A Validation of Six Wearable Devices for Estimating Sleep, Heart Rate and Heart Rate Variability in Healthy Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Gold-Standard Assessment of Sleep and Heart Rate Metrics
2.3.1. Polysomnography (PSG)
2.3.2. Electrocardiogram (ECG)
2.4. Alternative Assessment of Sleep and Heart Rate Metrics
2.4.1. Apple Watch S6 (Apple, Cupertino, CA, USA)
2.4.2. Garmin Forerunner 245 (Garmin, Kansas City, MO, USA)
2.4.3. Polar Vantage V (Polar, Kempele, Finland)
2.4.4. Oura Ring Generation 2 (Oura, Oulu, Finland)
2.4.5. WHOOP 3.0 (WHOOP, Boston, MA, USA)
2.4.6. Somfit (Compumedics, Melbourne, Australia)
2.5. Data Analysis
2.5.1. Sleep—Epoch-by-Epoch Comparisons
- Sensitivity for sleep (%) = TS/(TS + FW) × 100, i.e., the percentage of PSG sleep epochs correctly scored as sleep by the wearable device.
- Sensitivity for wake (%) = TW/(TW + FS) × 100, i.e., the percentage of PSG wake epochs correctly scored as wake by the wearable device (sometimes referred to as specificity).
- Agreement (%) = (TS + TW)/(TS + TW + FS + FW) × 100, i.e., the percentage of all PSG epochs correctly scored as sleep or wake by the wearable device.
- Sensitivity A for light sleep (%) = TL/(TL + FWN1N2 + FDN1N2 + FRN1N2) × 100, i.e., the percentage of PSG N1 or N2 epochs correctly scored as light sleep by the wearable device.
- Sensitivity B for deep sleep (%) = TD/(TD + FWD + FLD + FRD) × 100, i.e., the percentage of PSG N3 epochs correctly scored as deep sleep by the wearable device.
- Sensitivity B for REM sleep (%) = TR/(TR + FWR + FLR + FDR) × 100, i.e., the percentage of PSG REM epochs correctly scored as REM sleep by the wearable device.
- Sensitivity for wake (%) = TW/(TW + FLW + FDW + FRW) × 100, i.e., the percentage of PSG wake epochs correctly scored as wake by the wearable device (sometimes referred to as specificity).
- Agreement (%) = (TW + TL + TD + TR)/(TW + TL + TD + TR + FWN1N2 + FWN3 + FWR + FLW + FLN3 + FLR + FDW + FDN1N2 + FDR + FRW + FRN1N2 + FRN3) × 100, i.e., the percentage of all PSG epochs correctly scored as light sleep, deep sleep, REM sleep or wake, by the wearable device.
- A Somfit only—Somfit did not combine N1 and N2 into a single state of light sleep, so it was possible to calculate its separate sensitivity for N1 and N2.
- B Apple Watch only—Apple Watch combined N3 and REM into a single state of deep sleep, so its sensitivity for deep sleep was calculated as the percentage of PSG N3 or REM epochs correctly scored as deep sleep.
