Microwave Hyperthermia of Brain Tumors: A 2D Assessment Parametric Numerical Study
Abstract
:1. Introduction
2. Methods
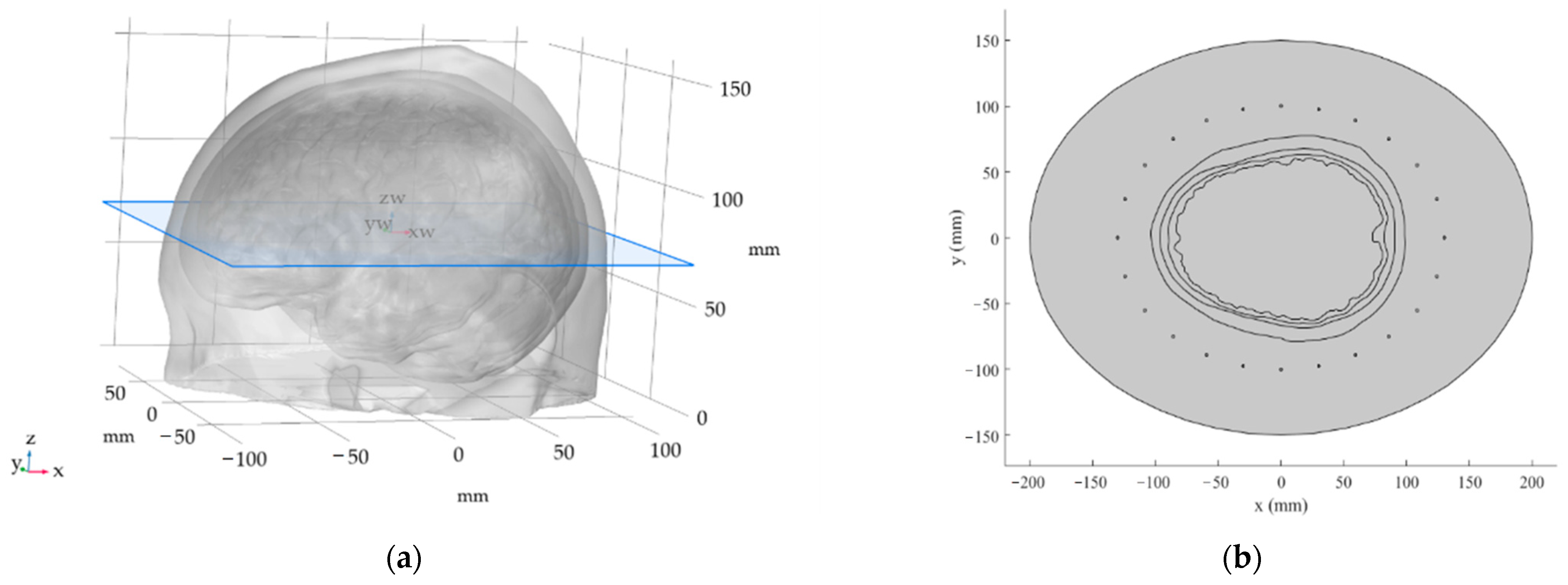
2.1. The 2D Head Phantom Models
Hyperthermia Targets: Size and Position
- Frontal lobe, with an incidence of 40%;
- Temporal lobe, with an incidence of 29%;
- Parietal and occipital lobe, with an incidence of 17%;
- Deep-seated tumors, with an incidence of 14%.
2.2. Numerical Simulations
2.3. SAR Optimization and Evaluation
- The maximization of the THQ, or the reduction of hot-spots;
- The maximization of the target coverage, or an improvement in TC25, TC50, and TC75.
3. Results
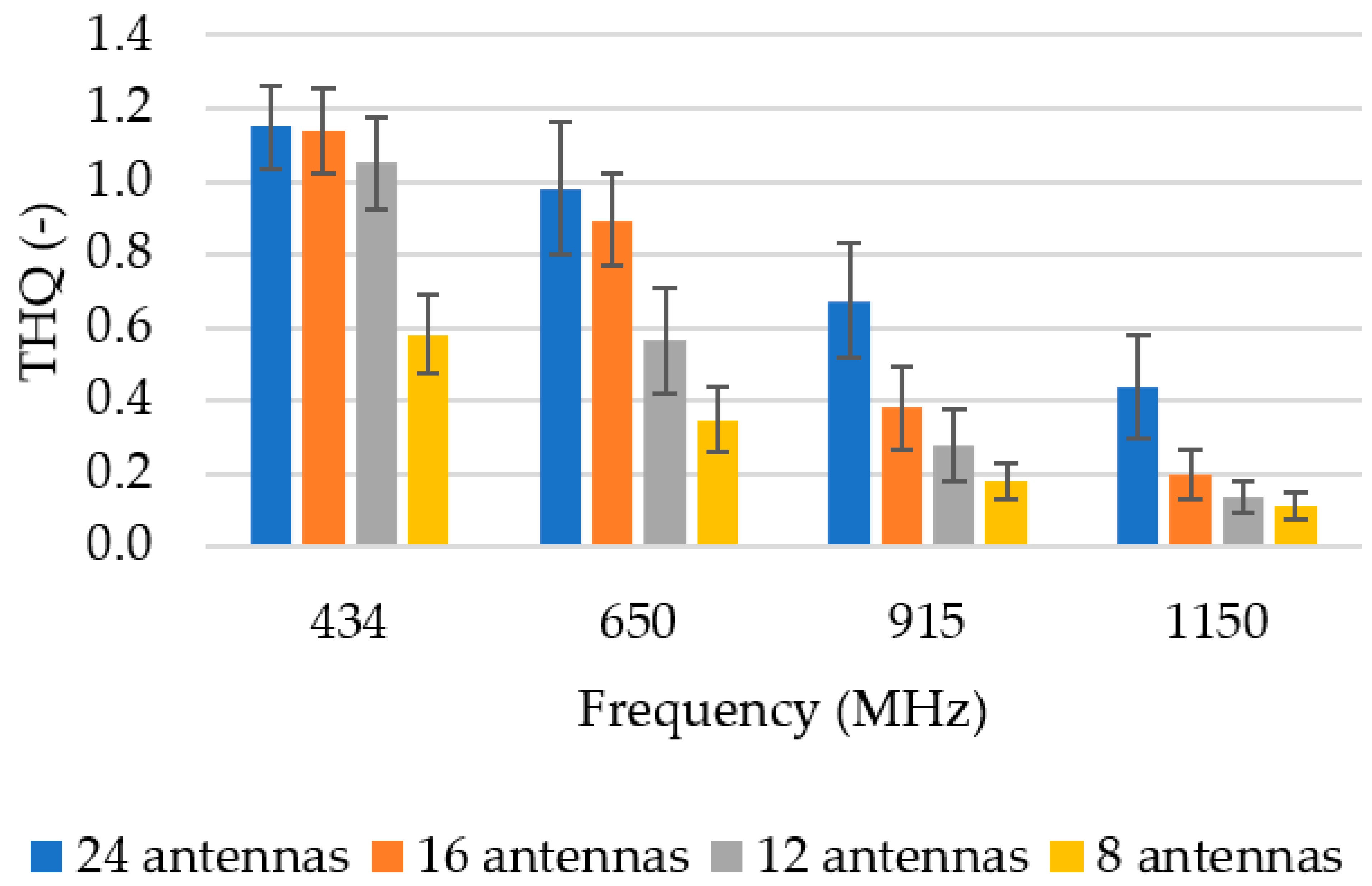
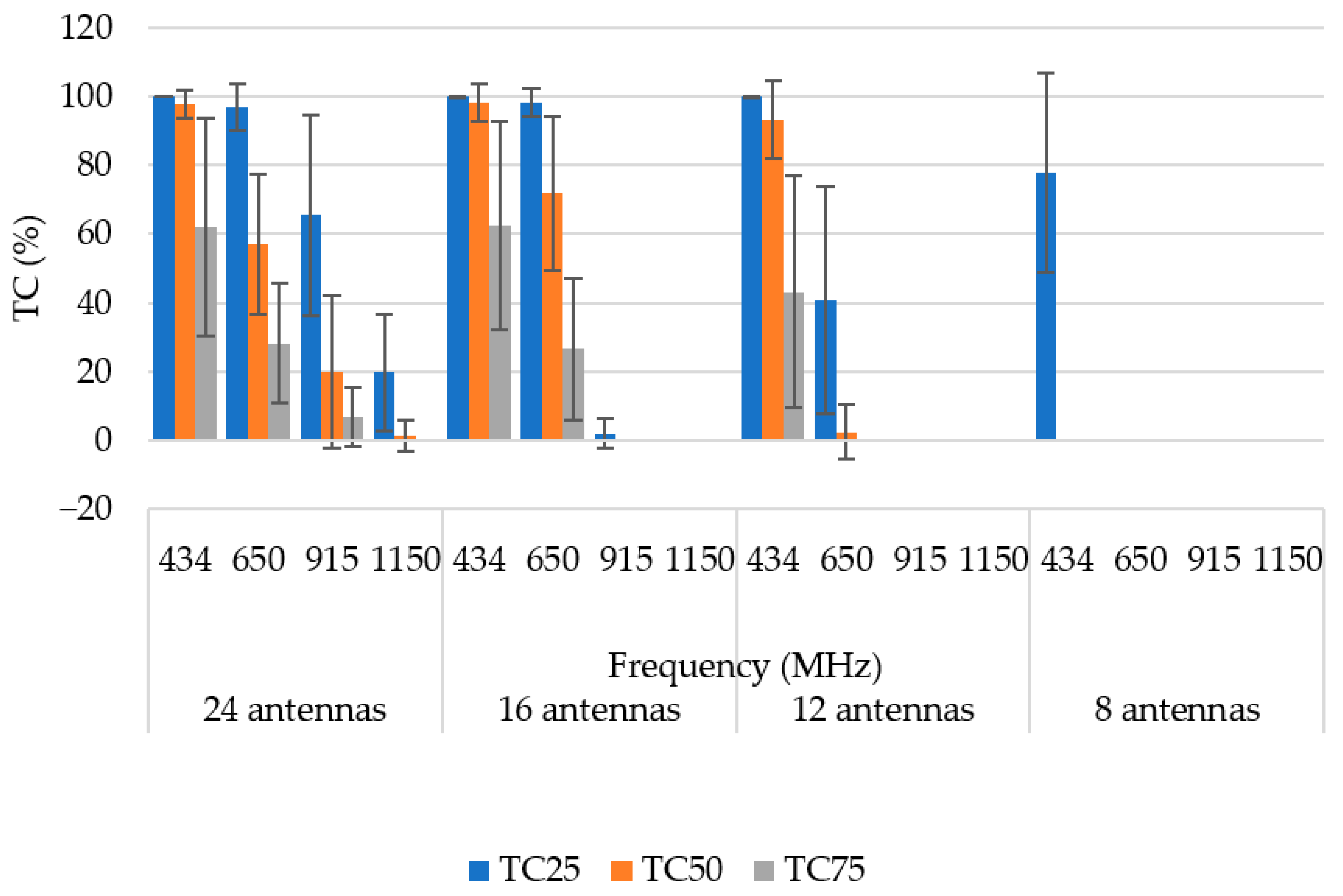
3.1. Operating Frequency
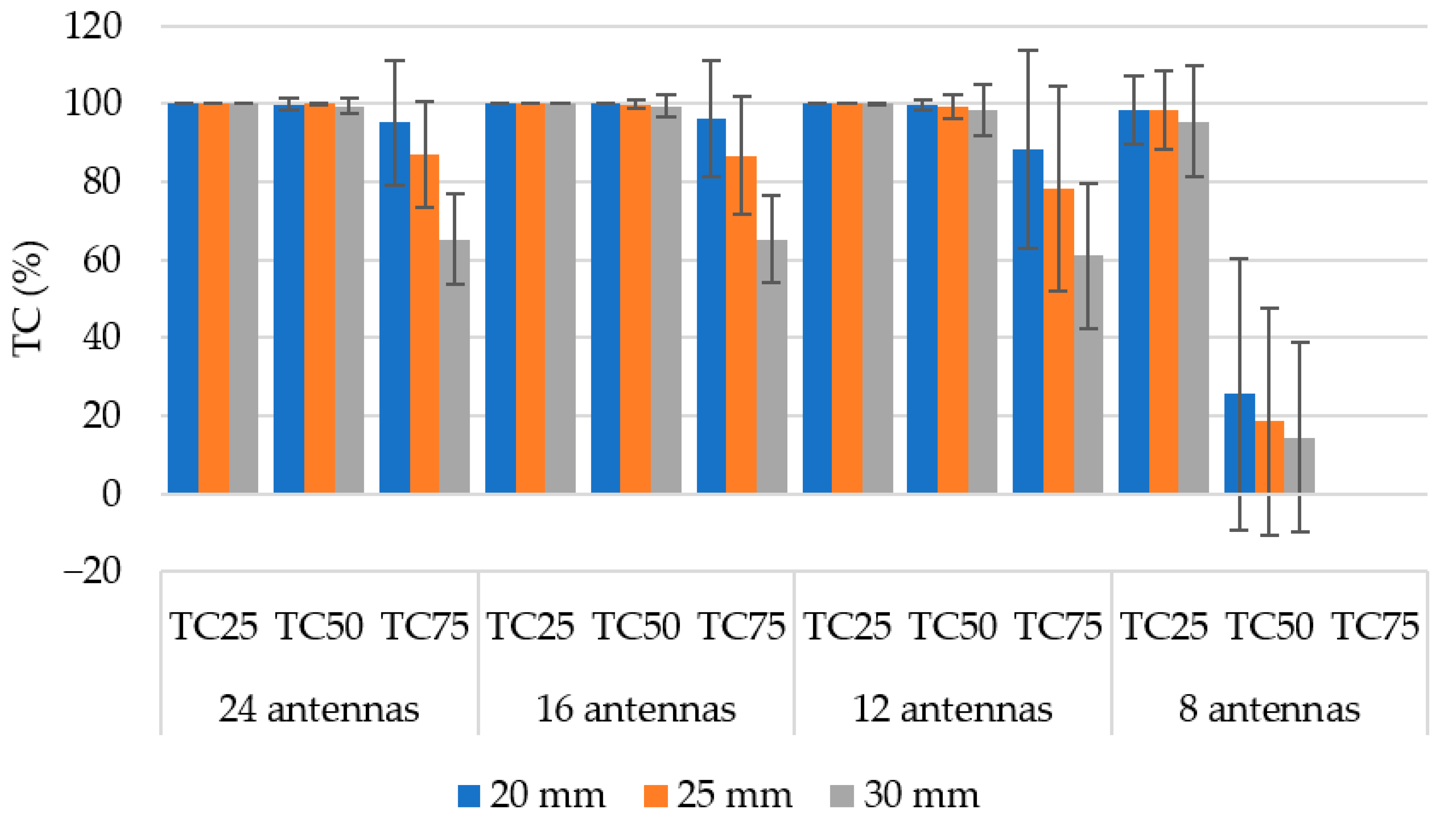
