Optimization of Printed Polyaniline Composites for Gas Sensing Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results
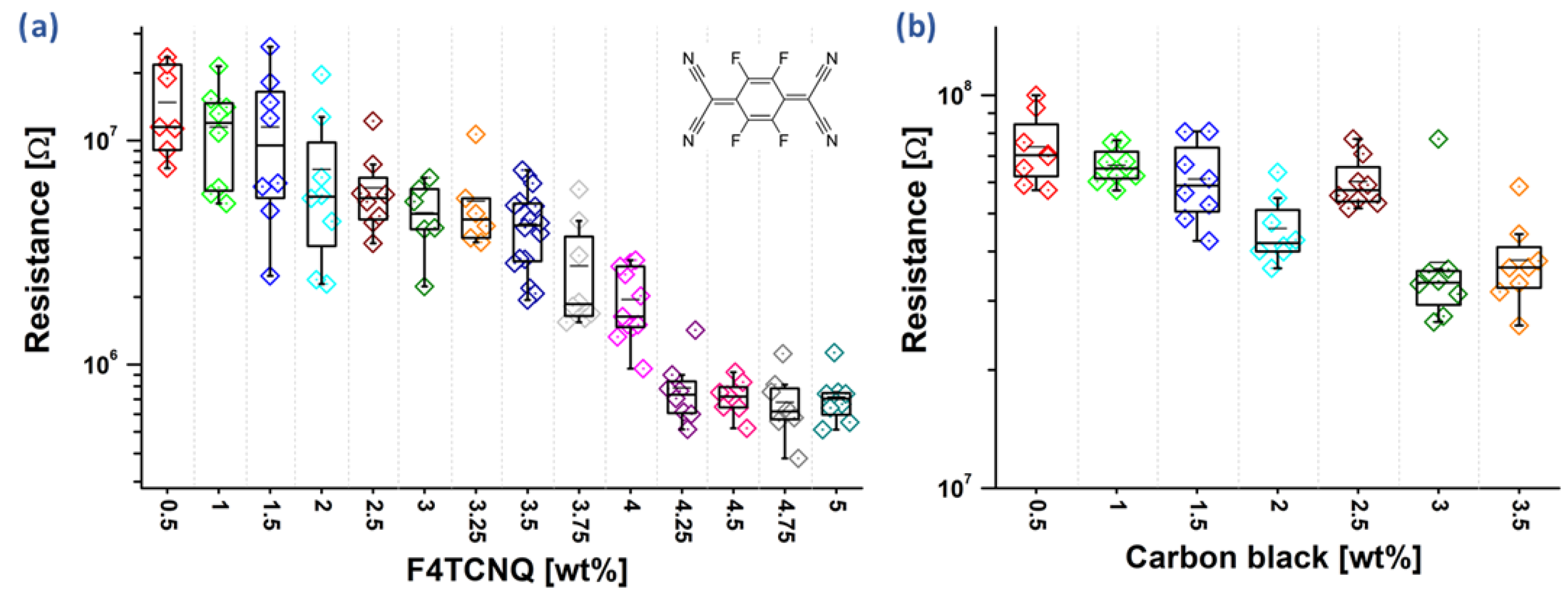
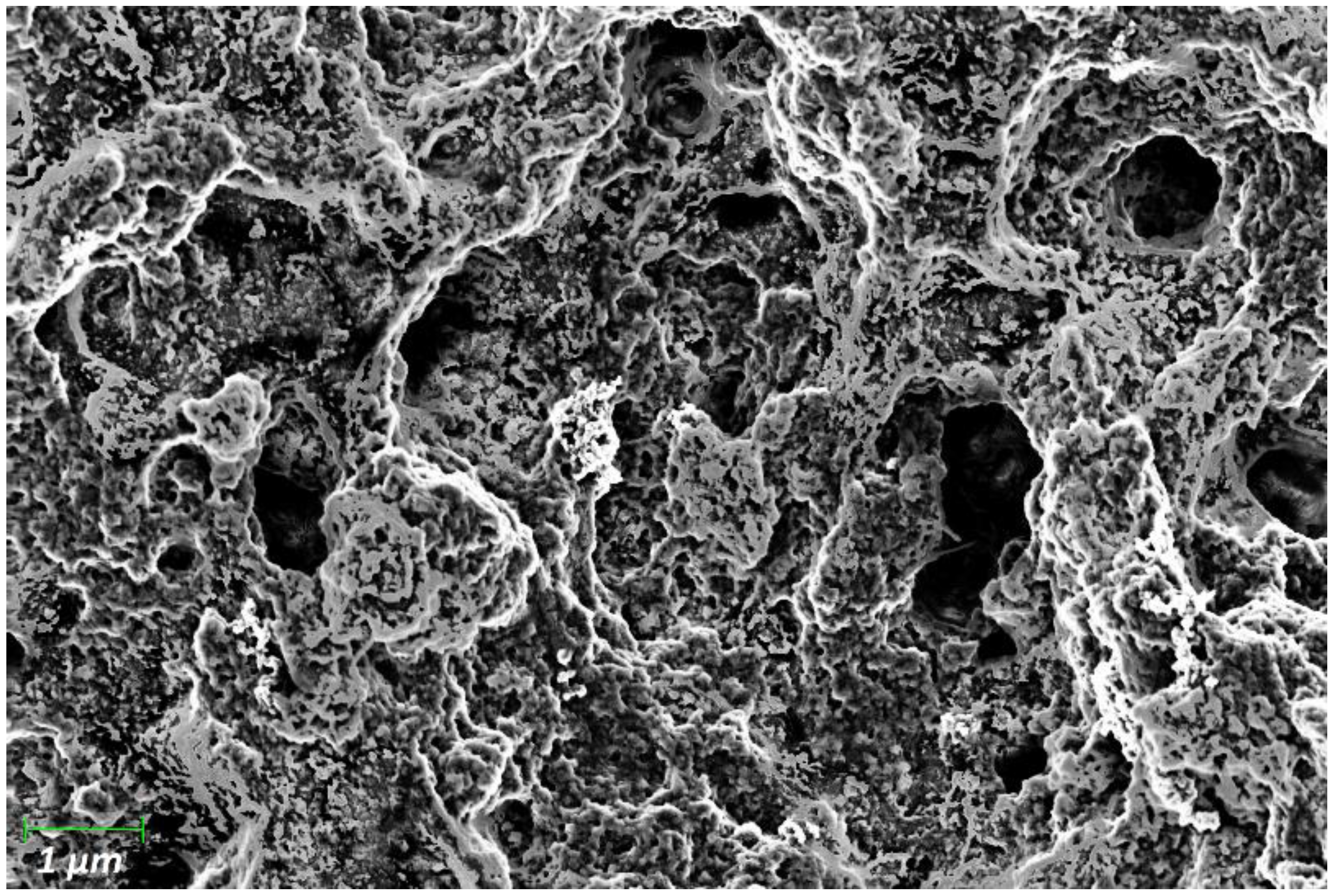
3.1. Resistance of Doped Polyaniline Films
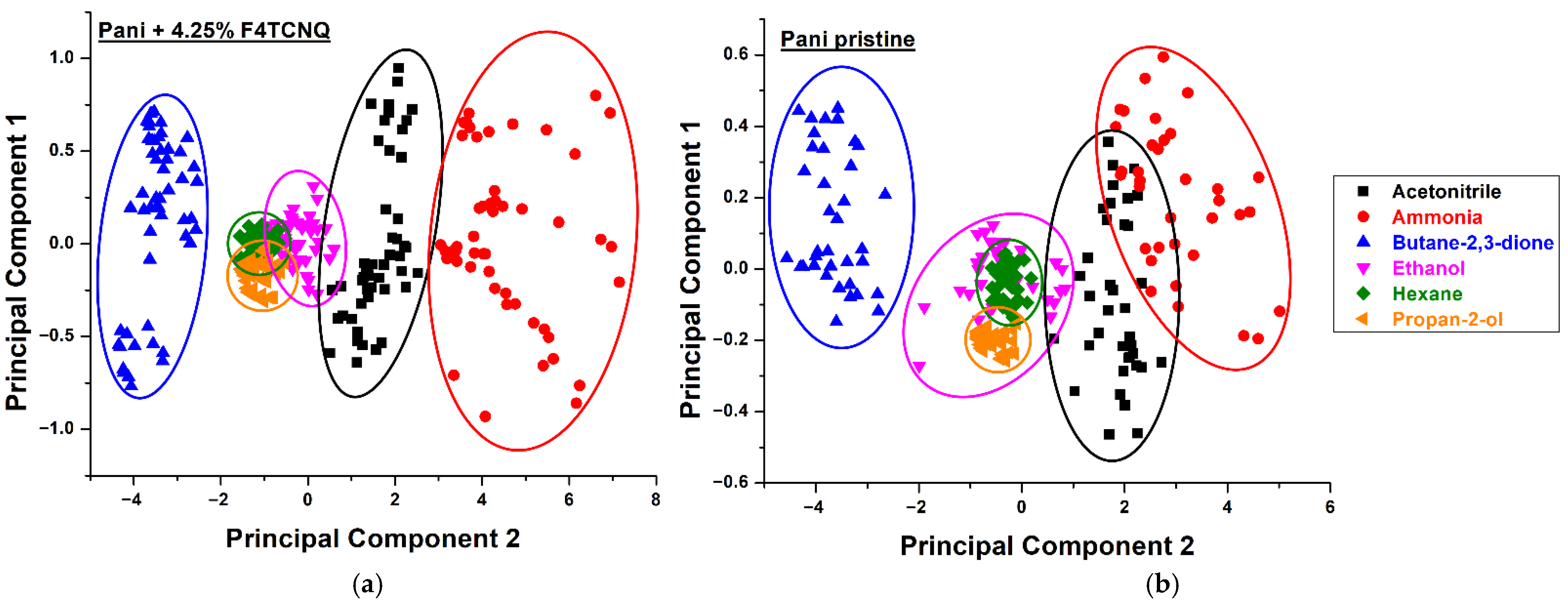
3.2. Responses to Analytes
3.2.1. Liquid Calibration Unit Measurements
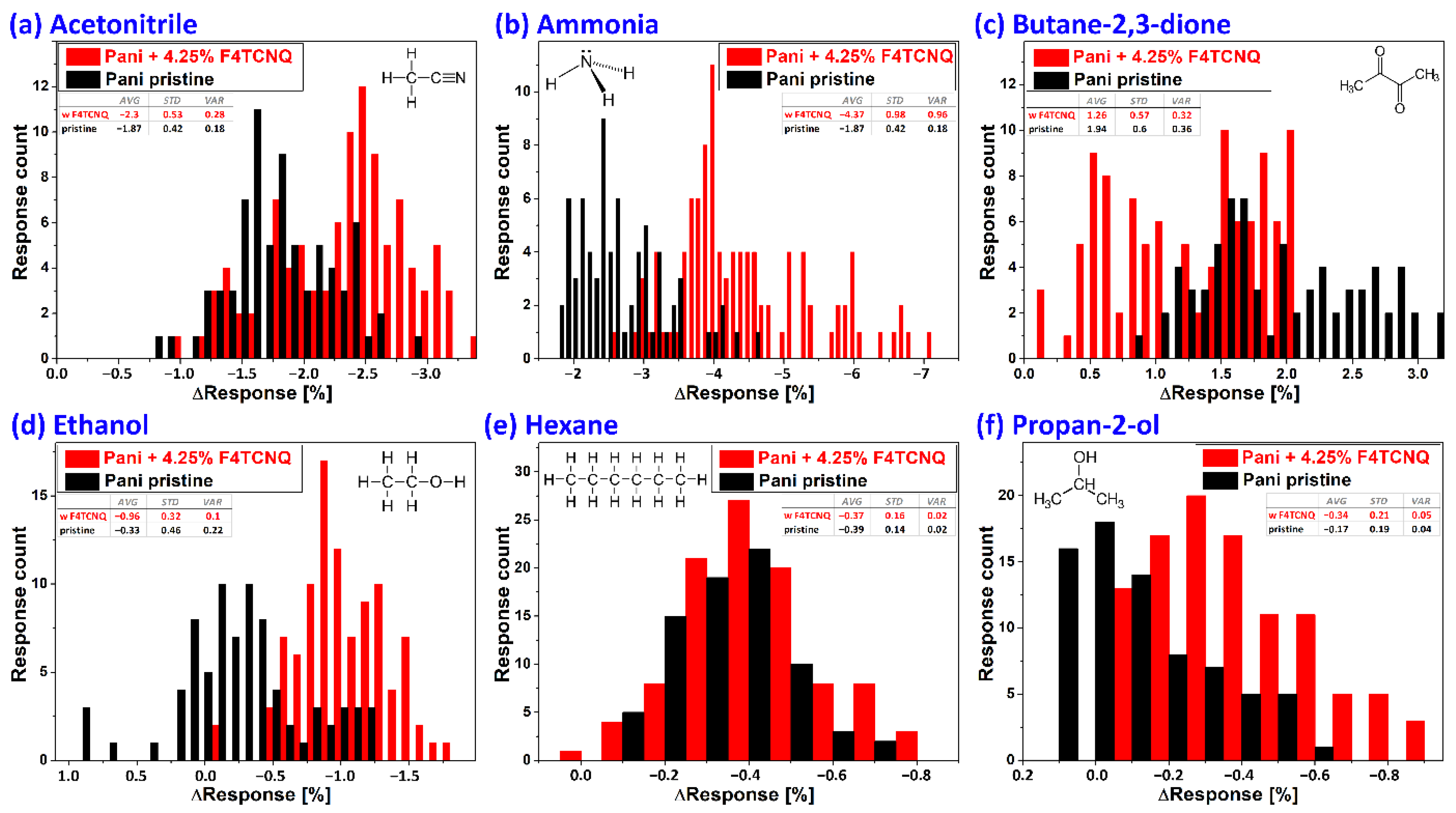
3.2.2. Closed Volume Measurements and Statistical Evaluation
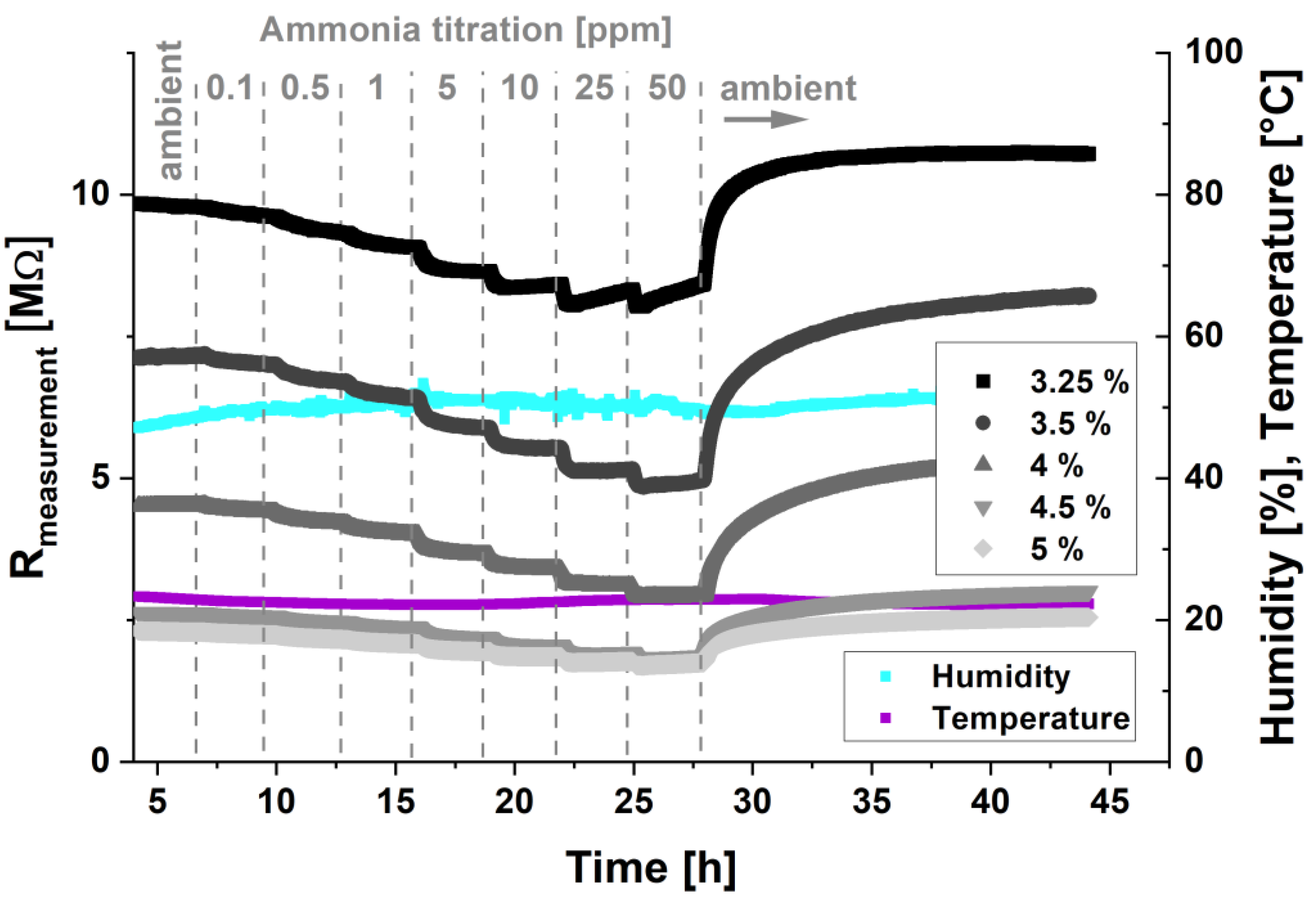
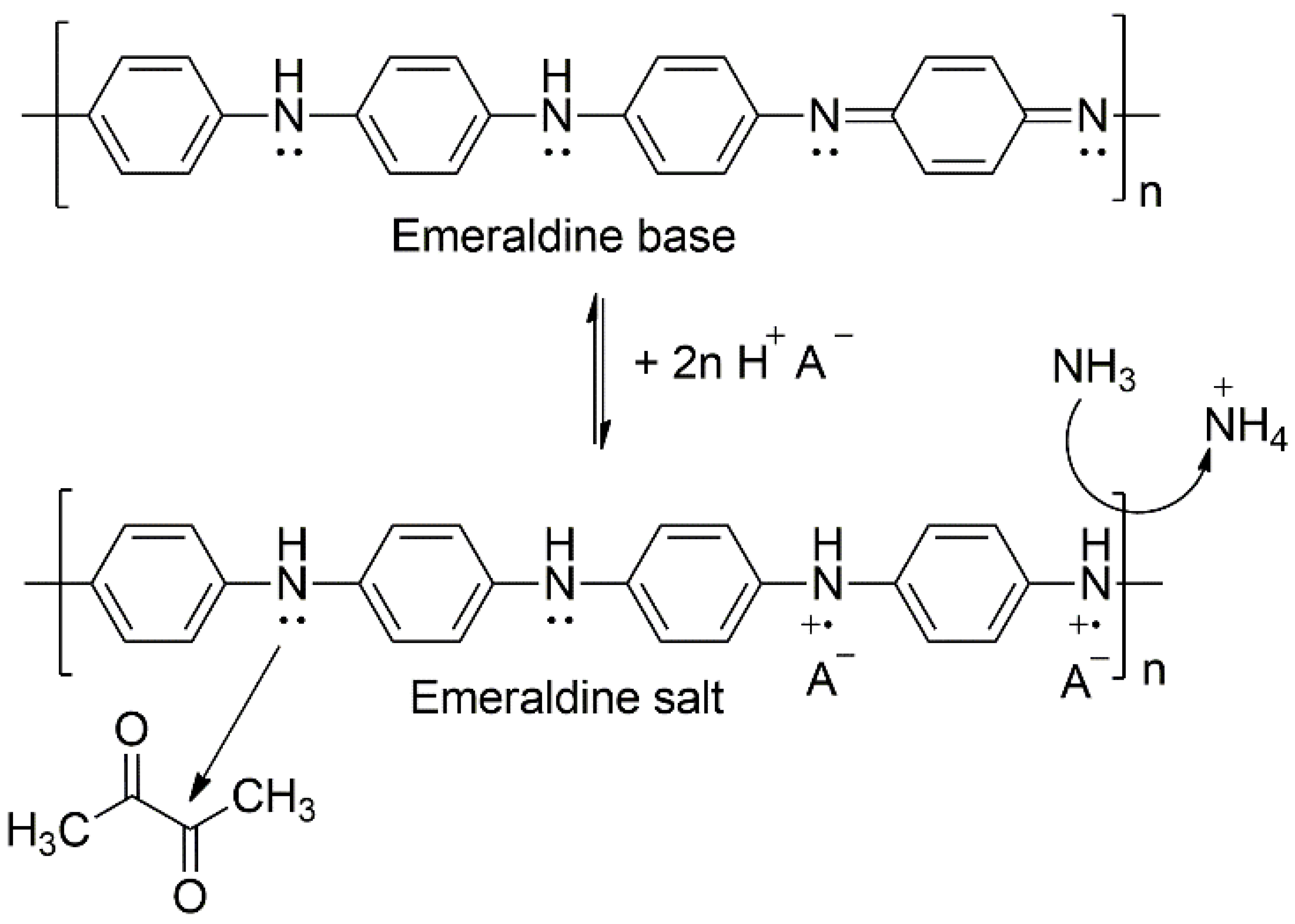
3.3. PANI Conductivity Changes with Relative Humidity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gardner, J.W.; Bartlett, P.N. A brief history of electronic noses. Sens. Actuators B Chem. 1994, 18, 210–211. [Google Scholar] [CrossRef]
- Tomić, M.; Šetka, M.; Vojkůvka, L.; Vallejos, S. VOCs sensing by metal oxides, conductive polymers, and carbon-based materials. Nanomaterials 2021, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Ogbeide, O.; Macadam, N.; Sun, Q.; Yu, W.; Li, Y.; Su, B.-L.; Hasan, T.; Huang, X.; Huang, W. Printed gas sensors. Chem. Soc. Rev. 2020, 49, 1756–1789. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, T.; Chen, F.; Sun, X.; Li, X.; Yu, Z.; Wan, P.; Chen, X. Hierarchical graphene–polyaniline nanocomposite films for high-performance flexible electronic gas sensors. Nanoscale 2016, 8, 12073–12080. [Google Scholar] [CrossRef] [PubMed]
- Wallace, G.G.; Spinks, G.M.; Kane-Maguire, L.A.P.; Teasdale, P.R. Conductive Electroactive Polymers, 2nd ed.; CRC Press LLC: Boca Raton, FL, USA, 2003. [Google Scholar]
- Zhang, T.; Cheng, Q.; Jiang, B.; Huang, Y. Design of the novel polyaniline/polysiloxane flexible nanocomposite film and its application in gas sensor. Compos. Part B Eng. 2020, 196, 108131. [Google Scholar] [CrossRef]