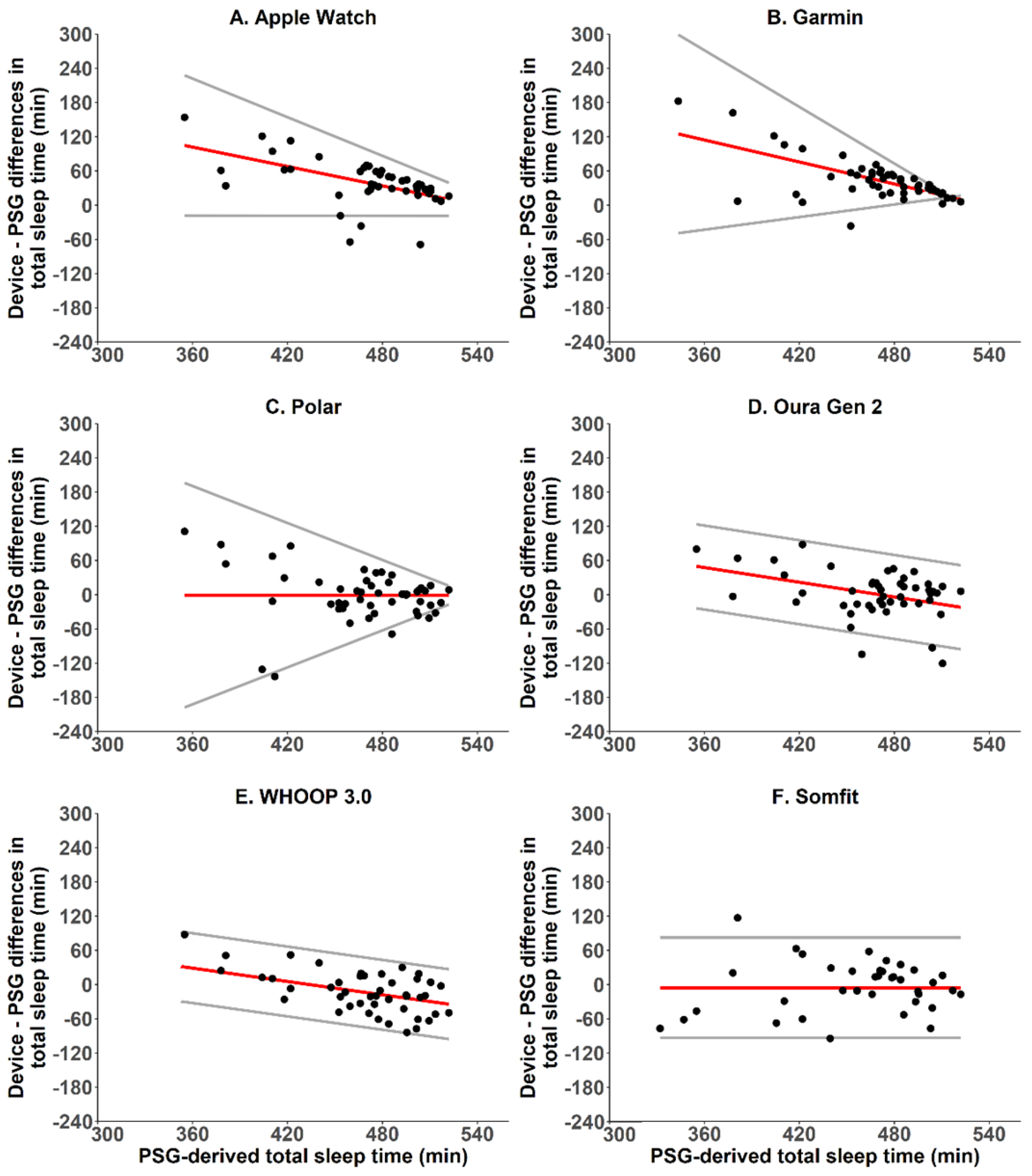
2.5.2. Sleep—Bland-Altman Analyses, Bias and Absolute Bias
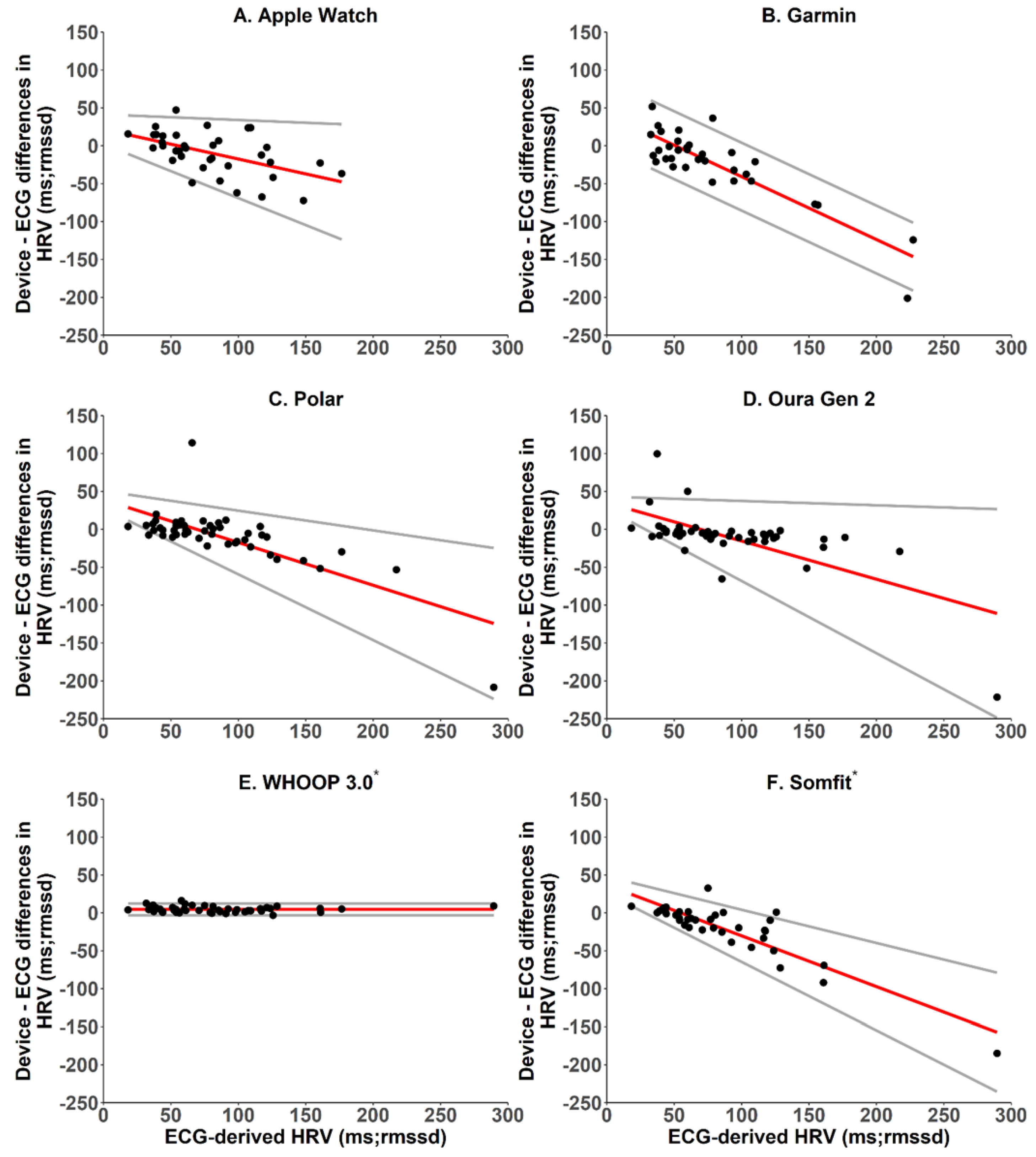
2.5.3. Heart Rate and Heart Rate Variability—Bland–Altman Analyses, Mean Bias and Absolute Bias
3. Results
3.1. Apple Watch S6
3.2. Garmin Forerunner 245 Music
3.3. Polar Vantage V
3.4. Oura Ring Generation 2
3.5. WHOOP 3.0
3.6. Somfit
4. Discussion
4.1. Sleep
4.2. Heart Rate and Heart Rate Variability
4.3. Boundary Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wearable Technology Market Size, Share & Trends Analysis Report by Product (Wrist-Wear, Eye-Wear & Head-Wear, Foot-Wear, Neck-Wear, Body-Wear), by Application, by Region, and Segment Forecasts, 2020–2027. Available online: https://www.researchandmarkets.com/reports/5124989/wearable-technology-market-size-share-and-trends (accessed on 17 June 2022).
