Polyvinyl Chloride Modified Carbon Paste Electrodes for Sensitive Determination of Levofloxacin Drug in Serum, Urine, and Pharmaceutical Formulations
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Solutions
2.2. Preparation of the LF–TPB Ion-Pair
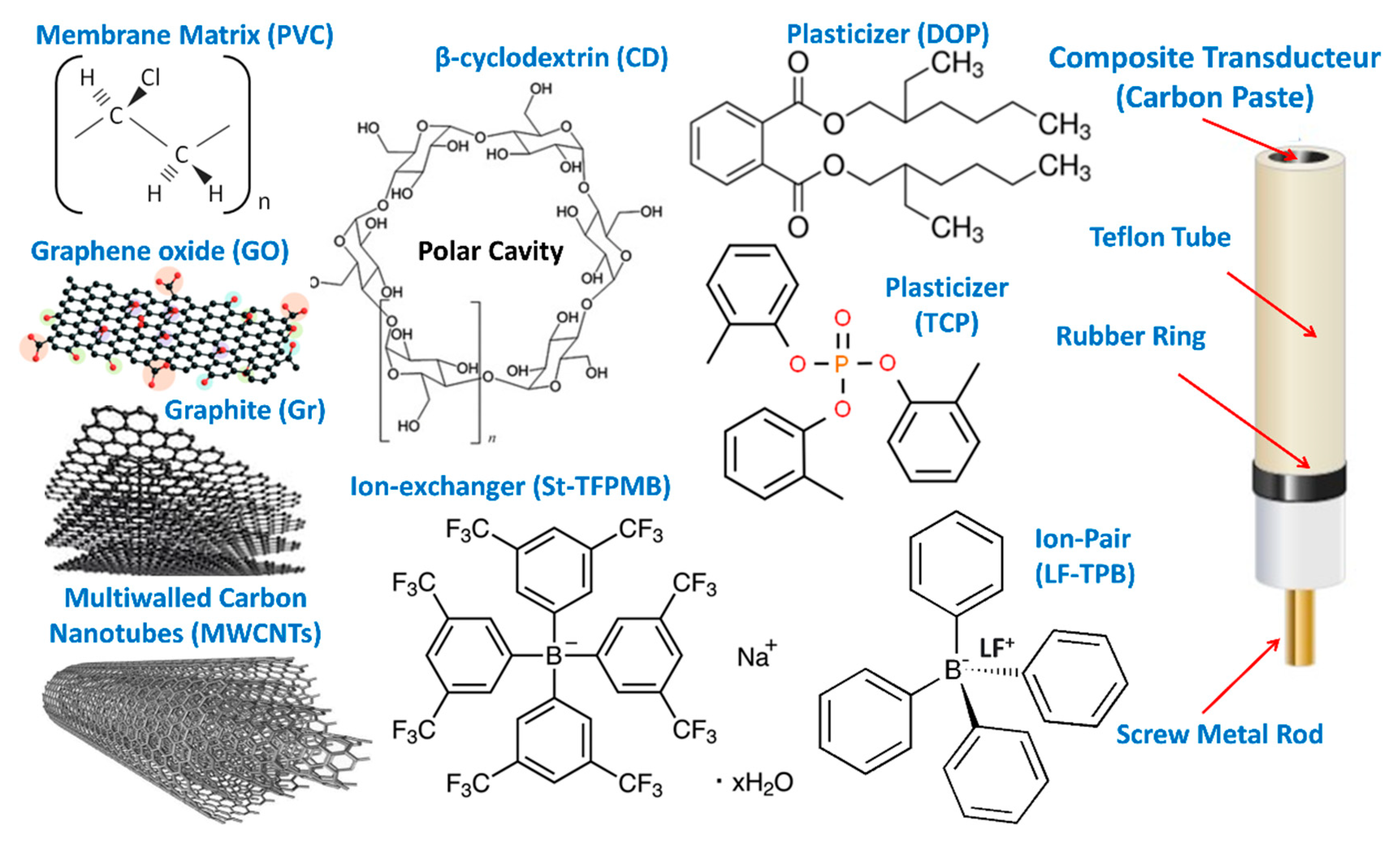
2.3. Preparation of the PVC Membrane Electrodes
2.4. Preparation of the Carbon Paste Electrodes
2.5. Preparation of the PVC Coated Carbon Paste Electrodes
2.6. Preparation of the PVC Plasticized Carbon Paste Electrodes
2.7. Electrochemical Measurements
2.8. Morf Test
2.9. Effect of pH and Selectivity
2.10. Practical Evaluation of the Prepared Electrodes
2.11. Statistical Analysis
3. Results and Discussion
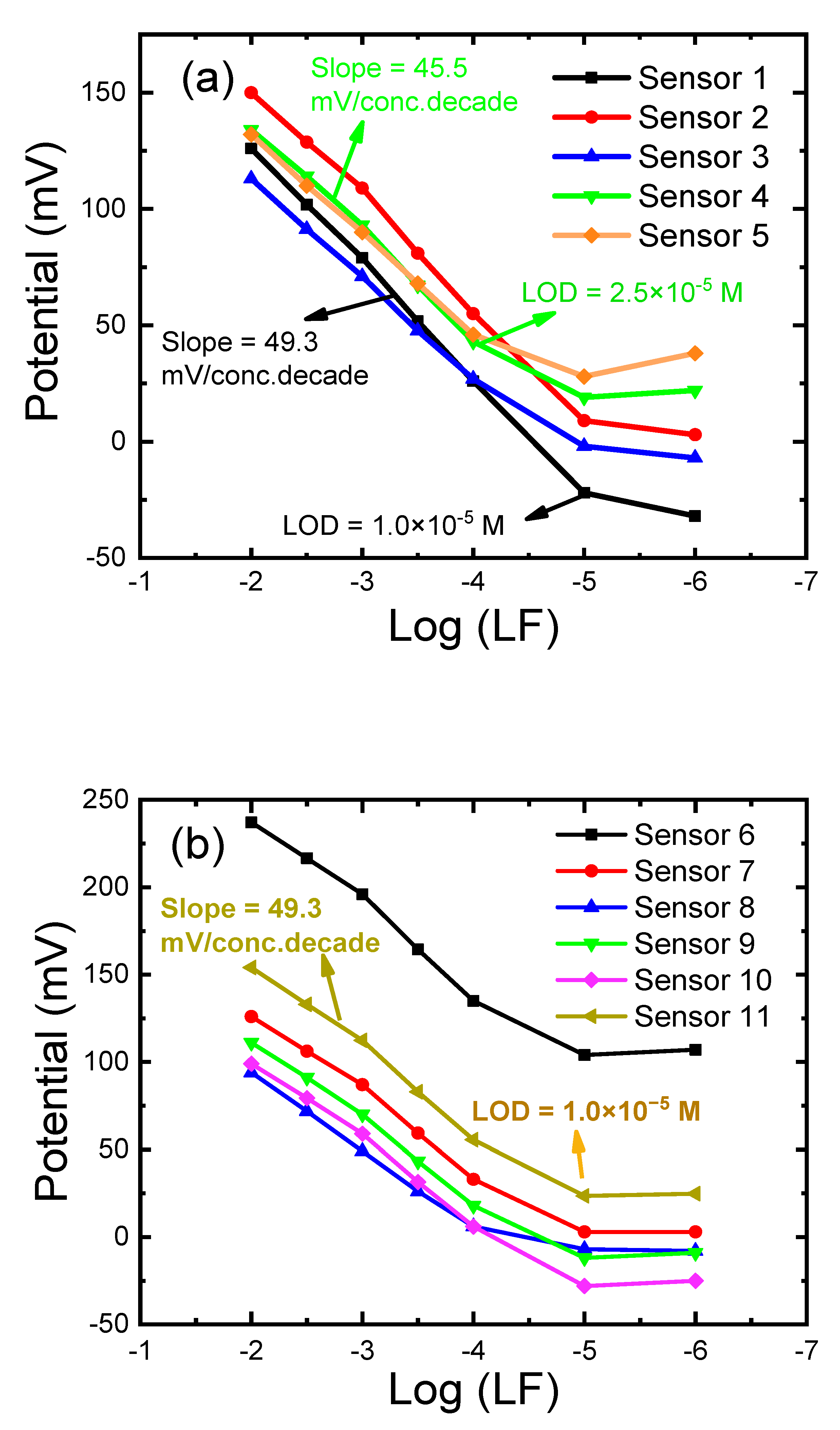
3.1. Optimization of the PVC Membrane Layer
3.2. Effect of the Carbon Paste Composition
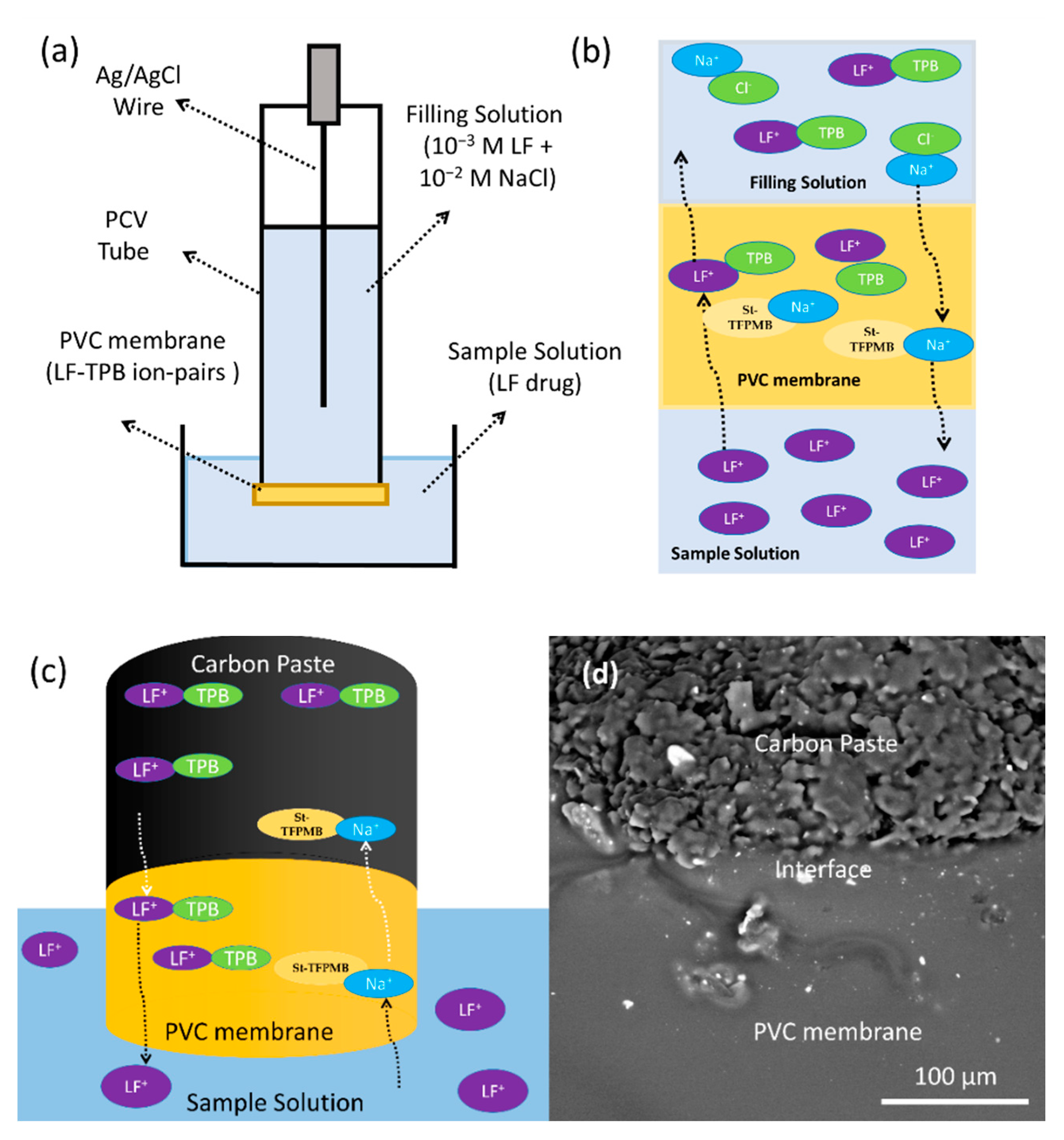
3.3. Effect of the PVC Modification on the CPEs Performance
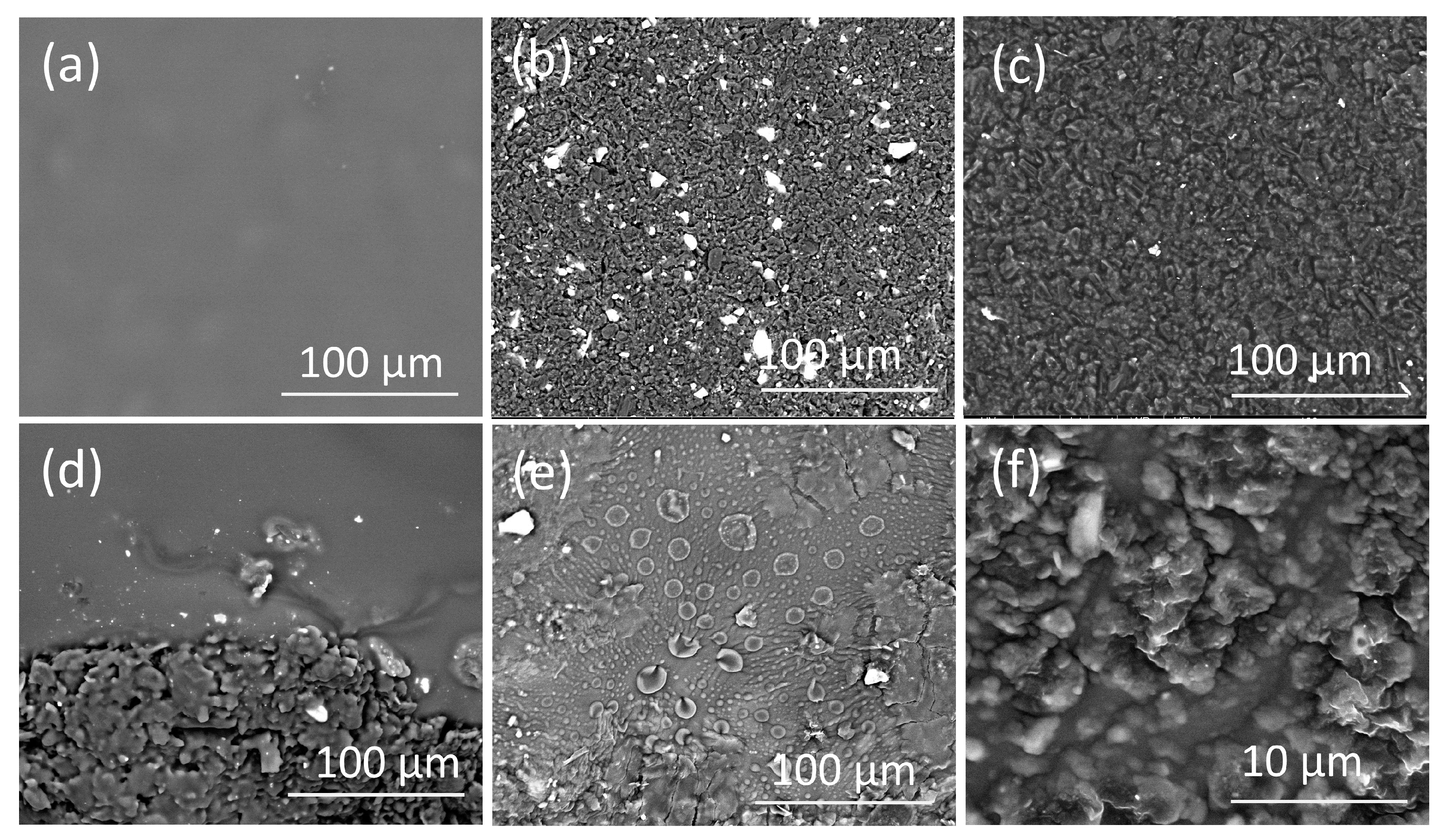
3.4. Surface Morphology of the PVC Modified CPEs
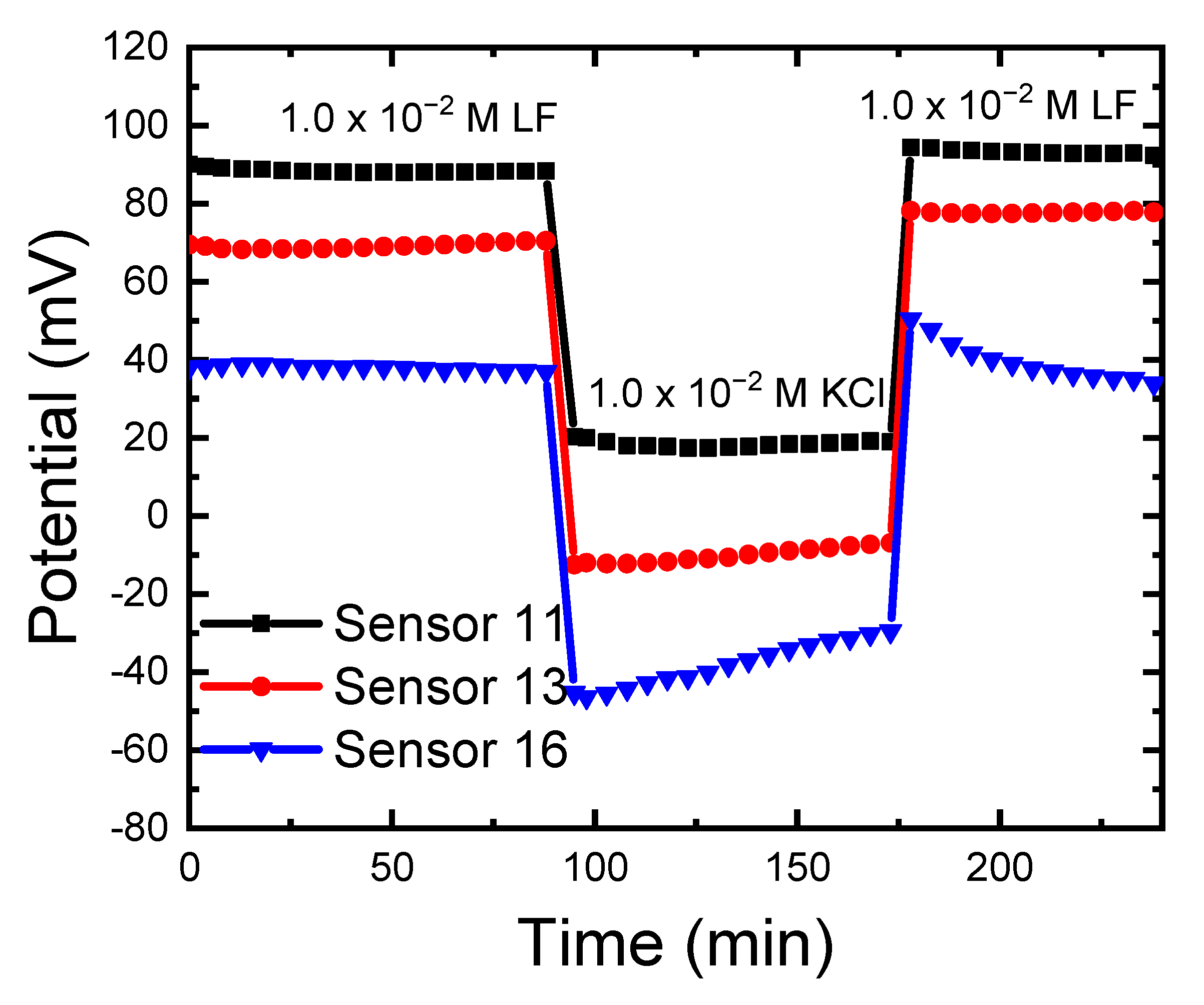
3.5. Morf Test to Study Water Penetration for the Different Sensors
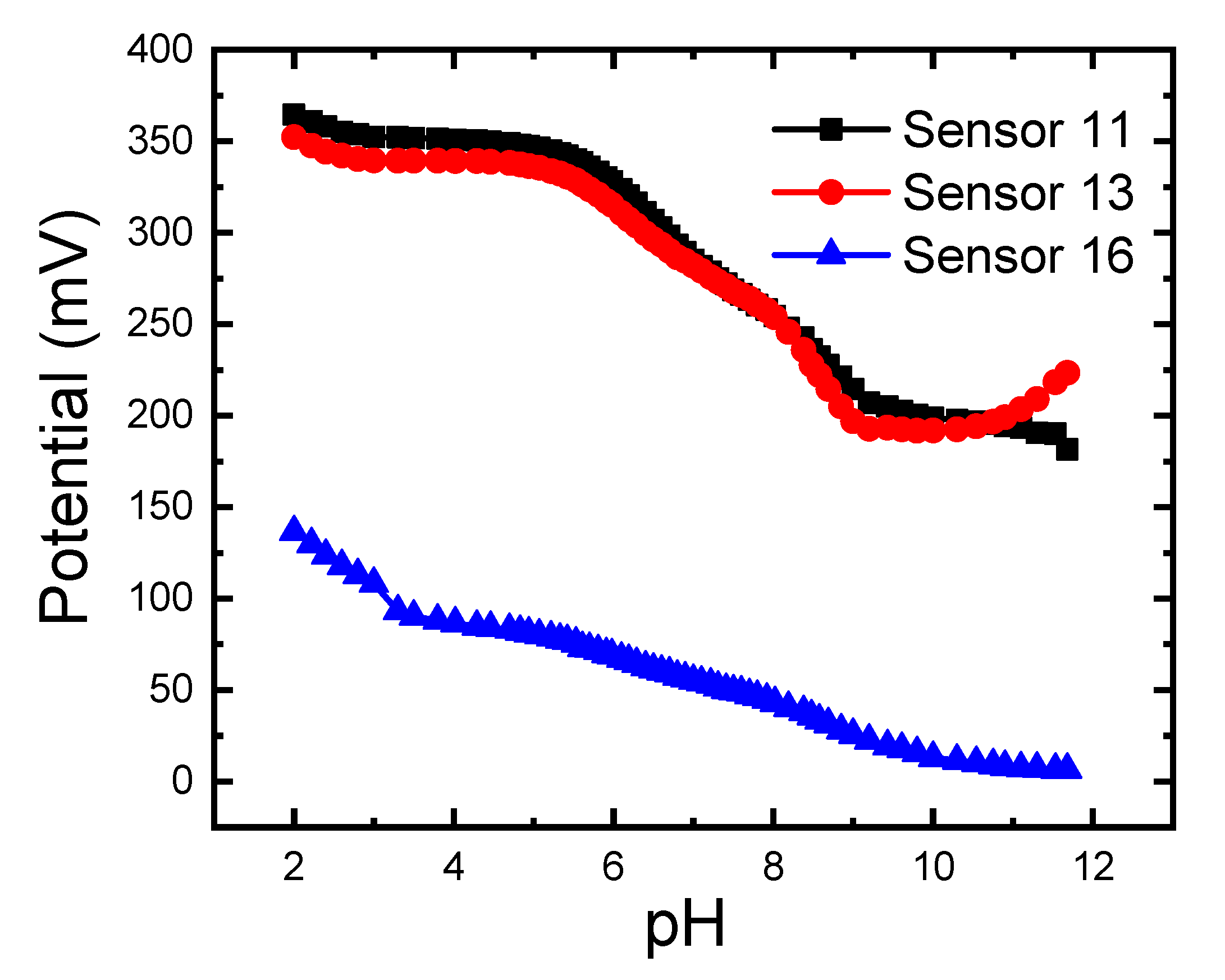
3.6. Effect of pH
3.7. Selectivity and Interference Study
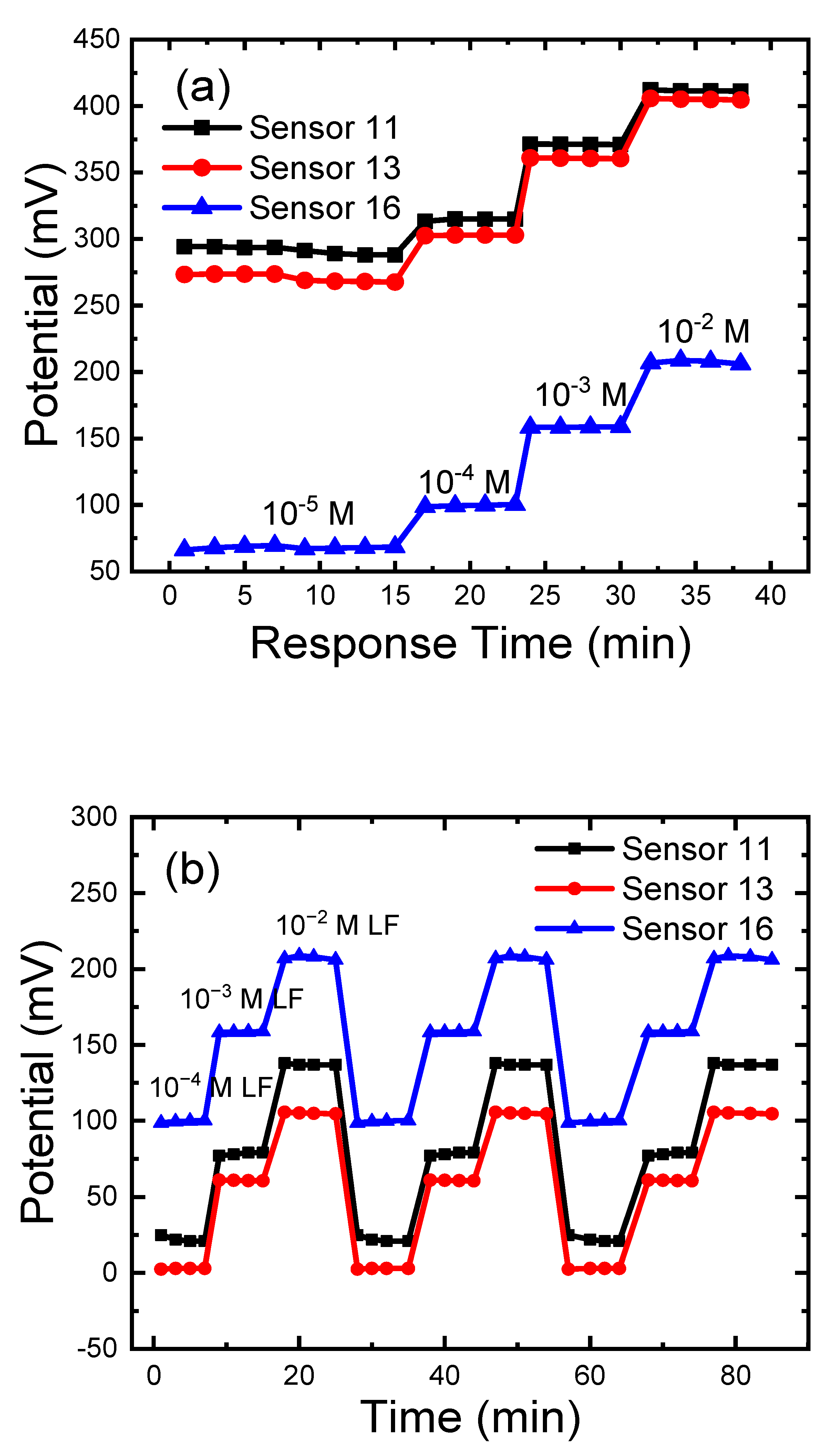
3.8. Response, Lifetime, and Reversibility
3.9. Analytical Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakker, E.; Bühlmann, P.; Pretsch, E. Carrier-based Ion-selective electrodes and bulk optodes. 1. General characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef] [PubMed]
- Švancara, I.; Vytřas, K.; Kalcher, K.; Walcarius, A.; Wang, J. Carbon paste electrodes in facts, numbers, and notes: A review on the occasion of the 50-years jubilee of carbon paste in electrochemistry and electroanalysis. Electroanalysis 2009, 21, 7–28. [Google Scholar] [CrossRef]