- Sarfraz, J.; Ihalainen, P.; Määttänen, A.; Peltonen, J.; Linden, M. Printed hydrogen sulfide gas sensor on paper substrate based on polyaniline composite. Thin Solid Film. 2013, 534, 621–628. [Google Scholar] [CrossRef]
- Vaghela, C.; Kulkarni, M.; Haram, S.; Karve, M.; Aiyer, R. Biopolymer-polyaniline composite for a wide range ammonia gas sensor. IEEE Sens. J. 2016, 16, 4318–4325. [Google Scholar] [CrossRef]
- Khened, B.; Prasad, M.; Sasikala, M. Synthesis, characterization and liquid petroleum gas sensing of ZnFe2O4 nanoribbons doped polyaniline nanocomposites. Sens. Lett. 2016, 14, 817–823. [Google Scholar] [CrossRef]
- Swe, M.M.; Eamsa-Ard, T.; Srikhirin, T.; Kerdcharoen, T. Monitoring the freshness level of beef using nanocomposite gas sensors in electronic nose. In Proceedings of the 2019 IEEE International Conference on Consumer Electronics-Asia (ICCE-Asia), Bangkok, Thailand, 12–14 June 2019; pp. 100–103. [Google Scholar]
- Le Maout, P.; Wojkiewicz, J.-L.; Redon, N.; Lahuec, C.; Seguin, F.; Dupont, L.; Mikhaylov, S.; Noskov, Y.; Ogurtsov, N.; Pud, A. Polyaniline nanocomposites based sensor array for breath ammonia analysis. Portable e-nose approach to non-invasive diagnosis of chronic kidney disease. Sens. Actuators B Chem. 2018, 274, 616–626. [Google Scholar] [CrossRef]
- Graboski, A.M.; Galvagni, E.; Manzoli, A.; Shimizu, F.M.; Zakrzevski, C.A.; Weschenfelder, T.A.; Steffens, J.; Steffens, C. Lab-made electronic-nose with polyaniline sensor array used in classification of different aromas in gummy candies. Food Res. Int. 2018, 113, 309–315. [Google Scholar] [CrossRef]