3.2. Number of Antenna Elements
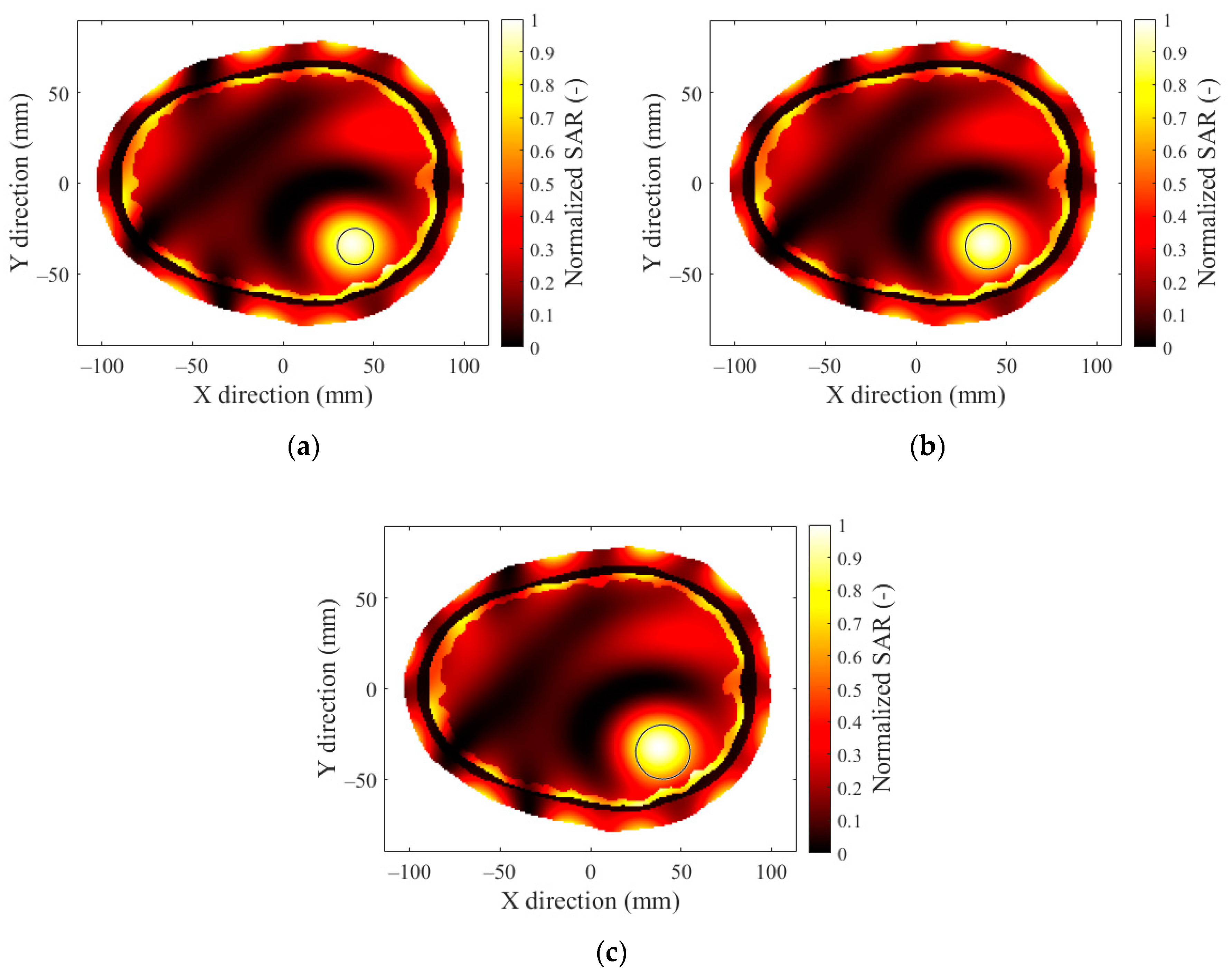
3.3. Hyperthermia Target Size
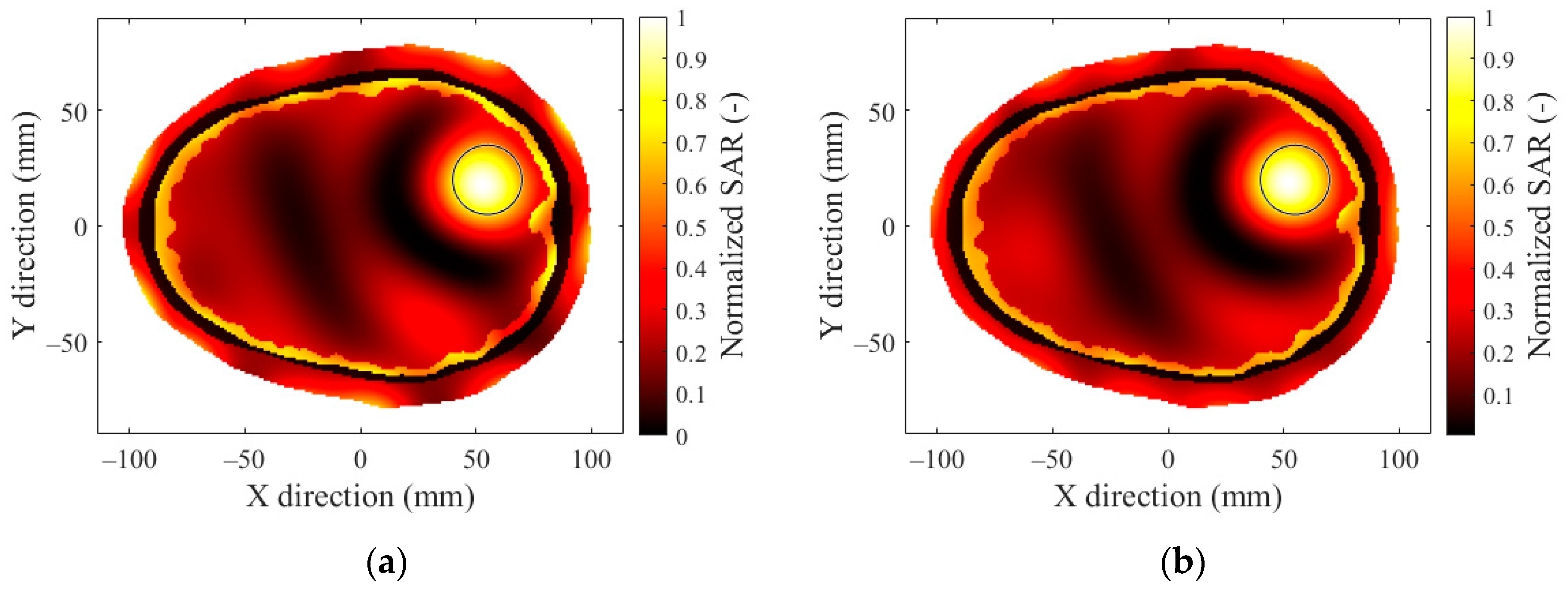
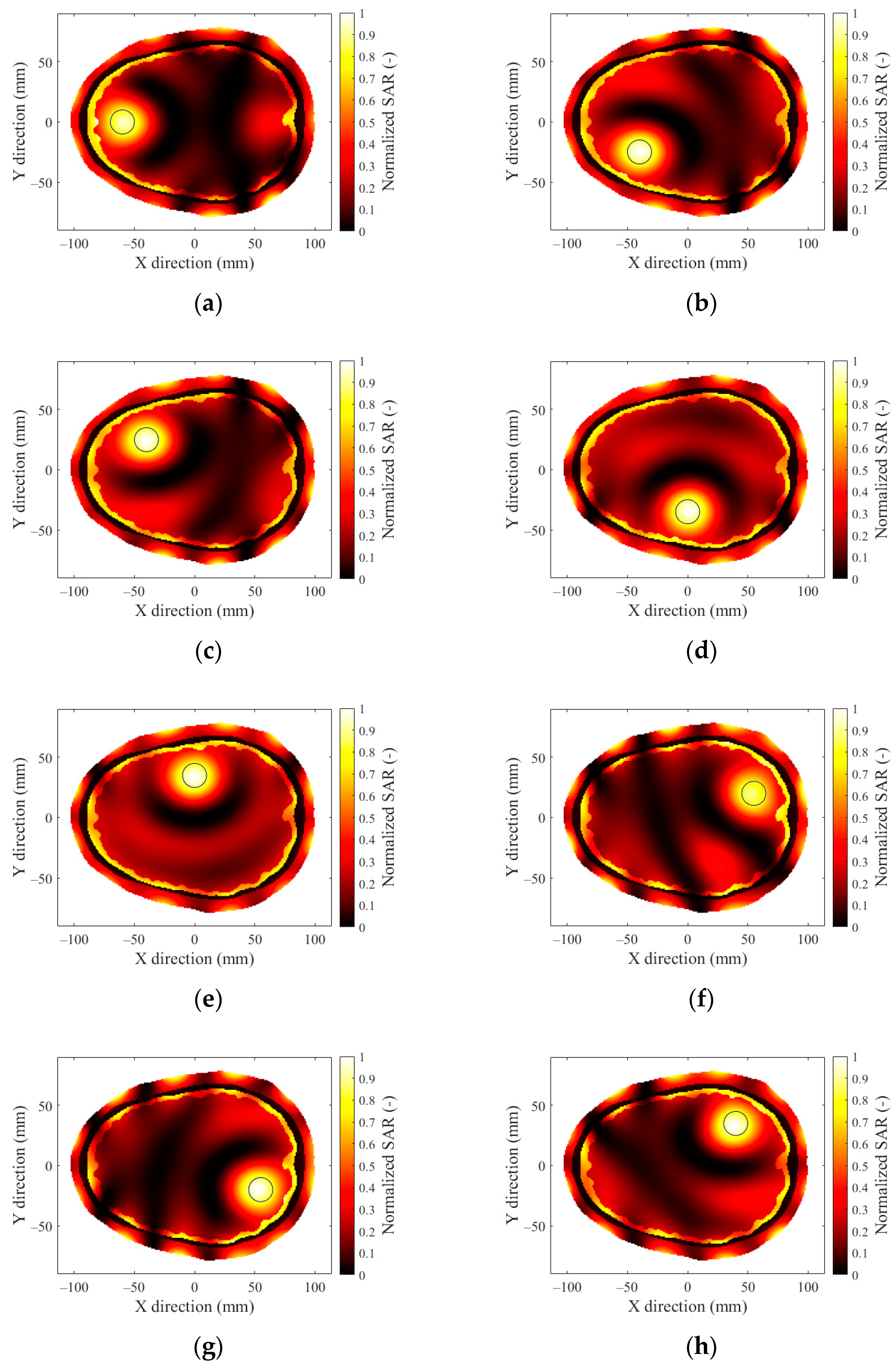
3.4. Effect of Hyperthermia Target Location
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
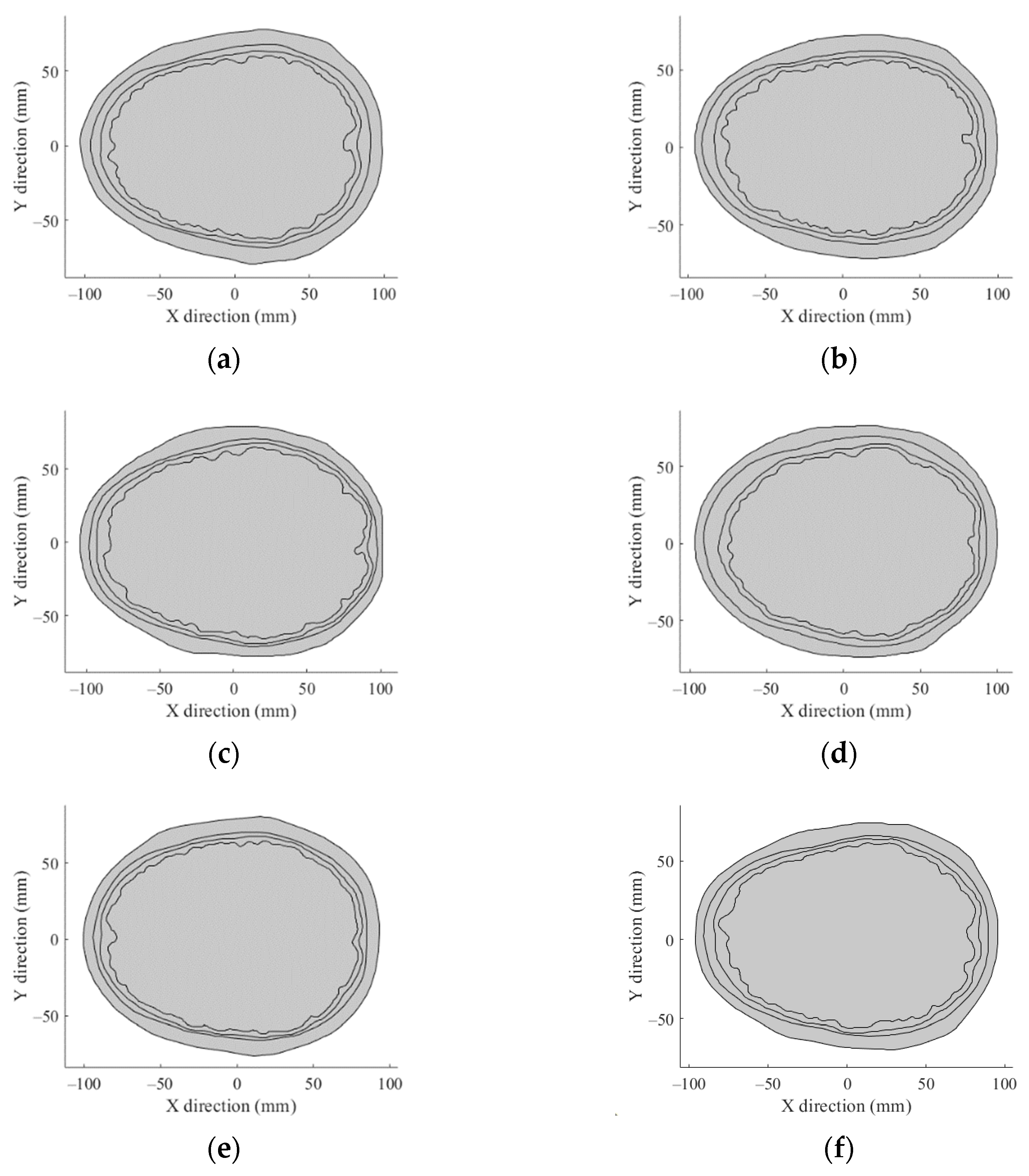

Appendix B

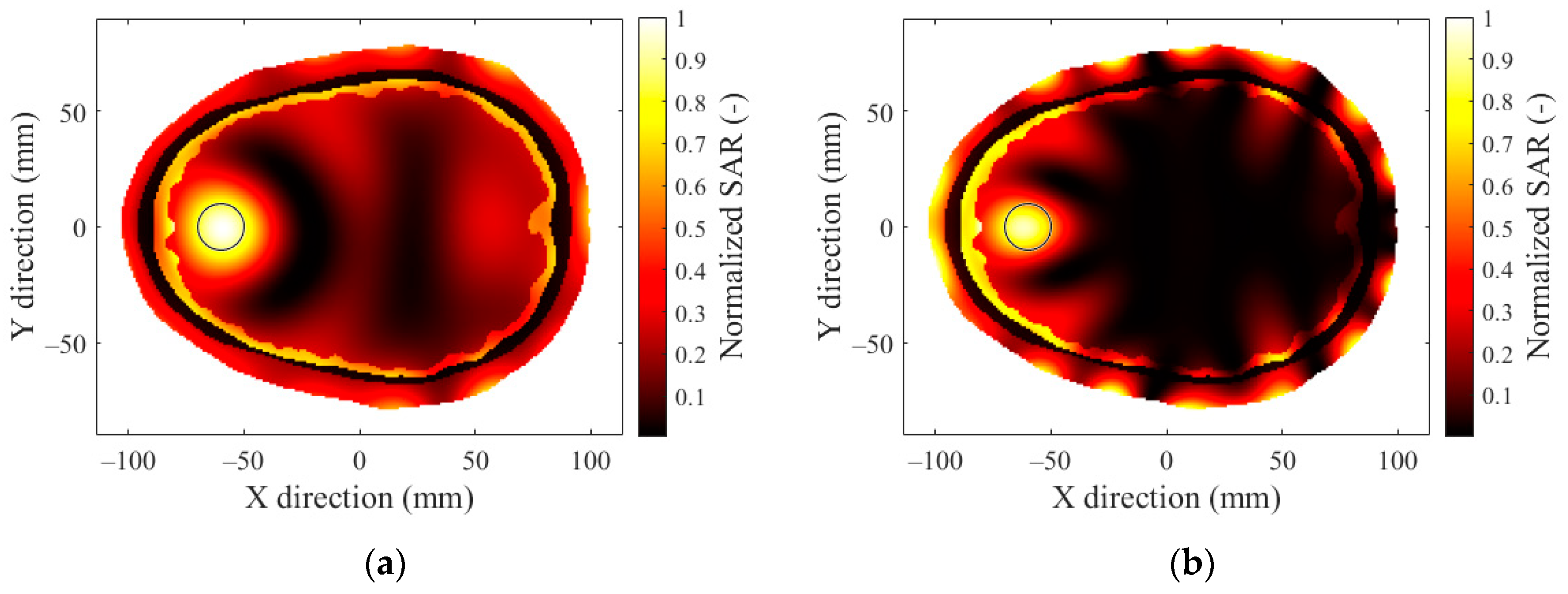
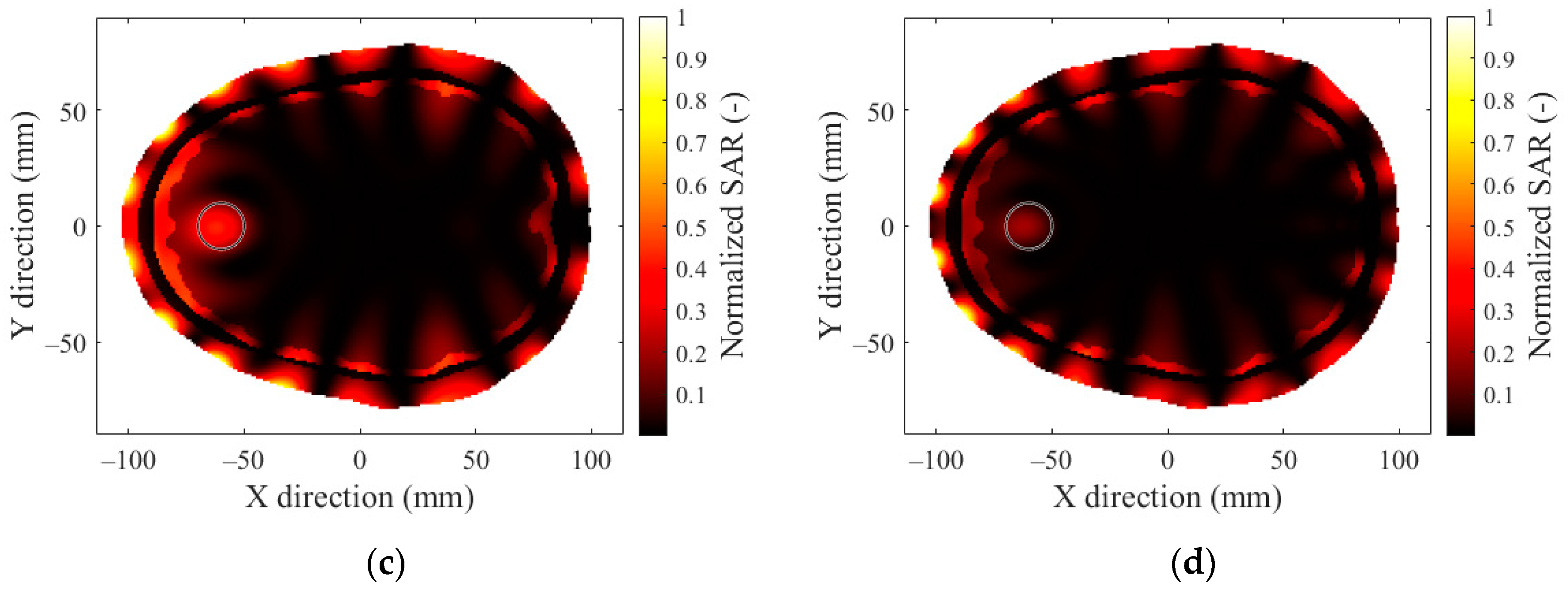
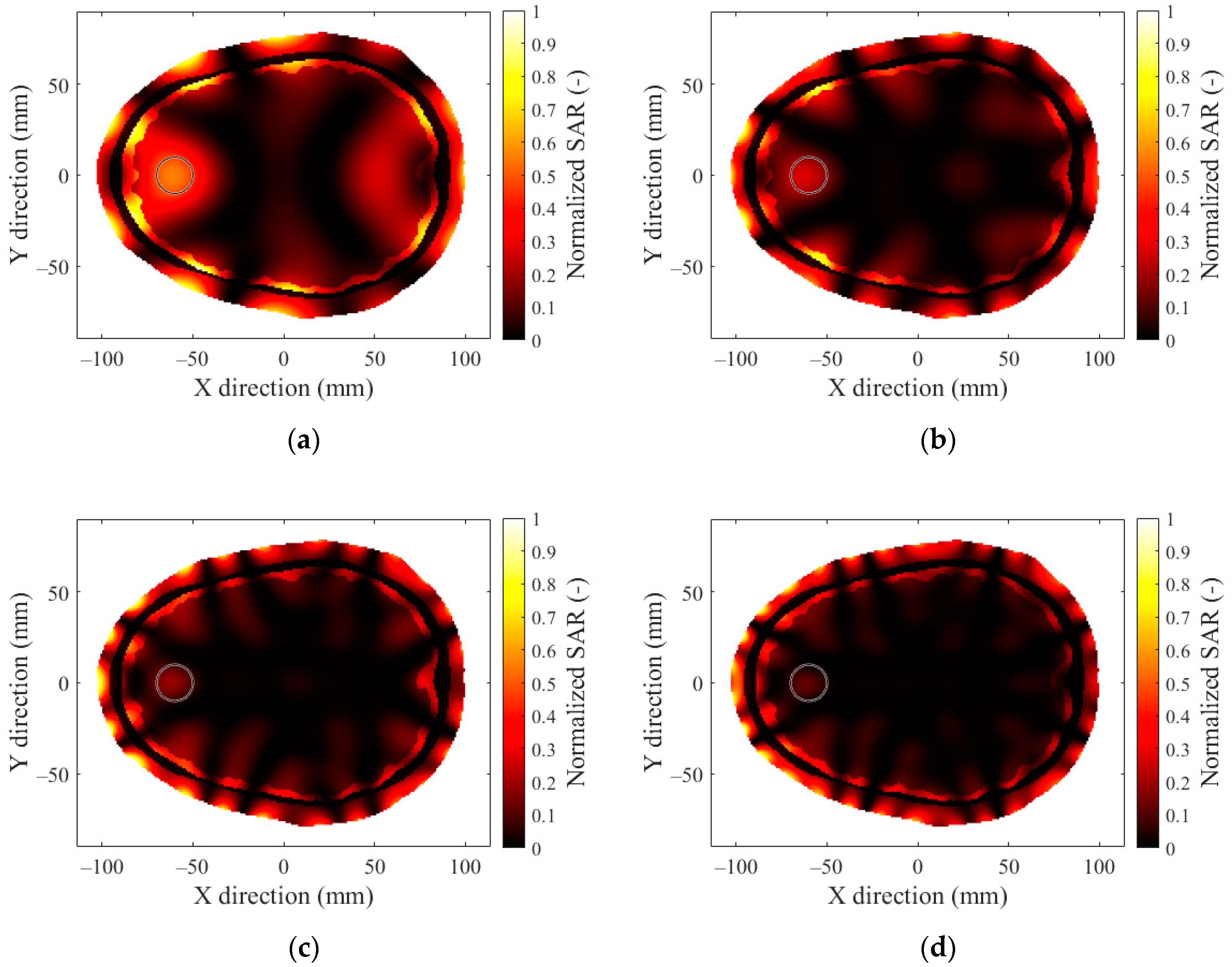
References
- Datta, N.R.; Ordóñez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients with Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef]
- Schooneveldt, G.; Trefná, H.D.; Persson, M.; de Reijke, T.M.; Blomgren, K.; Kok, H.P.; Crezee, H. Hyperthermia Treatment Planning Including Convective Flow in Cerebrospinal Fluid