Real-Time Assessment of Mandarin Crop Water Stress Index
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Soil Data
2.3. Water Stress Treatments
2.4. Fertilizer Application, Pest and Disease Management
2.5. CWSI Workswell Wiris Agro R Infrared Camera
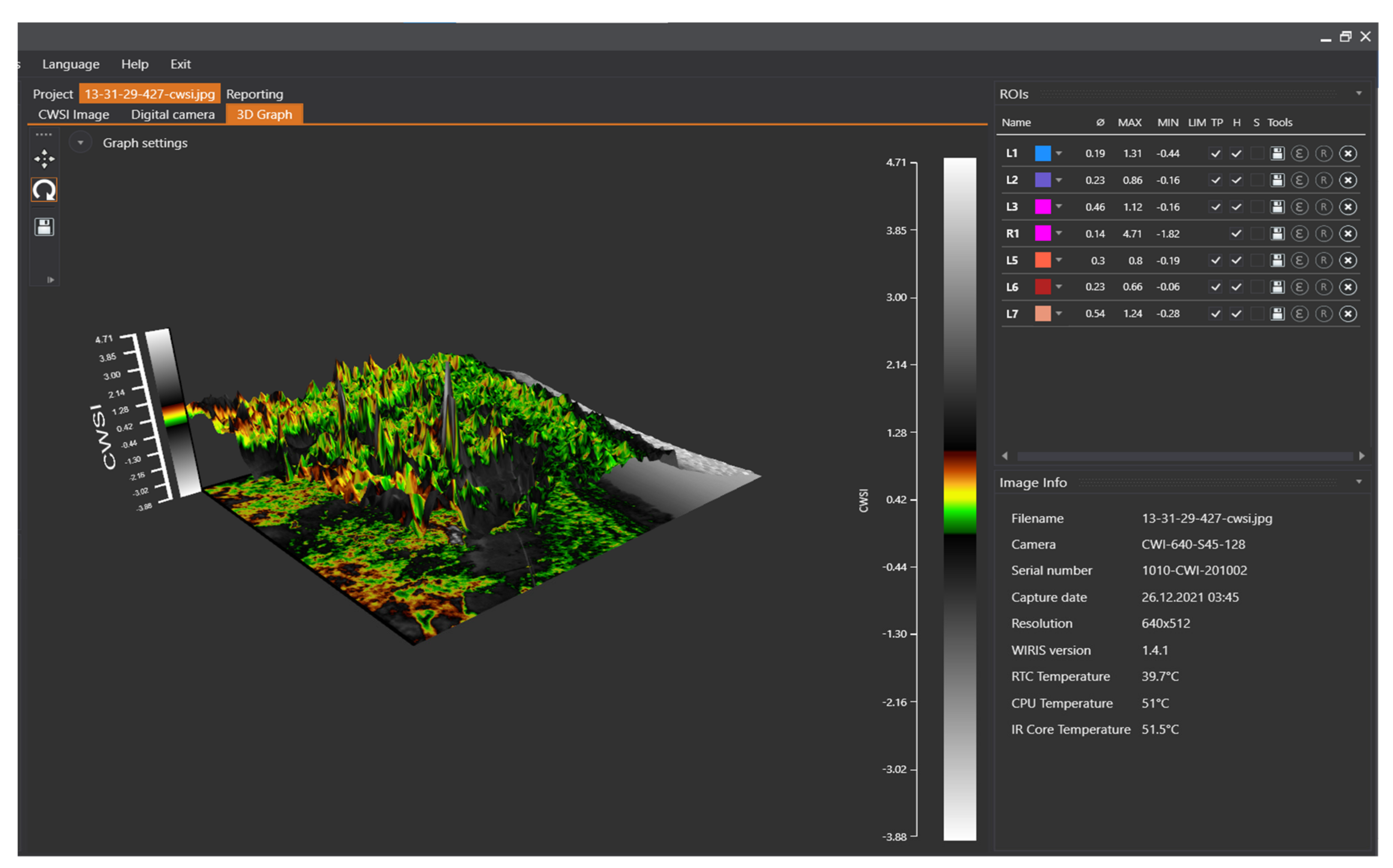
2.5.1. Camera Specification, Data Collection, and Processing
2.5.2. Camera Measurement Functions
2.6. Field Measurements
2.6.1. Image Acquisition
2.6.2. Canopy Temperature Measurement
2.6.3. Estimating the Relative Leaf Water Content (RLWC)
2.6.4. Estimating SPAD and the Nitrogen Sufficiency Index (NSI)
2.6.5. Estimating the CWSI Using an Empirical Method (the Idso Method)
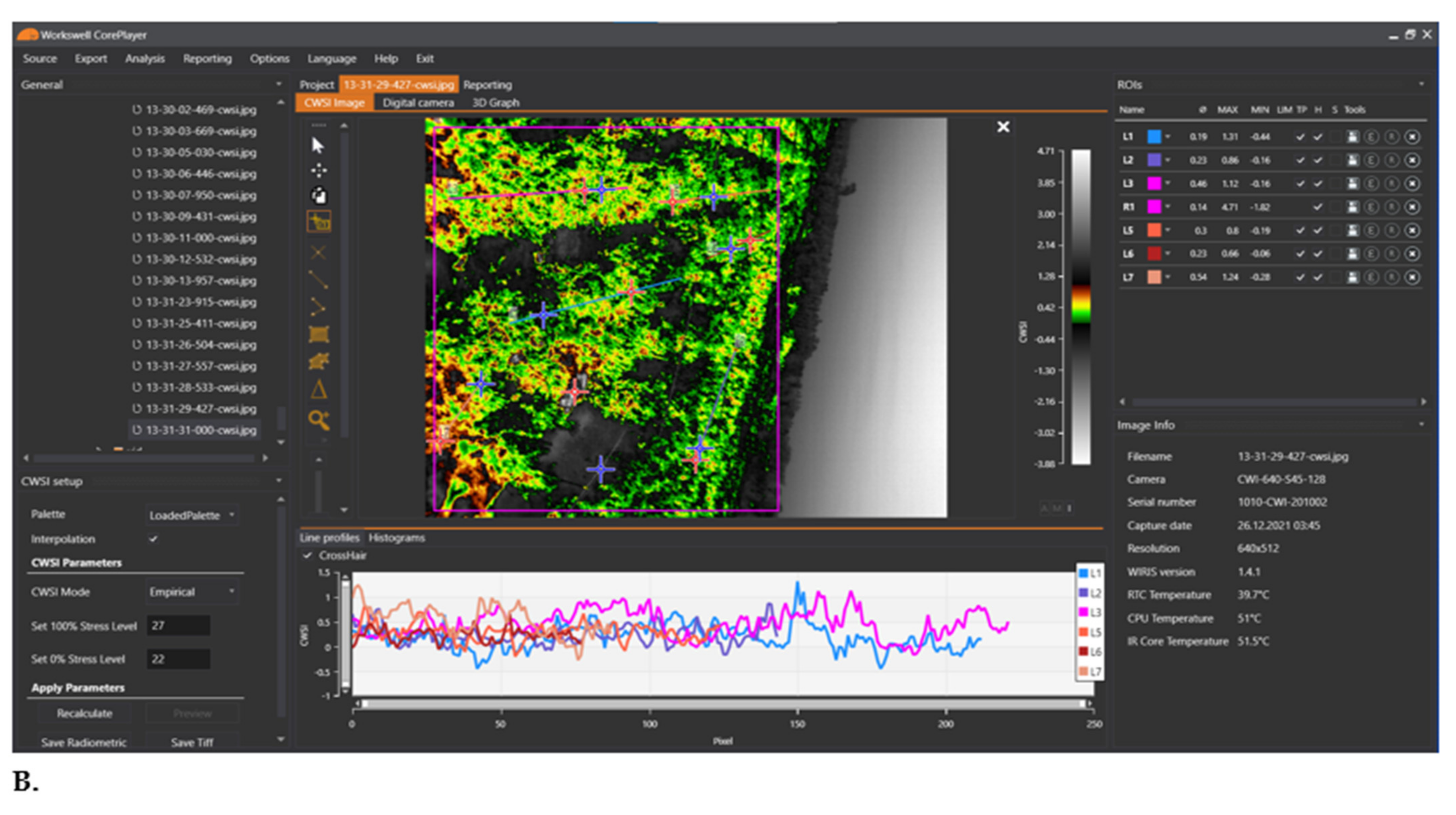
2.6.6. Estimating the CWSI with the Workswell WIRIS Agro R Infrared Camera
2.7. Data Analysis
3. Results
3.1. Soil Water Content Measurement
Variations in Soil Moisture Content
3.2. Plant-Based Indicators
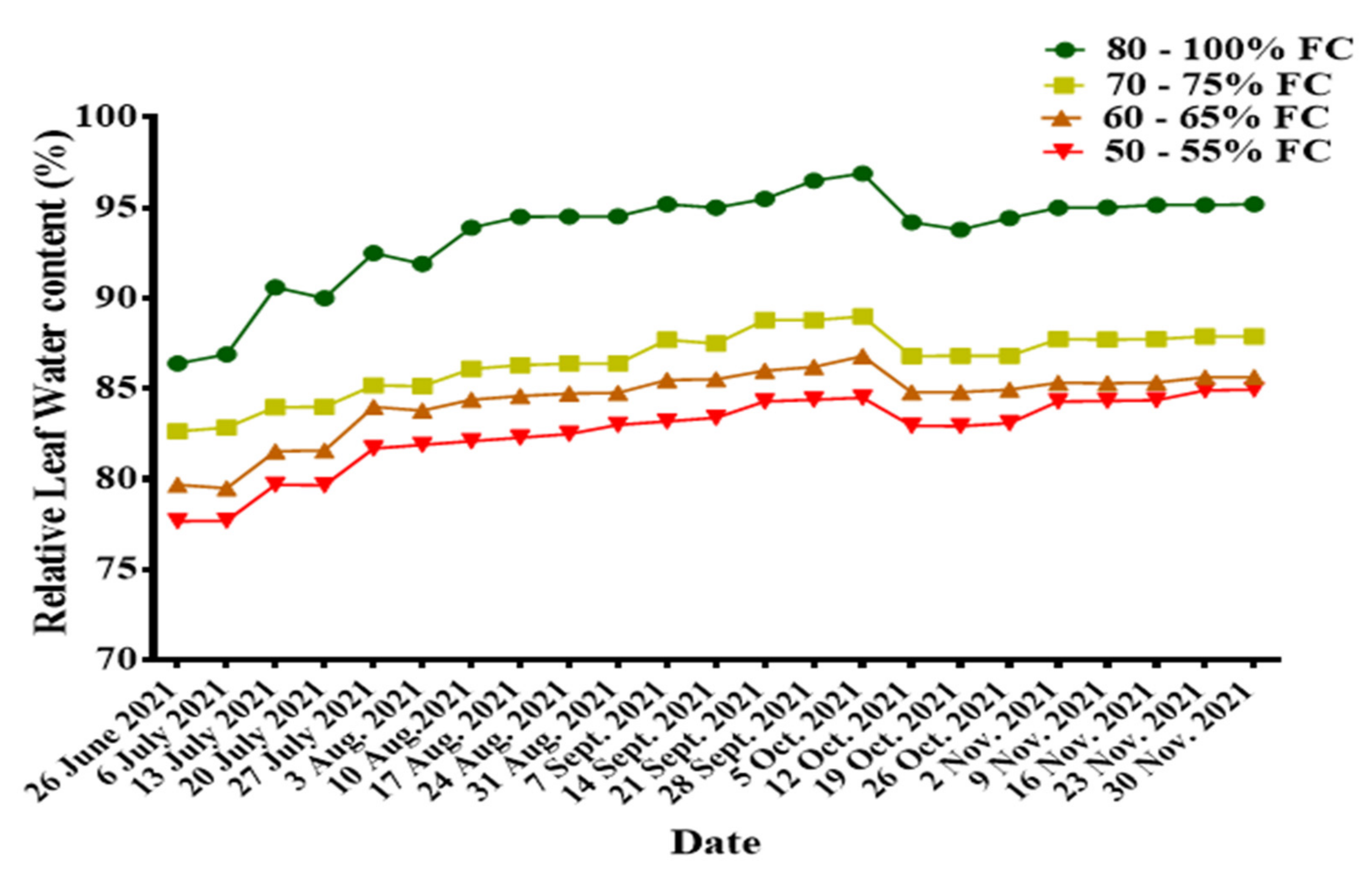
3.2.1. Relative Leaf Water Content (RLWC)
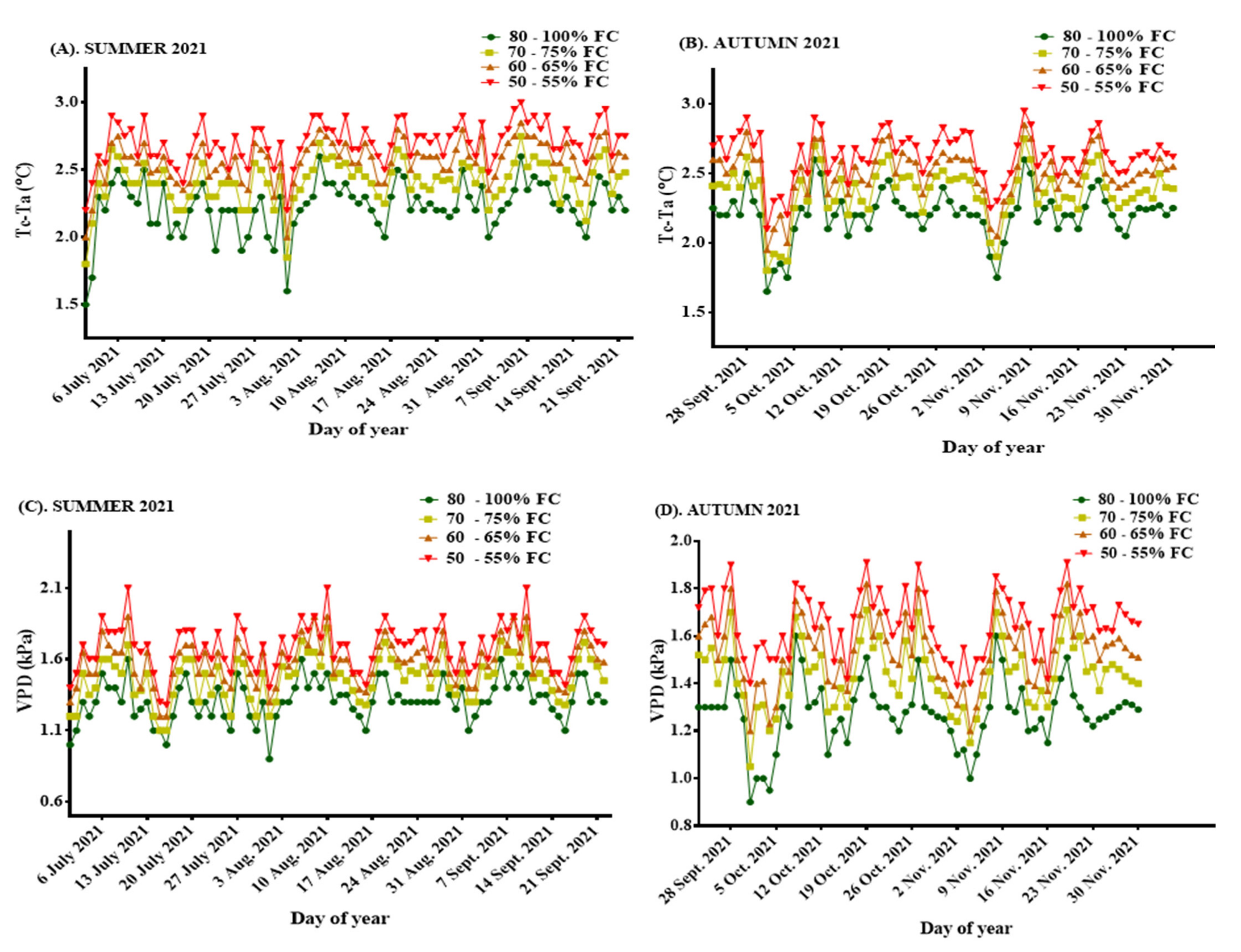
3.2.2. Leaf Temperature (°C)
3.2.3. Leaf Chlorophyll (mg g−1) Content and the Nitrogen Sufficiency Index
3.3. Mandarin Crop Water Stress Index (CWSI) Based on Idso Procedures and Baseline Equations
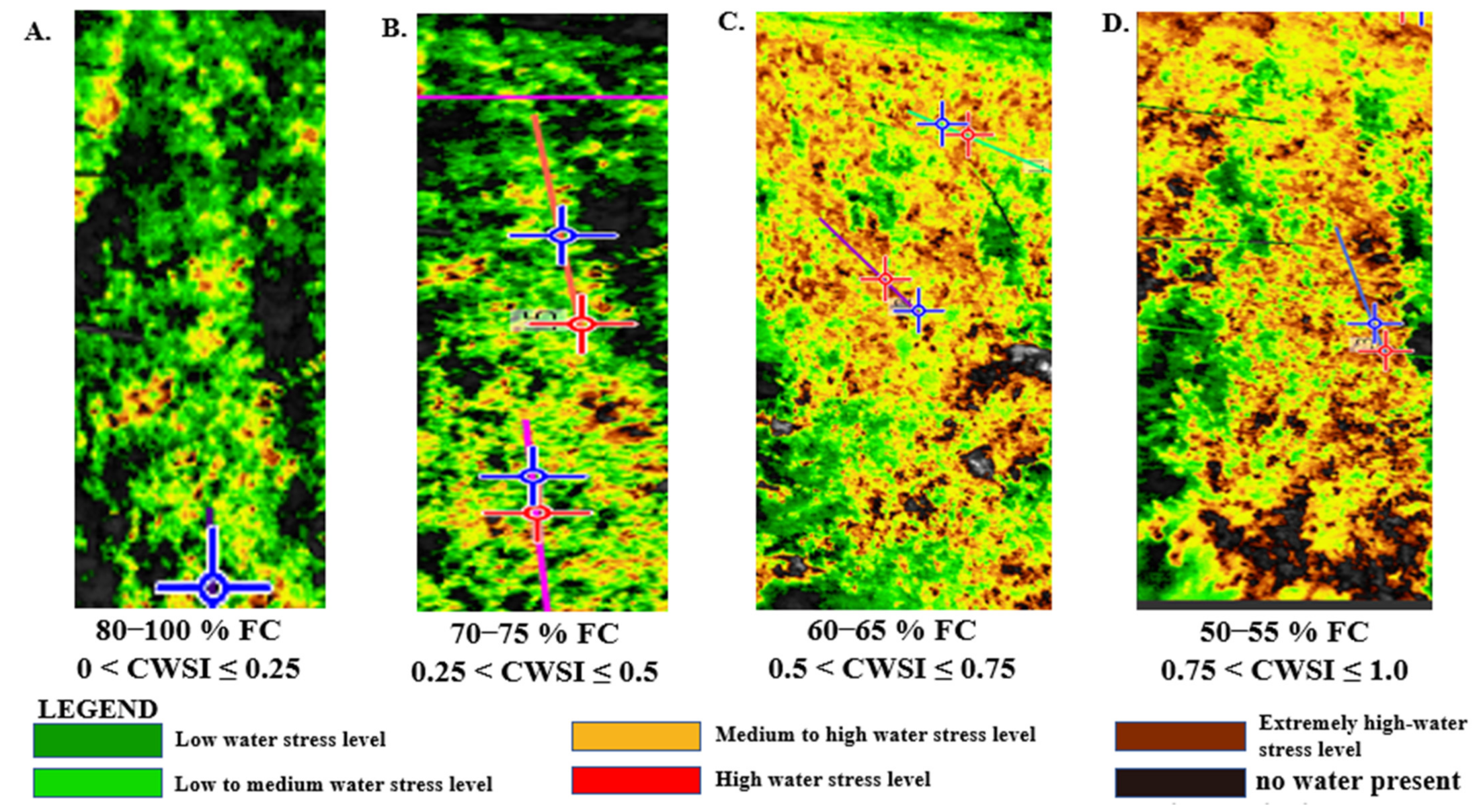
3.4. Mandarin CWSI Estimated from the Workswell WIRIS Agro R Infrared Camera
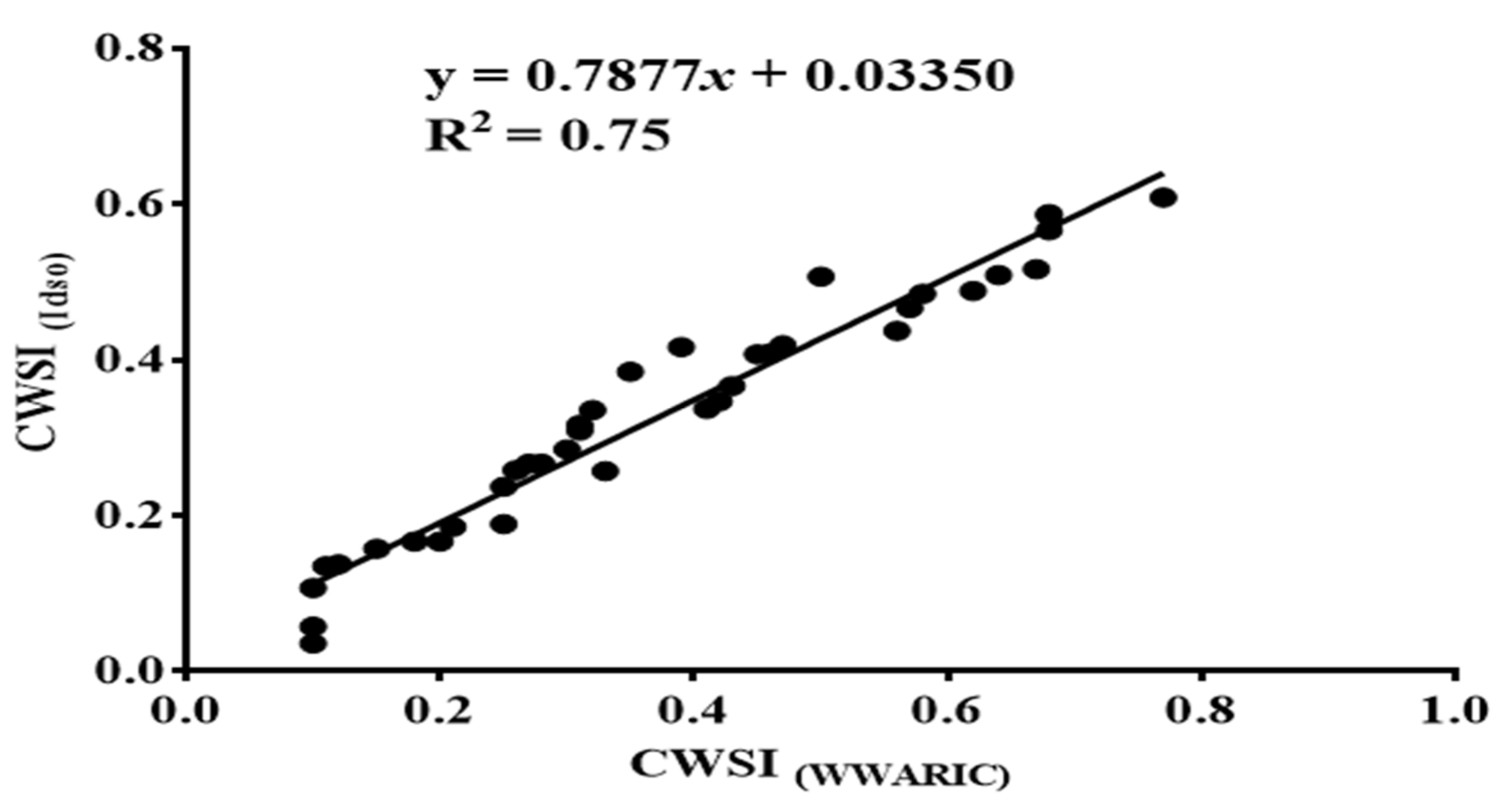
3.5. CWSI Based on the Idso Method and the WWARIC
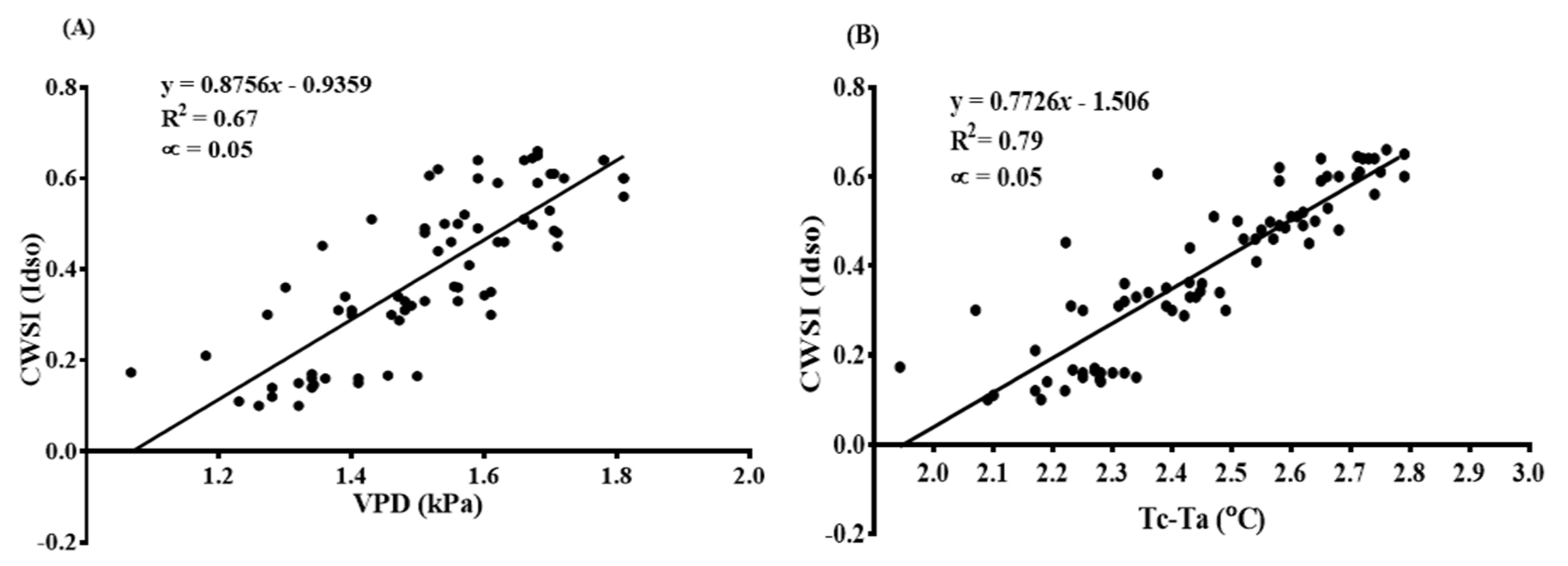
3.6. The CWSI and Plant-Based Water Stress Indicators (RLWC, Leaf Temperature, and Chlorophyll Content)
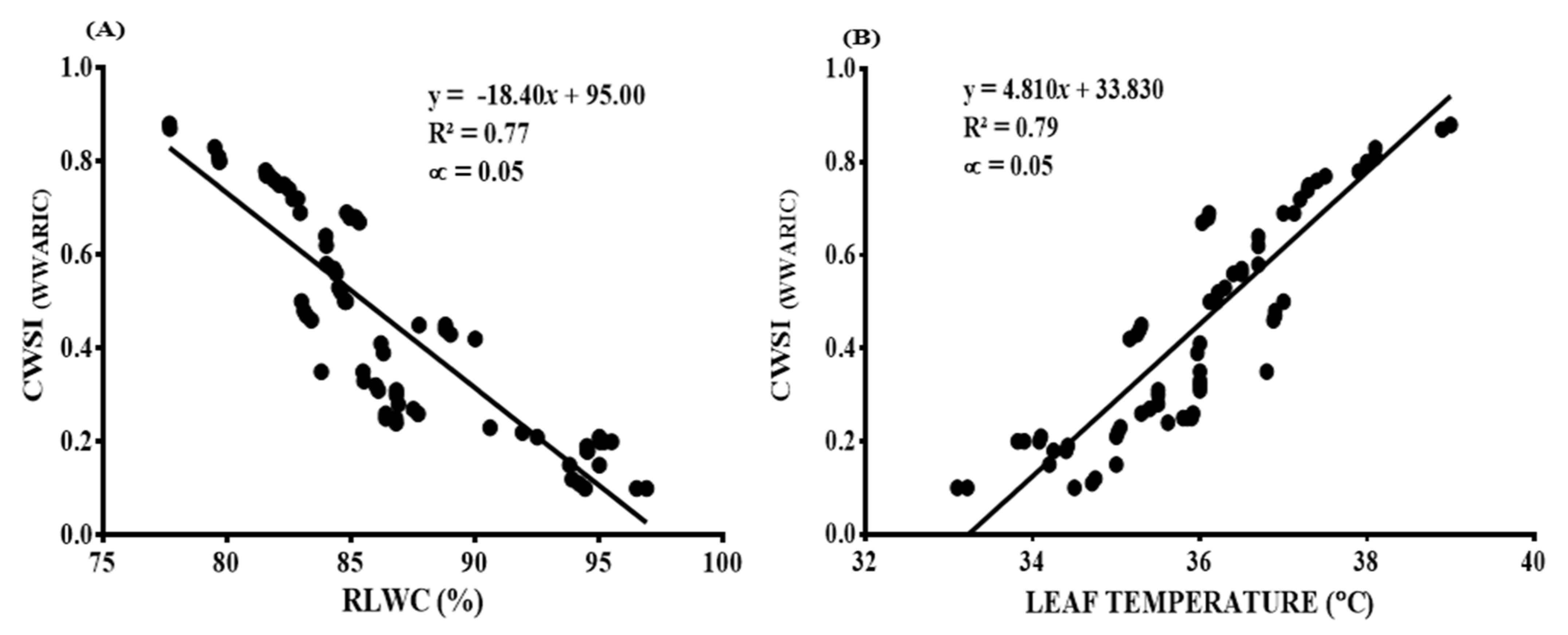
3.6.1. The CWSI Calculated by the Idso Method and Plant-Based Water Stress Indicators (RLWC, Leaf Temperature, and Chlorophyll Content)
3.6.2. The CWSI Estimated from the WWARIC and Plant-Based Water Stress Indicators (RLWC and Leaf Temperature)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Chen, J. Citrus Fruit-Cracking: Causes and Occurrence. Hortic. Plant J. 2017, 3, 255–260. [Google Scholar] [CrossRef]
- Blanco, V.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Influence of Regulated Deficit Irrigation and Environmental Conditions on Reproductive Response of Sweet Cherry Trees. Plants 2020, 9, 94. [Google Scholar] [CrossRef] [Green Version]
- Saitta, D.; Consoli, S.; Ferlito, F.; Torrisi, B.; Allegra, M.; Longo-Minnolo, G.; Ramírez-Cuesta, J.M.; Vanella, D. Adaptation of citrus orchards to deficit irrigation strategies. Agric. Water Manag. 2021, 247, 106734. [Google Scholar] [CrossRef]
- Kuscu, H.; Karasu, A.; Oz, M.; Demir, O.A.; Turgut, I. Effect of irrigation amounts applied with drip irrigation on maize evaporation, yield, water use efficiency and net return in a sub-humid climate. Turk. J. Field Crops 2013, 18, 13–19. [Google Scholar]