- Shirakawa, H. Nobel Lecture: The discovery of polyacetylene film—The dawning of an era of conducting polymers. Rev. Mod. Phys. 2001, 73, 713–718. [Google Scholar] [CrossRef] [Green Version]
- Malinauskas, M.; Žukauskas, A.; Hasegawa, S.; Hayasaki, Y.; Mizeikis, V.; Buividas, R.; Juodkazis, S. Ultrafast laser processing of materials: From science to industry. Light Sci. Appl. 2016, 5, e16133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.-Q.; Liu, Y.; Zhong, D.; Nikzad, S.; Liu, S.; Yu, Z.; Liu, D.; Wu, H.-C.; Zhu, C.; Li, J. Monolithic optical microlithography of high-density elastic circuits. Science 2021, 373, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Shahid, N.; Rappon, T.; Berta, W. Applications of artificial neural networks in health care organizational decision-making: A scoping review. PLoS ONE 2019, 14, e0212356. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Farha, F.; Li, Q.; Wan, Y.; Xu, Y.; Zhang, T.; Ning, H. Review on smart gas sensing technology. Sensors 2019, 19, 3760. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Kim, Y.; Kim, T.; Eom, T.H.; Kim, S.Y.; Jang, H.W. Chemoresistive materials for electronic nose: Progress, perspectives, and challenges. InfoMat 2019, 1, 289–316. [Google Scholar] [CrossRef] [Green Version]
- Duc, C.; Boukhenane, M.L.; Fagniez, T.; Khouchaf, L.; Redon, N.; Wojkiewicz, J.-L. Conductive Polymer Composites for Hydrogen Sulphide Sensors Working at Sub-PPM Level and Room Temperature. Sensors 2021, 21, 6529. [Google Scholar] [CrossRef]
- Li, W.; Hoa, N.D.; Cho, Y.; Kim, D.; Kim, J.-S. Nanofibers of conducting polyaniline for aromatic organic compound sensor. Sens. Actuators B Chem. 2009, 143, 132–138. [Google Scholar] [CrossRef]