for Brain Tumour Hyperthermia Treatment Using a Novel Dedicated Paediatric Brain Applicator. Cancers 2019, 11, 1183. [Google Scholar] [CrossRef]
- Sneed, P.K.; Stauffer, P.; McDermott, M.W.; Diederich, C.; Lamborn, K.R.; Prados, M.D.; Chang, S.; Weaver, K.A.; Spry, L.; Malec, M.K.; et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost ± hyperthermia for glioblastoma multiforme. Int. J. Radiat. Oncol. 1998, 40, 287–295. [Google Scholar] [CrossRef]
- Stea, B.; Rossman, K.; Kittelson, J.; Shetter, A.; Hamilton, A.; Cassady, J. Interstitial irradiation versus interstitial thermoradiotherapy for supratentorial malignant gliomas: A comparative survival analysis. Int. J. Radiat. Oncol. 1994, 30, 591–600. [Google Scholar] [CrossRef]
- Man, J.; Shoemake, J.D.; Ma, T.; Rizzo, A.E.; Godley, A.R.; Wu, Q.; Mohammadi, A.M.; Bao, S.; Rich, J.N.; Yu, J.S. Hyperthermia Sensitizes Glioma Stem-like Cells to Radiation by Inhibiting AKT Signaling. Cancer Res. 2015, 75, 1760–1769. [Google Scholar] [CrossRef]
- Paulides, M.M.; Bakker, J.F.; Neufeld, E.; Van Der Zee, J.; Jansen, P.P.; Levendag, P.C.; Van Rhoon, G.C. The HYPERcollar: A novel applicator for hyperthermia in the head and neck. Int. J. Hyperth. 2007, 23, 567–576. [Google Scholar] [CrossRef]
- Togni, P.; Rijnen, Z.; Numan, W.C.M.; Verhaart, R.F.; Bakker, J.F.; Van Rhoon, G.C.; Paulides, M.M. Electromagnetic redesign of the HYPERcollar applicator: Toward improved deep local head-and-neck hyperthermia. Phys. Med. Biol. 2013, 58, 5997–6009. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Ellsworth, J.; Turner, P. Feasibility of Heating Brain Tumors Using a 915 MHz Annular Phased-Array. IEEE Antennas Wirel. Propag. Lett. 2021, 20, 423–427. [Google Scholar] [CrossRef]
- Guérin, B.; Villena, J.F.; Polimeridis, A.G.; Adalsteinsson, E.; Daniel, L.; White, J.K.; Rosen, B.R.; Wald, L.L. Computation of ultimate SAR amplification factors for radiofrequency hyperthermia in non-uniform body models: Impact of frequency and tumour location. Int. J. Hyperth. 2017, 34, 87–100. [Google Scholar] [CrossRef]
- Takook, P.; Trefna, H.D.; Persson, M. Performance Evaluation of 2 Hyperthermia Applicators for Deep-Seated Brain Tumors. In Proceedings of the 2017 First IEEE MTT-S International Microwave Bio Conference (IMBIOC), Gothenburg, Sweden, 15–17 May 2017; pp. 1–3. [Google Scholar]
- Paulides, M.M.; Vossen, S.H.; Zwamborn, A.P.; van Rhoon, G.C. Theoretical investigation into the feasibility to deposit RF energy centrally in the head-and-neck region. Int. J. Radiat. Oncol. 2005, 63, 634–642. [Google Scholar] [CrossRef]
- Lee, E.G.R.; Hadimani, L.; Jiles, D.C. Population Head Model Repository; V1.0.; IT’IS Foundation: Zurich, Switzerland, 2016. [Google Scholar] [CrossRef]
- COMSOL. Multiphysics Reference Manual 2019; COMSOL Inc.: Burlington, MA, USA, 2019. [Google Scholar]
- Materialize 3-Matic, Reference guide Version 16.0; Leuven, Belgium, 2021.
- Larjavaara, S.; Mäntylä, R.; Salminen, T.; Haapasalo, H.; Raitanen, J.; Jääskeläinen, J.; Auvinen, A. Incidence of gliomas by anatomic location. Neuro-oncology 2007, 9, 319–325. [Google Scholar] [CrossRef]
- Prabhakar, R.; Haresh, K.; Ganesh, T.; Joshi, R.; Julka, P.; Rath, G. Comparison of computed tomography and magnetic resonance based target volume in brain tumors. J. Cancer Res. Ther. 2007, 3, 121–123. [Google Scholar] [CrossRef]
- Said, T.; Varadan, V.V. Variation of Cole-Cole Model Parameters with the Complex Permittivity of Biological Tissues. In Proceedings of the 2009 IEEE MTT-S International Microwave Symposium Digest, Boston, MA, USA, 7–12 June 2009; pp. 1445–1448. [Google Scholar]
- Hasgall, P.A.; Di Gennaro, F.; Baumgartner, C.; Neufeld, E.; Lloyd, B.; Gosselin, M.C.; Payne, D.; Klingenböck, A.; Kuster, N. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues; Version 4.1; IT’IS Foundation: Zurich, Switzerland, 2022. [Google Scholar] [CrossRef]
- MATLAB, version 9, 8.0.1323502 (R2020a); Natick, MA, USA, 2020.