- Pevnick, J.M.; Birkeland, K.; Zimmer, R.; Elad, Y.; Kedan, I. Wearable Technology for Cardiology: An Update and Framework for the Future. Trends Cardiovasc. Med. 2018, 28, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Piwek, L.; Ellis, D.A.; Andrews, S.; Joinson, A. The Rise of Consumer Health Wearables: Promises and Barriers. PLoS Med. 2016, 13, e1001953. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Li, L.; Zhang, H.; Guan, L.; Marques, C.; Savović, S.; Ortega, B.; Min, R.; Li, X. Low-Cost Plastic Optical Fiber Sensor Embedded in Mattress for Sleep Performance Monitoring. Opt. Fiber Technol. 2021, 64, 102541. [Google Scholar] [CrossRef]
- Kushida, C.A.; Littner, M.R.; Morgenthaler, T.; Alessi, C.A.; Bailey, D.; Coleman, J., Jr.; Friedman, L.; Hirshkowitz, M.; Kapen, S.; Kramer, M.; et al. Practice Parameters for the Indications for Polysomnography and Related Procedures: An Update for 2005. Sleep 2005, 28, 499–521. [Google Scholar] [CrossRef] [Green Version]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M. The AASM Manual for the Scoring of Sleep and Associated Events; American Academy of Sleep Medicine: Darien, IL, USA, 2015. [Google Scholar]
- de Zambotti, M.; Cellini, N.; Goldstone, A.; Colrain, I.M.; Baker, F.C. Wearable Sleep Technology in Clinical and Research Settings. Med. Sci. Sports Exerc. 2019, 51, 1538–1557. [Google Scholar] [CrossRef] [PubMed]
- Pollak, C.P.; Tryon, W.W.; Nagaraja, H.; Dzwonczyk, R. How Accurately Does Wrist Actigraphy Identify the States of Sleep and Wakefulness? Sleep 2001, 24, 957–965. [Google Scholar] [CrossRef]
- Sargent, C.; Lastella, M.; Romyn, G.; Versey, N.; Miller, D.J.; Roach, G.D. How Well Does a Commercially Available Wearable Device Measure Sleep in Young Athletes? Chronobiol. Int. 2018, 35, 754–758. [Google Scholar] [CrossRef]
- Miller, D.J.; Roach, G.D.; Lastella, M.; Scanlan, A.T.; Bellenger, C.R.; Halson, S.L.; Sargent, C. A Validation Study of a Commercial Wearable Device to Automatically Detect and Estimate Sleep. Biosensors 2021, 11, 185. [Google Scholar] [CrossRef]
- Plews, D.J.; Laursen, P.B.; Stanley, J.; Kilding, A.E.; Buchheit, M. Training Adaptation and Heart Rate Variability in Elite Endurance Athletes: Opening the Door to Effective Monitoring. Sports Med. 2013, 43, 773–781. [Google Scholar] [CrossRef]
- Plews, D.J.; Scott, B.; Altini, M.; Wood, M.; Kilding, A.E.; Laursen, P.B. Comparison of Heart-Rate-Variability Recording With Smartphone Photoplethysmography, Polar H7 Chest Strap, and Electrocardiography. Int. J. Sports Physiol. Perform. 2017, 12, 1324–1328. [Google Scholar] [CrossRef]
- de Zambotti, M.; Godino, J.G.; Baker, F.C.; Cheung, J.; Patrick, K.; Colrain, I.M. The Boom in Wearable Technology: Cause for Alarm or Just What Is Needed to Better Understand Sleep? Sleep 2016, 39, 1761–1762. [Google Scholar] [CrossRef] [Green Version]