- Zima, J.; Švancara, I.; Barek, J.; Vytřas, K. Recent advances in electroanalysis of organic compounds at carbon paste electrodes. Crit. Rev. Anal. Chem. 2009, 39, 204–227. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Salah, A.; Rizk, M.S.; Moustafa, H.; Bechelany, M.; Barhoum, A. Carbon-based nanosensors for salicylate determination in pharmaceutical preparations. Electroanalysis 2019, 31, 778–789. [Google Scholar] [CrossRef]
- El Nashar, R.M.; Ghani, N.T.A.; El Gohary, N.; Barhoum, A.; Madbouly, A. Molecularly imprinted polymers based biomimetic sensors for mosapride citrate detection in biological fluids. Mater. Sci. Eng. C 2017, 76, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Silvester, D.S. Recent advances in the use of ionic liquids for electrochemical sensing. Analyst 2011, 136, 4871–4882. [Google Scholar] [CrossRef] [PubMed]
- Alaviuhkola, T.; Bobacka, J.; Nissinen, M.; Rissanen, K.; Ivaska, A.; Pursiainen, J. Synthesis, characterization, and complexation of tetraarylborates with aromatic cations and their use in chemical sensors. Chem. A Eur. J. 2005, 11, 2071–2080. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Gamal, E.; Rizk, M.S.; El Nashar, R.M.; Anis, B.; Elnabawy, H.M.; Khalil, A.S.; Barhoum, A. t-Butyl calixarene/Fe2O3@MWCNTs composite-based potentiometric sensor for determination of ivabradine hydrochloride in pharmaceutical formulations. Mater. Sci. Eng. C 2020, 116, 111110. [Google Scholar] [CrossRef]