- Lin, C.W.; Hwang, B.J.; Lee, C.R. Methanol sensors based on the conductive polymer composites from polypyrrole and poly (vinyl alcohol). Mater. Chem. Phys. 1998, 55, 139–144. [Google Scholar] [CrossRef]
- Yang, S.; Park, S.; Bintinger, J.; Bonnassieux, Y.; Kymissis, I. P-99: Pneumatic Nozzle Printing as a Versatile Approach to Crystal Growth Management and Patterning of Printed Organic Thin Film Transistors. SID Symp. Dig. Tech. Pap. 2016, 47, 1502–1505. [Google Scholar] [CrossRef]
- Yang, S.; Bintinger, J.; Park, S.; Jain, S.; Alexandrou, K.; Fruhmann, P.; Besar, K.; Katz, H.; Kymissis, I. Inexpensive, Versatile, and Robust USB-Driven Sensor Platform. IEEE Sens. Lett. 2017, 1, 1–4. [Google Scholar] [CrossRef]
- Yang, S.; Park, S.; Bintinger, J.; Bonnassieux, Y.; Anthony, J.; Kymissis, I. Employing Pneumatic Nozzle Printing for Controlling the Crystal Growth of Small Molecule Organic Semiconductor for Field-Effect Transistors. Adv. Electron. Mater. 2018, 4, 1700534. [Google Scholar] [CrossRef]
- Chen, S.G.; Hu, J.W.; Zhang, M.Q.; Li, M.W.; Rong, M.Z. Gas sensitivity of carbon black/waterborne polyurethane composites. Carbon 2004, 42, 645–651. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Chen, Z.; Chen, S.; Sum, T.C.; Lin, J.; Shen, Z.X. Synergistic capacitive behavior between polyaniline and carbon black. Electrochim. Acta 2017, 230, 236–244. [Google Scholar] [CrossRef]
- Crowley, K.; O’Malley, E.; Morrin, A.; Smyth, M.R.; Killard, A.J. An aqueous ammonia sensor based on an inkjet-printed polyaniline nanoparticle-modified electrode. Analyst 2008, 133, 391–399. [Google Scholar] [CrossRef]
- Narkis, M.; Zilberman, M.; Siegmann, A. On the “curiosity” of electrically conductive melt processed doped-polyaniline/polymer blends versus carbon-black/polymer compounds. Polym. Adv. Technol. 1997, 8, 525–528. [Google Scholar] [CrossRef]