- Halson, S.L. Sleep Monitoring in Athletes: Motivation, Methods, Miscalculations and Why It Matters. Sports Med. 2019, 49, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.J.; Lastella, M.; Scanlan, A.T.; Bellenger, C.; Halson, S.L.; Roach, G.D.; Sargent, C. A Validation Study of the WHOOP Strap against Polysomnography to Assess Sleep. J. Sports Sci. 2020, 38, 2631–2636. [Google Scholar] [CrossRef] [PubMed]
- Iber, C. The AASM Manual for the Scoring of Sleep and Associated Events: Rules; Terminology and Technical Specification; American Academy of Sleep Medicine: Darien, IL, USA, 2007. [Google Scholar]
- Nielsen, T. Cognition in REM and NREM Sleep: A Review and Possible Reconciliation of Two Models of Sleep Mentation. Behav. Brain Sci. 2000, 23, 851–866. [Google Scholar] [CrossRef] [PubMed]
- Pichot, V.; Roche, F.; Celle, S.; Barthélémy, J.-C.; Chouchou, F. HRVanalysis: A Free Software for Analyzing Cardiac Autonomic Activity. Front. Physiol. 2016, 7, 557. [Google Scholar] [CrossRef]
- Menghini, L.; Cellini, N.; Goldstone, A.; Baker, F.C.; de Zambotti, M. A Standardized Framework for Testing the Performance of Sleep-Tracking Technology: Step-by-Step Guidelines and Open-Source Code. Sleep 2021, 44, zsaa170. [Google Scholar] [CrossRef]
- Magalang, U.J.; Chen, N.-H.; Cistulli, P.A.; Fedson, A.C.; Gíslason, T.; Hillman, D.; Penzel, T.; Tamisier, R.; Tufik, S.; Phillips, G.; et al. Agreement in the Scoring of Respiratory Events and Sleep among International Sleep Centers. Sleep 2013, 36, 591–596. [Google Scholar] [CrossRef] [Green Version]
- Kosmadopoulos, A.; Sargent, C.; Darwent, D.; Zhou, X.; Roach, G.D. Alternatives to Polysomnography (PSG): A Validation of Wrist Actigraphy and a Partial-PSG System. Behav. Res. Methods 2014, 46, 1032–1041. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Agreement between Methods of Measurement with Multiple Observations per Individual. J. Biopharm. Stat. 2007, 17, 571–582. [Google Scholar] [CrossRef] [Green Version]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Sargent, C.; Lastella, M.; Halson, S.L.; Roach, G.D. The Validity of Activity Monitors for Measuring Sleep in Elite Athletes. J. Sci. Med. Sport 2016, 19, 848–853. [Google Scholar] [CrossRef] [PubMed]

| Wearable Device | |||
|---|---|---|---|
| Sleep | Wake | ||
| PSG | Sleep | True Sleep (TS) | False Wake (FW) |
| Wake | False Sleep (FS) | True Wake (TW) | |
| Wearable Device (Garmin, Polar, Oura, WHOOP) | |||||
|---|---|---|---|---|---|
| Wake | Light | Deep | REM | ||
| PSG | Wake | True Wake (TW) | False Light (FLW) | False Deep (FDW) | False REM (FRW) |
| N1 or N2 | False Wake (FWN1N2) | True Light (TL) | False Deep (FDN1N2) | False REM (FRN1N2) | |
| N3 | False Wake (FWN3) | False Light (FLN3) | True Deep (TD) | False REM (FRN3) | |