- Sawy, A.M.; Barhoumbb, A.; Gaber, S.A.A.; El-Hallouty, S.M.; Shousha, W.G.; Maarouf, A.A.; Khalilaf, A.S. Insights of doxorubicin loaded graphene quantum dots: Synthesis, DFT drug interactions, and cytotoxicity. Mater. Sci. Eng. C 2021, 122, 111921. [Google Scholar] [CrossRef]
- Barhoum, A.; Shalan, A.E.; El-Hout, S.I.; Ali, G.A.M.; Abdelbasir, S.M.; Abu Serea, E.S.; Ibrahim, A.H.; Pal, K. A Broad Family of Carbon Nanomaterials: Classification, Properties, Synthesis, and Emerging Applications; Metzler, J.B., Ed.; Springer: New York, NY, USA, 2019; pp. 1–40. [Google Scholar]
- Barhoum, A.; Samyn, P.; Öhlund, T.; Dufresne, A. Review of recent research on flexible multifunctional nanopapers. Nanoscale 2017, 9, 15181–15205. [Google Scholar] [CrossRef]
- Zhang, K.; Barhoum, A.; Xiaoqing, C.; Li, H.; Samyn, P. Cellulose Nanofibers: Fabrication and Surface Functionalization Techniques. In Handbook of Nanofibers; Springer International Publishing: Midtown Manhattan, NY, USA, 2019; pp. 409–449. [Google Scholar] [CrossRef]
- Croom, K.F.; Goa, K.L. Levofloxacin: A review of its use in the treatment of bacterial infections in the United States. Drugs 2003, 63, 2769–2802. [Google Scholar] [CrossRef] [PubMed]
- Garrison, M.W. Comparative antimicrobial activity of levofloxacin and ciprofloxacin against Streptococcus pneumoniae. J. Antimicrob. Chemother. 2003, 52, 503–506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aguilar-Carrasco, J.C.; Hernández-Pineda, J.; Jimenez-Andrade, J.M.; Flores-Murrieta, F.J.; Carrasco-Portugal, M.D.C.; López-Canales, J.S. Rapid and sensitive determination of levofloxacin in microsamples of human plasma by high-performance liquid chromatography and its application in a pharmacokinetic study. Biomed. Chromatogr. 2014, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Malz, F.; Jancke, H. Validation of quantitative NMR. J. Pharm. Biomed. Anal. 2005, 38, 813–823. [Google Scholar] [CrossRef]