- Al-Ghobari, H.M.; Dewidar, A.Z. Integrating deficit irrigation into surface and subsurface drip irrigation as a strategy to save water in arid regions. Agric. Water Manag. 2018, 209, 55–61. [Google Scholar] [CrossRef]
- Fernandes-Silva, A.; Oliveira, M.; Paço, T.A.; Ferreira, I. Deficit Irrigation in Mediterranean Fruit Trees and Grapevines: Water Stress Indicators and Crop Responses. In Irrigation in Agroecosystems; Ondrašek, G., Ed.; IntechOpen: London, UK, 2018; pp. 52–85. [Google Scholar]
- Faghih, S.; Zamani, Z.; Fatahi, R.; Liaghat, A. Effects of deficit irrigation and kaolin application on vegetative growth and fruit traits of two early ripening apple cultivars. Biol. Res. 2019, 52, 43. [Google Scholar] [CrossRef] [Green Version]
- Fernández, J.E. Plant-based methods for irrigation scheduling of woody crops. Horticulturae 2017, 3, 35. [Google Scholar] [CrossRef] [Green Version]
- Romero-Trigueros, C.; Gambín, J.M.B.; Tortosa, P.A.N.; Cabañero, J.J.A.; Nicolás, E.N. Determination of Crop Water Stress Index by Infrared Thermometry in Grapefruit Trees Irrigated with Saline Reclaimed Water Combined with Deficit Irrigation. Remote Sens. 2019, 11, 757. [Google Scholar] [CrossRef] [Green Version]
- Ihuoma, S.O. The Use of Spectral Reflectance Data to Assess Plant Stress and Improve Irrigation Water Management. Ph.D. Thesis, McGill University, Montreal, Canada, 2020. [Google Scholar]
- Jones, H.G. Irrigation scheduling: Advantages and pitfalls of plant based methods. J. Exp. Bot. 2004, 55, 2427–2436. [Google Scholar] [CrossRef] [Green Version]
- Drechsler, K.; Kisekka, I.; Upadhyaya, S. A comprehensive stress indicator for evaluating plant water status in almond trees. Agric. Water Manag. 2019, 216, 214–223. [Google Scholar] [CrossRef]
- Berni, J.A.J.; Zarco-Tejada, P.J.; Sepulcre-Cantó, G.; Fereres, E.; Villalobos, F. Mapping canopy conductance and CWSI in olive orchards using high resolution thermal remote sensing imagery. Remote Sens. Environ. 2009, 113, 2380–2388. [Google Scholar] [CrossRef]
- Bastiaanssen, W.G.M.; Pelgrum, H.; Soppe, R.W.O.; Allen, R.G.; Thoreson, B.P.; de, C. Teixeira, A.H. Thermal-infrared technology for local and regional scale irrigation analyses in horticultural systems. Acta Hortic. 2006, 792, 33–46. [Google Scholar]
- Berni, J.A.; Zarco-Tejada, P.J.; Suárez, L.; Fereres, E. Thermal and narrowband multispectral remote sensing for vegetation monitoring from an unmanned aerial vehicle. IEEE Trans. Geosci. Remote Sens. 2009, 47, 722–738. [Google Scholar] [CrossRef] [Green Version]