| REM | False Wake (FWR) | False Light (FLR) | False Deep (FDR) | True REM (TR) | |
| Variable | Apple Watch | Garmin | Polar | Oura (Gen.2) | WHOOP (3.0) | Somfit |
|---|---|---|---|---|---|---|
| Two-state Analysis: | ||||||
| Sensitivity for sleep (%) | 97 | 98 | 92 | 94 | 90 | 92 |
| Sensitivity for wake (%) | 26 | 27 | 51 | 57 | 56 | 57 |
| Agreement (%) | 88 | 89 | 87 | 89 | 86 | 87 |
| Cohen’s Kappa (k) | 0.30 | 0.35 | 0.44 | 0.51 | 0.44 | 0.48 |
| Multi-state Analysis: | ||||||
| Sensitivity for N1 sleep (%) | – | – | – | – | – | 1 |
| Sensitivity for N2 sleep (%) | – | – | – | – | – | 79 |
| Sensitivity for light sleep (%) | 44 | 68 | 60 | 66 | 58 | – |
| Sensitivity for deep sleep (%) | – | 28 | 33 | 62 | 62 | 65 |
| Sensitivity for REM sleep (%) | – | 50 | 49 | 52 | 66 | 58 |
| Sensitivity for deep/REM sleep (%) | 71 | – | – | – | – | – |
| Sensitivity for wake (%) | 26 | 27 | 51 | 57 | 56 | 57 |
| Agreement (%) | 53 | 50 | 51 | 61 | 60 | 65 |
| Cohen’s Kappa (k) | 0.20 | 0.25 | 0.28 | 0.43 | 0.44 | 0.52 |
| Variable | Apple Watch | Garmin | Polar | Oura (Gen.2) | WHOOP (3.0) | Somfit |
|---|---|---|---|---|---|---|
| Bias: | ||||||
| Total sleep time (min) | 39.5 ± 41.5 | 43.8 ± 38.0 | −0.8 ± 45.3 | 1.5 ± 40.9 | −12.2 ± 36.3 | −5.5 ± 44.9 |
| N1 sleep (min) | – | – | – | – | – | −35.7 ± 17.1 |
| N2 sleep (min) | – | – | – | – | – | 57.1 ± 48.4 |
| Light sleep (min) | −35.4 ± 68.1 | 76.0 ± 57.4 | 41.8 ± 53.2 | 19.8 ± 57.4 | −15.6 ± 50.7 | – |
| Deep sleep (min) | – | −52.4 ± 64.2 | −40.4 ± 41.7 | 2.4 ± 56.4 | −19.6 ± 34.3 | −11.7 ± 32.2 |
| REM sleep (min) | – | 20.2 ± 64.1 | −2.2 ± 34.6 | −20.7 ± 35.3 | 22.9 ± 45.4 | −15.2 ± 34.6 |
| Deep/REM sleep (min) | 74.9 ± 73.8 | – | – | – | – | – |
| Wake (min) | −38.7 ± 33.2 | −44.2 ± 36.2 | −21.3 ± 34.6 | −3.1 ± 36.1 | 13.1 ± 32.6 | 11.2 ± 43.2 |
| Absolute Bias: | ||||||
| Total sleep time (min) | 48.1 ± 30.4 | 45.3 ± 36.3 | 31.2 ± 32.6 | 29.0 ± 28.6 | 30.3 ± 23.0 | 33.7 ± 27.1 |
| N1 sleep (min) | – | – | – | – | – | 35.7 ± 17.1 |
| N2 sleep (min) | – | – | – | – | – | 61.6 ± 42.3 |
| Light sleep (min) | 59.4 ± 48.1 | 81.6 ± 48.9 | 52.7 ± 42.1 | 48.8 ± 35.5 | 43.6 ± 29.5 | – |
| Deep sleep (min) | – | 72.4 ± 39.8 | 47.7 ± 32.8 | 42.4 ± 36.8 | 30.6 ± 24.4 | 28.5 ± 18.9 |
| REM sleep (min) | – | 54.5 ± 38.6 | 26.1 ± 22.5 | 32.8 ± 24.3 | 41.9 ± 28.5 | 29.5 ± 23.2 |
| Deep/REM sleep (min) | 86.8 ± 58.9 | – | – | – | – | – |
| Wake (min) | 42.0 ± 28.9 | 45.7 ± 34.3 | 26.0 ± 31.1 | 25.0 ± 26.0 | 28.0 ± 20.9 | 34.0 ± 28.4 |
| Apple Watch S6 | |||||||||||
| Wake | Light | Deep | |||||||||
| PSG | Wake | 26 | 51 | 23 | |||||||
| N1 or N2 | 4 | 44 | 52 | ||||||||
| N3 or REM | 3 | 26 | 71 | ||||||||
| Garmin Forerunner 245 Music | |||||||||||
| Wake | Light | Deep | REM | ||||||||
| PSG | Wake | 27 | 45 | 5 | 23 | ||||||
| N1 or N2 | 3 | 68 | 8 | 21 | |||||||
