A.; Kamen, G.; Boucher, J.P.; Inglis, J.G.; Gabriel, D.A. A Comparison of Statistical Models for Calculating Reliability of the Hoffmann Reflex. Meas. Phys. Educ. Exerc. Sci. 2010, 14, 164–175. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Casolo, A.; Negro, F.; Scorcelletti, M.; Bazzucchi, I.; Enoka, R.; Felici, F.; Farina, D. The Increase in Muscle Force after 4 Weeks of Strength Training Is Mediated by Adaptations in Motor Unit Recruitment and Rate Coding. J. Physiol. 2019, 597, 1873–1887. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Casolo, A.; Dideriksen, J.L.; Aagaard, P.; Felici, F.; Falla, D.; Farina, D. Lack of Increased Rate of Force Development after Strength Training Is Explained by Specific Neural, Not Muscular, Motor Unit Adaptations. J. Appl. Physiol. 2022, 132, 84–94. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Holobar, A.; Watanabe, K.; Takahashi, T.; Ueno, H.; Maeda, N.; Maruyama, H.; Tanaka, S.; Hyngstrom, A.S. Detecting Motor Unit Abnormalities in Amyotrophic Lateral Sclerosis Using High-Density Surface EMG. Clin. Neurophysiol. 2022, in press. [CrossRef]
- Chandra, S.; Suresh, N.L.; Afsharipour, B.; Rymer, W.Z.; Holobar, A. Anomalies of Motor Unit Amplitude and Territory after Botulinum Toxin Injection. J. Neural Eng. 2022, 19, 036041. [Google Scholar] [CrossRef]
- Hu, X.; Suresh, A.K.; Rymer, W.Z.; Suresh, N.L. Altered Motor Unit Discharge Patterns in Paretic Muscles of Stroke Survivors Assessed Using Surface Electromyography. J. Neural Eng. 2016, 13, 046025. [Google Scholar] [CrossRef] [PubMed]
- Noto, Y.; Watanabe, K.; Holobar, A.; Kitaoji, T.; Tsuji, Y.; Kojima, Y.; Kitani-Morii, F.; Mizuno, T.; Nakagawa, M. High-Density Surface Electromyography to Assess Motor Unit Firing Rate in Charcot-Marie-Tooth Disease Type 1A Patients. Clin. Neurophysiol. 2021, 132, 812–818. [Google Scholar] [PubMed]
- Scott, W.; Stevens, J.; Binder-Macleod, S.A. Human Skeletal Muscle Fiber Type Classifications. Phys. Ther. 2001, 81, 1810–1816. [Google Scholar] [CrossRef]
- Masuda, T.; De Luca, C.J. Technique for Detecting MUAP Propagation from High-Threshold Motor Units. J. Electromyogr. Kinesiol. 1991, 1, 75–80. [Google Scholar] [CrossRef]
- Watanabe, K.; Holobar, A.; Mita, Y.; Kouzaki, M.; Ogawa, M.; Akima, H.; Moritani, T. Effect of Resistance Training and Fish Protein Intake on Motor Unit Firing Pattern and Motor Function of Elderly. Front. Physiol. 2018, 9, 1733. [Google Scholar]
- Martinez-Valdes, E.; Negro, F.; Farina, D.; Falla, D. Divergent Response of Low-versus High-threshold Motor Units to Experimental Muscle Pain. J. Physiol. 2020, 598, 2093–2108. [Google Scholar] [CrossRef]
- Casolo, A.; Farina, D.; Falla, D.; Bazzucchi, I.; Felici, F.; Del Vecchio, A. Strength Training Increases Conduction Velocity of High-Threshold Motor Units. Med. Sci. Sports Exerc. 2020, 52, 955–967. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabriel, D.A. Teaching Essential EMG Theory to Kinesiologists and Physical Therapists Using Analogies Visual Descriptions, and Qualitative Analysis of Biophysical Concepts. Sensors 2022, 22, 6555. https://doi.org/10.3390/s22176555
Gabriel DA. Teaching Essential EMG Theory to Kinesiologists and Physical Therapists Using Analogies Visual Descriptions, and Qualitative Analysis of Biophysical Concepts. Sensors. 2022; 22(17):6555. https://doi.org/10.3390/s22176555
Chicago/Turabian StyleGabriel, David A. 2022. "Teaching Essential EMG Theory to Kinesiologists and Physical Therapists Using Analogies Visual Descriptions, and Qualitative Analysis of Biophysical Concepts" Sensors 22, no. 17: 6555. https://doi.org/10.3390/s22176555
APA StyleGabriel, D. A. (2022). Teaching Essential EMG Theory to Kinesiologists and Physical Therapists Using Analogies Visual Descriptions, and Qualitative Analysis of Biophysical Concepts. Sensors, 22(17), 6555. https://doi.org/10.3390/s22176555