- Stagakis, S.; González-Dugo, V.; Cid, P.; Guillén-Climent, M.L.; Zarco-Tejada, P.J. Monitoring water stress and fruit quality in an orange orchard under regulated deficit irrigation using narrow-band structural and physiological remote sensing indices. ISPRS J. Photogramm. Remote Sens. 2012, 71, 47–61. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Gimeno, M.A.; Bonet, L.; Provenzano, G.; Badal, E.; Intrigliolo, D.S.; Ballester, C. Assessment of yield and water productivity of clementine trees under surface and subsurface drip irrigation. Agric. Water Manag. 2018, 206, 209–216. [Google Scholar] [CrossRef]
- Romero-Trigueros, C.; Nortes, P.A.; Alarcón, J.J.; Hunink, J.E.; Parra, M.; Contreras, S.; Droogers, P.; Nicolás, E. Effects of saline reclaimed waters and deficit irrigation on Citrus physiology assessed by UAV remote sensing. Agric. Water Manag. 2017, 183, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhou, G. Estimation of vegetation water content using hyperspectral vegetation indices: A comparison of crop water indicators in response to water stress treatments for summer maize. BMC Ecol. 2019, 19, 18. [Google Scholar] [CrossRef] [Green Version]
- Ihuoma, S.O.; Madramootoo, C.A. Sensitivity of spectral vegetation indices for monitoring water stress in tomato plants. Comput. Electron. Agric. 2019, 163, 104860. [Google Scholar] [CrossRef]
- Stimson, H.C.; Breshears, D.D.; Ustin, S.L.; Kefauver, S.C. Spectral sensing of foliar water conditions in two co-occurring conifer species: Pinus edulis and Juniperus monosperma. Remote Sens. Environ. 2005, 96, 108–118. [Google Scholar] [CrossRef]
- Jones, H.G.; Stoll, M.; Santos, T.; de Sousa, C.; Chaves, M.M.; Grant, O.M. Use of infrared thermography for monitoring stomatal closure in the field: Application to grapevine. J. Exp. Bot. 2002, 53, 2249–2260. [Google Scholar] [CrossRef]
- Merlot, S.; Mustilli, A.C.; Genty, B.; North, H.; Lefebvre, V.; Sotta, B.; Vavasseur, A.; Giraudat, J. Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J. 2002, 30, 601–609. [Google Scholar] [CrossRef] [Green Version]
- Alordzinu, K.E.; Li, J.; Lan, Y.; Appiah, S.A.; Al Aasmi, A.; Wang, H. Rapid Estimation of Crop Water Stress Index on Tomato Growth. Sensors 2021, 21, 5142. [Google Scholar] [CrossRef] [PubMed]
- Poblete-Echeverría, C.; Espinace, D.; Sepúlveda-Reyes, D.; Zuñiga, M.; Sanchez, M. Analysis of crop water stress index (CWSI) for estimating stem water potential in grapevines: Comparison between natural reference and baseline approaches. Acta Hortic. 2017, 1150, 189–194. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.; Wang, W.; Ran, H.; Song, T.; Guo, Y. Evaluation of the Crop Water Stress Index as an Indicator for the Diagnosis of Grapevine Water Deficiency in Greenhouses. Horticulturae 2020, 6, 86. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.B.; Reginato, R.J.; Pinter, P.J. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- García-Tejero, I.; Durán-Zuazo, V.H.; Muriel-Fernández, J.L.; Martínez-García, G.; Jiménez-Bocanegra, J.A. Benefits of low-frequency irrigation in citrus orchards. Agron. Sustain. Dev. 2011, 31, 779–791. [Google Scholar] [CrossRef]