- Oh, E.H.; Song, H.S.; Park, T.H. Recent advances in electronic and bioelectronic noses and their biomedical applications. Enzym. Microb. Technol. 2011, 48, 427–437. [Google Scholar] [CrossRef]
- Yu, H.; Qiu, X.; Behzad, A.R.; Musteata, V.; Smilgies, D.-M.; Nunes, S.P.; Peinemann, K.-V. Asymmetric block copolymer membranes with ultrahigh porosity and hierarchical pore structure by plain solvent evaporation. Chem. Commun. 2016, 52, 12064–12067. [Google Scholar] [CrossRef]
- Zhang, A.; Bai, H.; Li, L. Breath figure: A nature-inspired preparation method for ordered porous films. Chem. Rev. 2015, 115, 9801–9868. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, V.F.; Costa, C.M.; Sencadas, V.; Botelho, G.; Gómez-Ribelles, J.L.; Lanceros-Mendez, S. Tailoring porous structure of ferroelectric poly (vinylidene fluoride-trifluoroethylene) by controlling solvent/polymer ratio and solvent evaporation rate. Eur. Polym. J. 2011, 47, 2442–2450. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, B.; Huang, L.; Huang, W.; Wang, Z.; Zhu, W.; Chen, Y.; Mao, Y.; Facchetti, A.; Marks, T.J. Breath figure–derived porous semiconducting films for organic electronics. Sci. Adv. 2020, 6, eaaz1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agah, A.; Vleugels, K.; Griffin, P.B.; Ronaghi, M.; Plummer, J.D.; Wooley, B.A. A High-Resolution Low-Power Incremental ΣΔ ADC with Extended Range for Biosensor Arrays. IEEE J. Solid-State Circuits 2010, 45, 1099–1110. [Google Scholar] [CrossRef]
- Hou, Y.; Soleimanpour, A.M.; Jayatissa, A.H. Low resistive aluminum doped nanocrystalline zinc oxide for reducing gas sensor application via sol–gel process. Sens. Actuators B Chem. 2013, 177, 761–769. [Google Scholar] [CrossRef]