- Locatelli, M.; Ciavarella, M.T.; Paolino, D.; Celia, C.; Fiscarelli, E.; Ricciotti, G.; Pompilio, A.; Di Bonaventura, G.; Grande, R.; Zengin, G.; et al. Determination of ciprofloxacin and levofloxacin in human sputum collected from cystic fibrosis patients using microextraction by packed sorbent-high performance liquid chromatography photodiode array detector. J. Chromatogr. A 2015, 1419, 58–66. [Google Scholar] [CrossRef]
- Szerkus, O.; Jacyna, J.; Gibas, A.; Sieczkowski, M.; Siluk, D.; Matuszewski, M.; Kaliszan, R.; Markuszewski, M. Robust HPLC–MS/MS method for levofloxacin and ciprofloxacin determination in human prostate tissue. J. Pharm. Biomed. Anal. 2017, 132, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Szerkus, O.; Jacyna, J.; Wiczling, P.; Gibas, A.; Sieczkowski, M.; Siluk, D.; Matuszewski, M.; Kaliszan, R.; Markuszewski, M. Ultra-high performance liquid chromatographic determination of levofloxacin in human plasma and prostate tissue with use of experimental design optimization procedures. J. Chromatogr. B 2016, 1029–1030, 48–59. [Google Scholar] [CrossRef]
- Van Toi, P.; Pouplin, T.; Tho, N.D.K.; Phuong, P.N.; Chau, T.T.H.; Thuong, N.T.T.; Heemskerk, D.; Hien, T.T.; Thwaites, G.E. High-performance liquid chromatography with time-programmed fluorescence detection for the quantification of Levofloxacin in human plasma and cerebrospinal fluid in adults with tuberculous meningitis. J. Chromatogr. B 2017, 1061–1062, 256–262. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Bair, M.-J.; Hu, C.-C. Determination of levofloxacin in human urine with capillary electrophoresis and fluorescence detector. J. Chin. Chem. Soc. 2007, 54, 991–995. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Cao, J.-T.; Tian, W.; Zheng, Y.-L. Determination of levofloxacin and norfloxacin by capillary electrophoresis with electrochemiluminescence detection and applications in human urine. Electrophoresis 2008, 29, 3207–3212. [Google Scholar] [CrossRef]
- Maleque, M.; Hasan, R.; Hossen, F.; Safi, S. Development and validation of a simple UV spectrophotometric method for the determination of levofloxacin both in bulk and marketed dosage formulations. J. Pharm. Anal. 2012, 2, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Pravallika, K.E.; Bhavya, M.; Ravi, P.; Hemavathi, K.; Lalitha Kumari, D. Quantitative determination of levofloxacin hemihydrate in bulk and tablets by UV-spectrophotometry and first order derivative methods. Asian J. Pharm. Anal. Med. Chem. 2014, 2, 176–182. [Google Scholar]
- Al-Momani, I.F. Flow injection spectrophotometric determination of the antibacterial levofloxacin in tablets and human urine. Anal. Lett. 2006, 39, 741–750. [Google Scholar] [CrossRef]
- Altiokka, G.; Atkosar, Z.; Can, N. The determination of levofloxacin by flow injection analysis using UV detection, potentiometry, and conductometry in pharmaceutical preparations. J. Pharm. Biomed. Anal. 2002, 30, 881–885. [Google Scholar] [CrossRef]
- Salem, A.A.; Mossa, H.A. Method validation and determinations of levofloxacin, metronidazole and sulfamethoxazole in an aqueous pharmaceutical, urine and blood plasma samples using quantitative nuclear magnetic resonance spectrometry. Talanta 2012, 88, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Shanin, I.A.; Thuy, N.T.D.; Eremin, S.A. Determination of levofloxacin (the Levorotatory Stereoisomer of Ofloxacin) in milk by an indirect enzyme-linked immunosorbent assay. Mosc. Univ. Chem. Bull. 2014, 69, 136–141. [Google Scholar] [CrossRef]