- Stagno, F.; Roccuzzo, G.; Allegra, M.; Intrigliolo, F.; Parisi, R.; Cirelli, G.; Consoli, S.; Barbagallo, S. Deficit irrigation strategies: Preliminary assessment on a sicilian young orange orchard. Acta Hortic. 2015, 1065, 1713–1718. [Google Scholar] [CrossRef]
- Consoli, S.; Stagno, F.; Vanella, D.; Boaga, J.; Cassiani, G.; Roccuzzo, G. Partial root-zone drying irrigation in orange orchards: Effects on water use and crop production characteristics. Eur. J. Agron. 2017, 82, 190–202. [Google Scholar] [CrossRef]
- Consoli, S.; Stagno, F.; Roccuzzo, G.; Cirelli, G.L.; Intrigliolo, F. Sustainable management of limited water resources in a young orange orchard. Agric. Water Manag. 2014, 132, 60–68. [Google Scholar] [CrossRef]
- Wright, G.C. Irrigating Citrus Trees; University of Arizona: Tucson, AZ, USA, 2000. [Google Scholar]
- Swain, S. Growing Citrus in the North Bay; University of California: Berkeley, CA, USA, 2012. [Google Scholar]
- Carr, M.K.V. The Water Relations and Irrigation Requirements of Citrus (Citrus Spp.): A Review. Exp. Agric. 2012, 48, 347–377. [Google Scholar] [CrossRef]
- Khedkar, D.D. Minimal Volume Irrigation Scheduling for Citrus Cultivation in Warud Tehsil, Maharashtra, India. Agric. Agric. Sci. Procedia 2015, 4, 365–371. [Google Scholar] [CrossRef]
- Idso, S.B.; Jackson, R.D.; Pinter, P.J.; Reginato, R.J.; Hatfield, J.H. Normalizing the stress degree-day parameter for environmental variability. Agric. Meteorol. 1981, 24, 45–55. [Google Scholar] [CrossRef]
- Idso, S.B. Non-water stressed baselines: A key to measuring and interpreting plant water stress. Agric. Meteorol. 1982, 27, 59–70. [Google Scholar] [CrossRef]
- Cai, L.; Xu, Z.; Ren, M.; Guo, Q.; Hu, X.; Hu, G.; Wan, H.; Peng, P. Source identification of eight hazardous heavy metals in agricultural soils of Huizhou, Guangdong Province, China. Ecotoxicol. Environ. Saf. 2012, 78, 2–8. [Google Scholar] [CrossRef]
- Fahad, M.; Wajid, S.A.; Ahmad, A.; Cheema, M.J.M. Response of wheat cultivars to deficit irrigation under semiarid conditions of Faisalabad. Int. J. Agric. Biol. 2019, 21, 1004–1012. [Google Scholar]
- Khan, S.; Rasool, A.; Irshad, S.; Hafeez, M.B.; Ali, M.; Saddique, M.; Asif, M.; Hasnain, Z.; Naseem, S.; Alwahibi, M.S.; et al. Potential soil moisture deficit: A useful approach to save water with enhanced growth and productivity of wheat crop. J. Water Clim. Change 2021, 12, 2515–2525. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, Q.; Zheng, C. Best hyperspectral indices for tracing leaf water status as determined from leaf dehydration experiments. Ecol. Indic. 2015, 54, 96–100. [Google Scholar] [CrossRef]