| N3 | 1 | 61 | 28 | 10 | |||||||
| REM | 1 | 42 | 7 | 50 | |||||||
| Polar Vantage V | |||||||||||
| Wake | Light | Deep | REM | ||||||||
| PSG | Wake | 51 | 31 | 5 | 13 | ||||||
| N1 or N2 | 9 | 60 | 14 | 17 | |||||||
| N3 | 7 | 53 | 33 | 7 | |||||||
| REM | 5 | 43 | 3 | 49 | |||||||
| Oura Ring Generation 2 | |||||||||||
| Wake | Light | Deep | REM | ||||||||
| PSG | Wake | 57 | 30 | 6 | 7 | ||||||
| N1 or N2 | 9 | 66 | 15 | 10 | |||||||
| N3 | 2 | 32 | 62 | 4 | |||||||
| REM | 6 | 37 | 5 | 52 | |||||||
| WHOOP 3.0 | |||||||||||
| Wake | Light | Deep | REM | ||||||||
| PSG | Wake | 56 | 28 | 2 | 14 | ||||||
| N1 or N2 | 12 | 58 | 10 | 20 | |||||||
| N3 | 2 | 32 | 62 | 4 | |||||||
| REM | 9 | 23 | 2 | 66 | |||||||
| Somfit | |||||||||||
| Wake | N1 | N2 | N3 | REM | |||||||
| PSG | Wake | 57 | 24 | 1 | 6 | 12 | |||||
| N1 | 26 | 1 | 53 | 3 | 17 | ||||||
| N2 | 7 | 1 | 80 | 7 | 5 | ||||||
| N3 | 5 | 1 | 25 | 68 | 1 | ||||||
| REM | 8 | 1 | 26 | 5 | 61 | ||||||
| Variable | Apple Watch | Garmin | Polar | Oura (Gen.2) | WHOOP (3.0) * | Somfit * |
|---|---|---|---|---|---|---|
| Bias: | ||||||
| Heart Rate (bpm) | 0.5 ± 2.1 | 5.0 ± 12.8 | −1.1 ± 2.2 | 0.1 ± 4.5 | −0.3 ± 1.0 | 2.2 ± 6.5 |
| HRV (RMSSD, ms) | −9.6 ± 28.1 | −22.4 ± 46.9 | −8.7 ± 38.0 | −10.2 ± 39.4 | −4.5 ± 3.9 | −20.5 ± 37.2 |
| Absolute Bias: | ||||||
| Heart Rate (bpm) | 1.5 ± 1.5 | 5.4 ± 12.6 | 1.5 ± 1.9 | 1.8 ± 4.1 | 0.7 ± 0.8 | 2.6 ± 6.3 |
| HRV (RMSSD, ms) | 22.5 ± 19.2 | 33.1 ± 39.9 | 18.8 ± 34.0 | 18.9 ± 35.9 | 4.7 ± 3.6 | 24.0 ± 35.0 |
| Limits of Agreement: | ||||||
| Heart Rate (bpm) | ±4.0 | ±25.0 | ±4.4 | ±8.8 | ±1.9 | ±12.7 |
| HRV (RMSSD, ms) | ±55.2 | ±92.0 | ±74.5 | ±77.2 | ±7.6 | ±72.9 |
| Intraclass Correlations: | ||||||
| Heart Rate (bpm) | 0.96 | 0.41 | 0.93 | 0.85 | 0.99 | 0.65 |
| HRV (RMSSD, ms) | 0.67 | 0.24 | 0.65 | 0.63 | 0.99 | 0.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, D.J.; Sargent, C.; Roach, G.D. A Validation of Six Wearable Devices for Estimating Sleep, Heart Rate and Heart Rate Variability in Healthy Adults. Sensors 2022, 22, 6317. https://doi.org/10.3390/s22166317
Miller DJ, Sargent C, Roach GD. A Validation of Six Wearable Devices for Estimating Sleep, Heart Rate and Heart Rate Variability in Healthy Adults. Sensors. 2022; 22(16):6317. https://doi.org/10.3390/s22166317
Chicago/Turabian StyleMiller, Dean J., Charli Sargent, and Gregory D. Roach. 2022. "A Validation of Six Wearable Devices for Estimating Sleep, Heart Rate and Heart Rate Variability in Healthy Adults" Sensors 22, no. 16: 6317. https://doi.org/10.3390/s22166317
APA StyleMiller, D. J., Sargent, C., & Roach, G. D. (2022). A Validation of Six Wearable Devices for Estimating Sleep, Heart Rate and Heart Rate Variability in Healthy Adults. Sensors, 22(16), 6317. https://doi.org/10.3390/s22166317