- Rzaij, J.M.; Habubi, N.F. Room temperature gas sensor based on La2O3 doped CuO thin films. Appl. Phys. A 2020, 126, 560. [Google Scholar] [CrossRef]
- Bintinger, J.; Aspermair, P.; Reiner-Rozman, C. Patent, Verfahren zur Herstellung Eines Sensors Sowie Sensor 2022. AT524446 A4, 15 June 2022. [Google Scholar]
- Kukla, A.L.; Shirshov, Y.M.; Piletsky, S.A. Ammonia sensors based on sensitive polyaniline films. Sens. Actuators B Chem. 1996, 37, 135–140. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Zhu, S.; Zhou, Z.; Yao, Y.; Quan, W.; Liu, B. Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite. Sens. Actuators B Chem. 2013, 178, 485–493. [Google Scholar] [CrossRef]
- Chabukswar, V.V.; Pethkar, S.; Athawale, A.A. Acrylic acid doped polyaniline as an ammonia sensor. Sens. Actuators B Chem. 2001, 77, 657–663. [Google Scholar] [CrossRef]
- Tanguy, N.R.; Thompson, M.; Yan, N. A review on advances in application of polyaniline for ammonia detection. Sens. Actuators B Chem. 2018, 257, 1044–1064. [Google Scholar] [CrossRef]
- Yang, L.; Xu, X.; Liu, M.; Chen, C.; Cui, J.; Chen, X.; Wu, K.; Sun, D. Wearable and flexible bacterial cellulose/polyaniline ammonia sensor based on a synergistic doping strategy. Sens. Actuators B Chem. 2021, 334, 129647. [Google Scholar] [CrossRef]
- Saad Ali, S.; Pauly, A.; Brunet, J.; Varenne, C.; Ndiaye, A.L. MWCNTs/PMMA/PS composites functionalized PANI: Electrical characterization and sensing performance for ammonia detection in a humid environment. Sens. Actuators B Chem. 2020, 320, 128364. [Google Scholar] [CrossRef]