- Fuchs, M. Infrared measurement of canopy temperature and detection of plant water stress. Theor. Appl. Clim. 1990, 42, 253–261. [Google Scholar] [CrossRef]
- Ihuoma, S.O.; Madramootoo, C.A. Narrow-band reflectance indices for mapping the combined effects of water and nitrogen stress in tomato crops. Biosyst. Eng. 2020, 192, 133–143. [Google Scholar] [CrossRef]
- Rashid, A.; Stark, J.C.; Tanveer, A.; Mustafa, T. Use of Canopy Temperature Measurements as a Screening Tool for Drought Tolerance in Spring Wheat. J. Agron. Crop Sci. 1999, 182, 231–237. [Google Scholar] [CrossRef]
- Pieczynski, M.; Marczewski, W.; Hennig, J.; Dolata, J.; Bielewicz, D.; Piontek, P.; Wyrzykowska, A.; Krusiewicz, D.; Strzelczyk-Zyta, D.; Konokpa-Postupolska, D.; et al. Down-regulation of CBP80 gene expression as a strategy to engineer a drought-tolerant potato. Plant Biotechnol. J. 2013, 11, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tremblay, N.; Liang, Y. A corn nitrogen status indicator less affected by soil water content. Agron. J. 2011, 103, 890–898. [Google Scholar] [CrossRef]
- Jamshidi, S.; Zand-Parsa, S.; Kamgar-Haghighi, A.A.; Shahsavar, A.R.; Niyogi, D. Evapotranspiration, crop coefficients, and physiological responses of citrus trees in semi-arid climatic conditions. Agric. Water Manag. 2020, 227, 105838. [Google Scholar] [CrossRef]
- Jamshidi, S.; Zand-Parsa, S.; Niyogi, D. Assessing Crop Water Stress Index of Citrus Using In-Situ Measurements, Landsat, and Sentinel-2 Data. Int. J. Remote Sens. 2021, 42, 1893–1916. [Google Scholar] [CrossRef]
- Katsoulas, N.; Elvanidi, A.; Ferentinos, K.P.; Kacira, M.; Bartzanas, T.; Kittas, C. Crop reflectance monitoring as a tool for water stress detection in greenhouses: A review. Biosyst. Eng. 2016, 151, 374–398. [Google Scholar] [CrossRef]
- Paltineanu, C.; Septar, L.; Moale, C. Crop Water Stress in Peach Orchards and Relationships with Soil Moisture Content in a Chernozem of Dobrogea. J. Irrig. Drain. Eng. 2013, 139, 20–25. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Tanriverdi, C.; Atilgan, A.; Degirmenci, H.; Akyuz, A. Comparasion of Crop Water Stress Index (CWSI) and Water Deficit Index (WDI) by Using Remote Sensing (RS). Infrastruct. Ecol. Rural Areas 2017, 879–894. [Google Scholar] [CrossRef]
- Magney, T.S.; Vierling, L.A.; Eitel, J.U.; Huggins, D.R.; Garrity, S.R. Response of high frequency Photochemical Reflectance Index (PRI) measurements to environmental conditions in wheat. Remote Sens. Environ. 2016, 173, 84–97. [Google Scholar] [CrossRef]