- Bellizzi, G.G.; Drizdal, T.; Van Rhoon, G.C.; Crocco, L.; Isernia, T.; Paulides, M.M. Predictive value of SAR based quality indicators for head and neck hyperthermia treatment quality. Int. J. Hyperth. 2019, 36, 455–464. [Google Scholar] [CrossRef]
- Paulides, M.M.; Verduijn, G.M.; Van Holthe, N. Status quo and directions in deep head and neck hyperthermia. Radiat. Oncol. 2016, 11, 1–14. [Google Scholar] [CrossRef]
- Gaffoglio, R.; Righero, M.; Giordanengo, G.; Zucchi, M.; Vecchi, G. Fast Optimization of Temperature Focusing in Hyperthermia Treatment of Sub-Superficial Tumors. IEEE J. Electromagn. RF Microwaves Med. Biol. 2020, 5, 286–293. [Google Scholar] [CrossRef]
- Lee, H.K.; Antell, A.G.; Perez, C.A.; Straube, W.L.; Ramachandran, G.; Myerson, R.J.; Emami, B.; Molmenti, E.P.; Buckner, A.; Lockett, M.A. Superficial hyperthermia and irradiation for recurrent breast carcinoma of the chest wall: Prognostic factors in 196 tumors. Int. J. Radiat. Oncol. 1998, 40, 365–375. [Google Scholar] [CrossRef]
- Kroesen, M.; van Holthe, N.; Sumser, K.; Chitu, D.; Vernhout, R.; Verduijn, G.; Franckena, M.; Hardillo, J.; van Rhoon, G.; Paulides, M. Feasibility, SAR Distribution, and Clinical Outcome upon Reirradiation and Deep Hyperthermia Using the Hypercollar3D in Head and Neck Cancer Patients. Cancers 2021, 13, 6149. [Google Scholar] [CrossRef]









| Hyperthermia Target Size | Diameter (mm) |
|---|---|
| Small | 20 |
| Medium | 25 |
| Large | 30 |
| Tissue | Maximum Element Size (mm) | Minimum Element Size (mm) |
|---|---|---|
| Scalp | 5.5 | 0.0225 |
| Skull | 9.4 | 0.09 |
| CSF | 4 | 0.09 |
| Brain tissue | 4.9 | 0.09 |
| Deionized water | 5.2 | 0.09 |
| Tissue | Density (kg/m3) | Frequency (MHz) | Dielectric Parameters | |
|---|---|---|---|---|
| Electric Conductivity (S/m) | Permittivity (-) | |||
| Scalp | 1109 | 434 | 0.53 | 38.17 |
| 650 | 0.58 | 36.78 | ||
| 915 | 0.64 | 35.88 | ||
| 1150 | 0.70 | 35.37 | ||
| Skull | 1908 | 434 | 0.09 | 13.07 |
| 650 | 0.12 | 12.72 | ||
| 915 | 0.15 | 12.44 | ||
| 1150 | 0.18 | 12.23 | ||
| CSF | 1007 | 434 | 2.26 | 70.63 |
| 650 | 2.32 | 69.33 | ||
| 915 | 2.42 | 68.60 | ||
| 1150 | 2.53 | 68.18 | ||
| Brain tissue | 1046 | 434 | 1.62 | 48.46 |
| 650 | 1.70 | 46.28 | ||
| 915 | 1.79 | 44.86 | ||
| 1150 | 1.88 | 44.06 | ||
| Deionized water | 1000 | 434 | 0.04 | 84.42 |
| 650 | 0.10 | 84.37 | ||
| 915 | 0.19 | 84.28 | ||
| 1150 | 0.30 | 84.17 | ||
| Frequency (MHz) | Target Coverage (%) | ||||
|---|---|---|---|---|---|
| 24-Antenna System | 16-Antenna System | 12-Antenna System | 8-Antenna System | ||
| TC25 | 434 | 100 ± 0 | 100 ± 0.02 | 100 ± 0.03 | 97.4 ± 11.35 |
| 650 | 98.6 ± 4.86 | 99.32 ± 2.16 | 83.8 ± 23 | 34.28 ± 26.66 | |
| 915 | 78.56 ± 27.64 | 41.48 ±27.23 | 11.83 ± 19.22 | 0 ± 0 | |
| 1150 | 51.76 ± 28.95 | 1.69 ± 5.5 | 0 ± 0 | 0 ± 0 | |
| TC50 | 434 | 99.76 ± 1.42 | 99.79 ± 1.79 | 99.22 ± 4.28 | 19.55 ± 30.08 |
| 650 | 84.61 ± 16.49 | 86.45 ± 14.93 | 21.59 ± 26.71 | 0 ± 0 | |
| 915 | 45.61 ± 24.64 | 1.76 ± 6.36 | 0 ± 0 | 0 ± 0 | |
| 1150 | 10.99 ± 16.30 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| TC75 | 434 | 82.54 ± 18.71 | 82.71 ± 18.88 | 75.87 ± 26.29 | 0 ± 0 |
| 650 | 45.53 ± 19.66 | 42.74 ± 21.32 | 0.45 ± 2.63 | 0 ± 0 | |
| 915 | 12.89 ± 13.22 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 1150 | 1.18 ± 4.61 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redr, J.; Pokorny, T.; Drizdal, T.; Fiser, O.; Brunat, M.; Vrba, J.; Vrba, D. Microwave Hyperthermia of Brain Tumors: A 2D Assessment Parametric Numerical Study. Sensors 2022, 22, 6115. https://doi.org/10.3390/s22166115
Redr J, Pokorny T, Drizdal T, Fiser O, Brunat M, Vrba J, Vrba D. Microwave Hyperthermia of Brain Tumors: A 2D Assessment Parametric Numerical Study. Sensors. 2022; 22(16):6115. https://doi.org/10.3390/s22166115
Chicago/Turabian StyleRedr, Jan, Tomas Pokorny, Tomas Drizdal, Ondrej Fiser, Matous Brunat, Jan Vrba, and David Vrba. 2022. "Microwave Hyperthermia of Brain Tumors: A 2D Assessment Parametric Numerical Study" Sensors 22, no. 16: 6115. https://doi.org/10.3390/s22166115
APA StyleRedr, J., Pokorny, T., Drizdal, T., Fiser, O., Brunat, M., Vrba, J., & Vrba, D. (2022). Microwave Hyperthermia of Brain Tumors: A 2D Assessment Parametric Numerical Study. Sensors, 22(16), 6115. https://doi.org/10.3390/s22166115