- Ganeev, A.A.; Gubal, A.R.; Lukyanov, G.N.; Arseniev, A.I.; Barchuk, A.A.; Jahatspanian, I.E.; Gorbunov, I.S.; Rassadina, A.A.; Nemets, V.M.; Nefedov, A.O. Analysis of exhaled air for early-stage diagnosis of lung cancer: Opportunities and challenges. Russ. Chem. Rev. 2018, 87, 904. [Google Scholar] [CrossRef]
- Mackay, D.; van Wesenbeeck, I. Correlation of chemical evaporation rate with vapor pressure. Environ. Sci. Technol. 2014, 48, 10259–10263. [Google Scholar] [CrossRef] [PubMed]
- Hasan, B.M.S.; Abdulazeez, A.M. A review of principal component analysis algorithm for dimensionality reduction. J. Soft Comput. Data Min. 2021, 2, 20–30. [Google Scholar]
- Buehler, M.G.; Ryan, M.A. Temperature and humidity dependence of a polymer-based gas sensor. In Proceedings of the Electro-Optical Technology for Remote Chemical Detection and Identification II, Orlando, FL, USA, 21 April 1997; Volume 3082, pp. 40–48. [Google Scholar] [CrossRef]
- Liu, M.-Q.; Wang, C.; Kim, N.-Y. High-sensitivity and low-hysteresis porous mimtype capacitive humidity sensor using functional polymer mixed with TiO2 microparticles. Sensors 2017, 17, 284. [Google Scholar] [CrossRef]
- Jacobs, I.E.; Wang, F.; Hafezi, N.; Medina-Plaza, C.; Harrelson, T.F.; Li, J.; Augustine, M.P.; Mascal, M.; Moulé, A.J. Quantitative dedoping of conductive polymers. Chem. Mater. 2017, 29, 832–841. [Google Scholar] [CrossRef] [Green Version]
- MacDiarmid, A.G. “Synthetic metals”: A novel role for organic polymers (Nobel lecture). Angew. Chem. Int. Ed. 2001, 40, 2581–2590. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef] [Green Version]
- Ray, A.; Richter, A.F.; MacDiarmid, A.G.; Epstein, A.J. Polyaniline: Protonation/deprotonation of amine and imine sites. Synth. Met. 1989, 29, 151–156. [Google Scholar] [CrossRef]