- Veysi, S.; Naseri, A.A.; Hamzeh, S.; Bartholomeus, H. A satellite based crop water stress index for irrigation scheduling in sugarcane fields. Agric. Water Manag. 2017, 189, 70–86. [Google Scholar] [CrossRef]
- Yang, Y.; Ling, P.; Fleisher, D.H.; Timlin, D.J.; Reddy, V.R. Non-contacting techniques for plant drought stress detection. Trans. ASABE 2008, 51, 1483–1492. [Google Scholar] [CrossRef]
- Espinoza, C.Z.; Khot, L.R.; Sankaran, S.; Jacoby, P.W. High resolution multispectral and thermal remote sensing-based water stress assessment in subsurface irrigated grapevines. Remote Sens. 2017, 9, 961. [Google Scholar] [CrossRef] [Green Version]
- Gerhards, M.; Schlerf, M.; Mallick, K.; Udelhoven, T. Challenges and Future Perspectives of Multi- Hyperspectral Thermal Infrared Remote Sensing for Crop Water-Stress Detection: A Review. Remote Sens. 2019, 11, 1240. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhou, G. Deriving a light use efficiency estimation algorithm using in situ hyperspectral and eddy covariance measurements for a maize canopy in Northeast China. Ecol. Evol. 2017, 7, 4735–4744. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Dugo, V.; Zarco-Tejada, P.J.; Fereres, E. Applicability and limitations of using the crop water stress index as an indicator of water deficits in citrus orchards. Agric. For. Meteorol. 2014, 198–199, 94–104. [Google Scholar] [CrossRef]
- Testi, L.; Goldhamer, D.; Iniesta, F.; Salinas, M. Crop water stress index is a sensitive water stress indicator in pistachio trees. Irrig. Sci. 2008, 26, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Sepaskhah, A.R.; Kashefipour, S.M. Evapotranspiration and crop coefficient of sweet lime under drip irrigation. Agric. Water Manag. 1995, 27, 331–340. [Google Scholar] [CrossRef]
- DeJonge, K.C.; Taghvaeian, S.; Trout, T.J.; Comas, L.H. Comparison of canopy temperature-based water stress indices for maize. Agric. Water Manag. 2015, 156, 51–62. [Google Scholar] [CrossRef]
- Taghvaeian, S.; Comas, L.; DeJonge, K.C.; Trout, T.L. Conventional and simplified canopy temperature indices predict water stress in sunflower. Agric. Water Manag. 2014, 144, 69–80. [Google Scholar] [CrossRef]
- Dahlgren, R.P.; Vanderbilt, V.C.; Daughtry, C.S.T. Estimates of Leaf Relative Water Content from Optical Polarization Measurements. In Proceedings of the 2017 American Geophysical Union Fall Meeting, New Orleans, LA, USA, 11–15 December 2017; Volume 2017. Abstract A21B-2160. [Google Scholar]
- Swain, S.; Rundquist, D.; Arkebauer, T.; Narumalani, S.; Wardlow, B. Non-invasive estimation of relative water content in soybean leaves using infrared thermography. Isr. J. Plant Sci. 2012, 60, 25–36. [Google Scholar] [CrossRef]