- Kiefer, D.; Kroon, R.; Hofmann, A.I.; Sun, H.; Liu, X.; Giovannitti, A.; Stegerer, D.; Cano, A.; Hynynen, J.; Yu, L. Double doping of conjugated polymers with monomer molecular dopants. Nat. Mater. 2019, 18, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Tigelaar, D.M.; Lee, W.; Bates, K.A.; Saprigin, A.; Prigodin, V.N.; Cao, X.; Nafie, L.A.; Platz, M.S.; Epstein, A.J. Role of Solvent and Secondary Doping in Polyaniline Films Doped with Chiral Camphorsulfonic Acid: Preparation of a Chiral Metal. Chem. Mater. 2002, 14, 1430–1438. [Google Scholar] [CrossRef]
- Mishra, R.K.; Choi, G.-J.; Choi, H.-J.; Gwag, J.-S. ZnS Quantum Dot Based Acetone Sensor for Monitoring Health-Hazardous Gases in Indoor/Outdoor Environment. Micromachines 2021, 12, 598. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reiner-Rozman, C.; Pichler, B.; Madi, V.; Weißenböck, P.; Hegedüs, T.; Aspermair, P.; Bintinger, J. Optimization of Printed Polyaniline Composites for Gas Sensing Applications. Sensors 2022, 22, 5379. https://doi.org/10.3390/s22145379
Reiner-Rozman C, Pichler B, Madi V, Weißenböck P, Hegedüs T, Aspermair P, Bintinger J. Optimization of Printed Polyaniline Composites for Gas Sensing Applications. Sensors. 2022; 22(14):5379. https://doi.org/10.3390/s22145379
Chicago/Turabian StyleReiner-Rozman, Ciril, Bernhard Pichler, Vivien Madi, Petra Weißenböck, Thomas Hegedüs, Patrik Aspermair, and Johannes Bintinger. 2022. "Optimization of Printed Polyaniline Composites for Gas Sensing Applications" Sensors 22, no. 14: 5379. https://doi.org/10.3390/s22145379
APA StyleReiner-Rozman, C., Pichler, B., Madi, V., Weißenböck, P., Hegedüs, T., Aspermair, P., & Bintinger, J. (2022). Optimization of Printed Polyaniline Composites for Gas Sensing Applications. Sensors, 22(14), 5379. https://doi.org/10.3390/s22145379