- Pirzad, A.; Shakiba, M.R.; Zehtab-Salmasi, S.; Mohammadi, S.A.; Darvishzadeh, R.; Samadi, A. Effect of water stress on leaf relative water content, chlorophyll, proline and soluble carbohydrates in Matricaria chamomilla L. J. Med. Plants Res. 2011, 5, 2483–2488. [Google Scholar]
- Carroll, D.A.; Hansen, N.C.; Hopkins, B.G.; DeJonge, K.C. Leaf temperature of maize and Crop Water Stress Index with variable irrigation and nitrogen supply. Irrig. Sci. 2017, 35, 549–560. [Google Scholar] [CrossRef]
- Sanchez, R.A.; Hall, A.J.; Trapani, N.; de Hunau, R.C. Effects of water stress on the chlorophyll content, nitrogen level and photosynthesis of leaves of two maize genotypes. Photosynth. Res. 1983, 4, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Chutia, J.; Borah, S.P. Water Stress Effects on Leaf Growth and Chlorophyll Content but Not the Grain Yield in Traditional Rice (Oryza sativa Linn.) Genotypes of Assam, India II. Protein and Proline Status in Seedlings under PEG Induced Water Stress. Am. J. Plant Sci. 2012, 3, 971–980. [Google Scholar] [CrossRef] [Green Version]
- Khayatnezhad, M.; Gholamin, R. The effect of drought stress on leaf chlorophyll content and stress resistance in maize cultivars (Zea mays). Afr. J. Microbiol. Res. 2012, 6, 2844–2848. [Google Scholar] [CrossRef]
- Shahenshah; Isoda, A. Effects of Water Stress on Leaf Temperature and Chlorophyll Fluorescence Parameters in Cotton and Peanut. Plant Prod. Sci. 2010, 13, 269–278. [Google Scholar] [CrossRef]
- Jensen, A.M.; McKee, M.; Chen, Y. Procedures for processing thermal images using low-cost microbolometer cameras for small unmanned aerial systems. In Proceedings of the 2014 IEEE Geoscience and Remote Sensing Symposium, Quebec City, QC, Canada, 13–18 July 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 2629–2632. [Google Scholar]
- Khanal, S.; Fulton, J.; Shearer, S. An overview of current and potential applications of thermal remote sensing in precision agriculture. Comput. Electron. Agric. 2017, 139, 22–32. [Google Scholar] [CrossRef]
- Maes, W.; Huete, A.; Steppe, K. Optimizing the processing of UAV-based thermal imagery. Remote Sens. 2017, 9, 476. [Google Scholar] [CrossRef] [Green Version]
- Aragon, B.; Johansen, K.; Parkes, S.; Malbetéau, Y.; Al-Mashharawi, S.; Al-Amoudi, T.; Andrade, C.F.; Turner, D.; Lucieer, A.; McCabe, M.F. A calibration procedure for field and UAV-based uncooled thermal infrared instruments. Sensors 2020, 20, 3316. [Google Scholar] [CrossRef]
- Stutsel, B.; Johansen, K.; Malbéteau, Y.M.; McCabe, M.F. Detecting Plant Stress Using Thermal and Optical Imagery from an Unoccupied Aerial Vehicle. Front. Plant Sci. 2021, 12, 734944. [Google Scholar] [CrossRef]







| Soil Physical Properties | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depth (cm) | Textural Analysis | Field Capacity (%) | Permanent Wilting Point (%) | Bulk Density (gcm−3) | ||||||||||
| Sand (%) | Clay (%) | Silt (%) | Textural Class | |||||||||||
| 0–30 | 72.80 | 20.00 | 7.20 | Loamy sand | 22.60 ± 0.1 | 10.35 ± 0.1 | 1.47 ± 0.1 | |||||||
| 30–60 | 73.80 | 19.20 | 7.02 | Loamy sand | 24.20 ± 0.1 | 10.30 ± 0.1 | 1.48 ± 0.1 | |||||||
| 60–90 | 69.70 | 19.10 | 11.20 | Loamy sand | 22.10 ± 0.1 | 10.27 ± 0.1 | 1.53 ± 0.1 | |||||||
| Average | 72.10 | 19.43 | 8.47 | Loamy sand | 22.97 ± 0.1 | 10.31 ± 0.1 | 1.49 ± 0.1 | |||||||
| Soil Chemical Properties | ||||||||||||||
| Parameter | pH | O.M (g/kg) | Total N (g/kg) | Total K (g/kg) | Total P (g/kg) | Alkalized N (mg/kg) | Available P (mg/kg) | Available K (mg/kg) | ||||||
| Values | 4.90 | 8.83 | 0.42 | 2.27 | 0.37 | 39.81 | 85.91 | 72.89 | ||||||
| Season | Summer | Autumn |
|---|---|---|
| Air temperature (℃) | 32.5 | 28.0 |
| Relative humidity (%) | 79.3 | 75.0 |
| Vapor pressure deficit (kPa) | 1.5 | 1.5 |
| Season | Irrigation Period (Days) | Irrigation Amount (mm) | |||
|---|---|---|---|---|---|
| 80–100% FC | 70–75% FC | 60–65% FC | 50–55% FC | ||
| Summer | June (9 days) | - | - | - | - |
| July (31 days) | 24.05 | 17.44 | 15.03 | 12.63 | |
| August (31 days) | 57.20 | 41.50 | 35.70 | 30.00 | |
| September (22 days) | 24.39 | 18.60 | 15.60 | 13.70 | |
| Total for summer | 105.64 | 77.54 | 66.33 | 56.33 | |
| Autumn | September (7 days) | 9.40 | 6.00 | 5.80 | 5.94 |
| October (31 days) | 32.80 | 24.04 | 21.20 | 17.96 | |
| November (30 days) | 36.80 | 26.44 | 23.07 | 19.91 | |
| Total for autumn | 79.00 | 56.48 | 50.07 | 43.81 | |
| Total irrigation amount | 184.64 | 134.02 | 116.40 | 100.14 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appiah, S.A.; Li, J.; Lan, Y.; Darko, R.O.; Alordzinu, K.E.; Al Aasmi, A.; Asenso, E.; Issaka, F.; Afful, E.A.; Wang, H.; et al. Real-Time Assessment of Mandarin Crop Water Stress Index. Sensors 2022, 22, 4018. https://doi.org/10.3390/s22114018
Appiah SA, Li J, Lan Y, Darko RO, Alordzinu KE, Al Aasmi A, Asenso E, Issaka F, Afful EA, Wang H, et al. Real-Time Assessment of Mandarin Crop Water Stress Index. Sensors. 2022; 22(11):4018. https://doi.org/10.3390/s22114018
Chicago/Turabian StyleAppiah, Sadick Amoakohene, Jiuhao Li, Yubin Lan, Ransford Opoku Darko, Kelvin Edom Alordzinu, Alaa Al Aasmi, Evans Asenso, Fuseini Issaka, Ebenezer Acheampong Afful, Hao Wang, and et al. 2022. "Real-Time Assessment of Mandarin Crop Water Stress Index" Sensors 22, no. 11: 4018. https://doi.org/10.3390/s22114018
APA StyleAppiah, S. A., Li, J., Lan, Y., Darko, R. O., Alordzinu, K. E., Al Aasmi, A., Asenso, E., Issaka, F., Afful, E. A., Wang, H., & Qiao, S. (2022). Real-Time Assessment of Mandarin Crop Water Stress Index. Sensors, 22(11), 4018. https://doi.org/10.3390/s22114018