- Gaber, M.; Abu Shawish, H.M.; Khedr, A.M.; Abed-Almonem, K.I. Determination of benzalkonium chloride preservative in pharmaceutical formulation of eye and ear drops using new potentiometric sensors. Mater. Sci. Eng. C 2012, 32, 2299–2305. [Google Scholar] [CrossRef]
- Rizka, N.M.H.; EL-Sayedb, F.A.; El-Sawadib, F.A. Determination of levofloxacin and lomefloxacin—Egyptian Society of Analytical Chemistry (EGSAC). Egypt J. Anal. Chem. 2012, 21, 81–92. [Google Scholar]
- Ghani, N.E.T.A.; Abdel-Haleem, F.M.; Mahmoud, S.; El Nashar, R.M. Electrochemical detection of the different species of levofloxacin using PVC, carbon paste and screen-printed electrodes: Effect of pH. J. Anal. Test. 2018, 2, 175–183. [Google Scholar] [CrossRef]
- Kłosińska-Szmurło, E.; Grudzień, M.; Betlejewska-Kielak, K.; Pluciński, F.; Biernacka, J.; Mazurek, A.P. Physicochemical properties of lomefloxacin, levofloxacin, and moxifloxacin relevant to the biopharmaceutics classification system. Acta Chim. Slov. 2014, 61, 827–834. [Google Scholar]
- Babić, S.; Horvat, A.J.; Pavlović, D.M.; Kaštelan-Macan, M. Determination of pKa values of active pharmaceutical ingredients. TrAC Trends Anal. Chem. 2007, 26, 1043–1061. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Shehab, O.R. Comparative study of carbon paste, screen printed, and PVC potentiometric sensors based on copper-sulphamethazine schiff base complex for determination of iodide—Experimental and theoretical approaches. Electroanalysis 2015, 28, 800–807. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.; Madbouly, A.; El Nashar, R.; Abdel-Ghani, N. Molecularly imprinted polymer-based bulk optode for the determination of itopride hydrochloride in physiological fluids. Biosens. Bioelectron. 2016, 85, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Kim, K.K.; Benayad, A.; Yoon, S.; Park, H.K.; Jung, I.; Jin, M.H.; Jeong, H.; Kim, J.M.; Choi, J.; et al. Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Badr, I.H.A.; Rizk, M.S. Potentiometric anion selectivity and analytical applications of polymer membrane electrodes based on novel Mn(III)- and Mn(IV)-salophen complexes. Electroanalysis 2016, 28, 2922–2929. [Google Scholar] [CrossRef]
- Kormosh, Z.; Hunka, I.; Bazel, Y.; Matviychuk, O. Potentiometric determination of ketoprofen and piroxicam at a new PVC electrode based on ion associates of Rhodamine 6G. Mater. Sci. Eng. C 2010, 30, 997–1002. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Rizk, M.S. Highly selective thiocyanate optochemical sensor based on manganese(III)-salophen ionophore. Mater. Sci. Eng. C 2017, 75, 682–687. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Saad, M.; Rizk, M.S. Development of new potentiometric sensors for the determination of proguanil hydrochloride in serum and urine. Chin. Chem. Lett. 2016, 27, 857–863. [Google Scholar] [CrossRef]
- El-Beshlawy, M.M.; Abdel-Haleem, F.M.; Barhoum, A. Molecularly imprinted potentiometric sensor for nanomolar determination of pioglitazone hydrochloride in pharmaceutical formulations. Electroanalysis 2021, 202060141. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Gamal, E.; Rizk, M.S.; Madbouly, A.; El Nashar, R.M.; Anis, B.; Elnabawy, H.M.; Khalil, A.S.G.; Barhoum, A. Molecularly imprinted electrochemical sensor-based Fe2O3@MWCNTs for ivabradine drug determination in pharmaceutical formulation, serum, and urine samples. Front. Bioeng. Biotechnol. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Ali, T.A.; Mohamed, G.G.; Al-Sabagh, A.; Migahed, M. A New screen-printed ion selective electrode for determination of citalopram hydrobromide in pharmaceutical formulation. Chin. J. Anal. Chem. 2014, 42, 565–572. [Google Scholar] [CrossRef]
- Fibbioli, M.; Morf, W.E.; Badertscher, M.; De Rooij, N.F.; Pretsch, E. Potential drifts of solid-contacted ion-selective electrodes due to zero-current ion fluxes through the sensor membrane. Electroanalysis 2000, 1286–1292. [Google Scholar] [CrossRef]
- Yuan, D.; Anthis, A.H.C.; Afshar, M.G.; Pankratova, N.; Cuartero, M.; Crespo, G.A.; Bakker, E. All-solid-state potentiometric sensors with a multiwalled carbon nanotube inner transducing layer for anion detection in environmental samples. Anal. Chem. 2015, 87, 8640–8645. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.; Abdel-Haleem, F. Plastic membrane electrodes for the determination of flavoxate hydrochloride and cyclopentolate hydrochloride. Electrochim. Acta 2010, 55, 5592–5597. [Google Scholar] [CrossRef]
- Abu Shawish, H.M.; Almonem, K.I.A.; Saadeh, S.M.; Al-Lham, W.S. Determination of haloperidol drug in ampoules and in urine samples using a potentiometric modified carbon paste electrode. Meas. J. Int. Meas. Confed. 2016, 78, 180–186. [Google Scholar] [CrossRef]
- Umezawa, Y.; Bühlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric selectivity coefficients of ion-selective electrodes. Part I. inorganic cations (technical report). Pure Appl. Chem. 2000, 72, 1851–2082. [Google Scholar] [CrossRef]
- Shao, Y.; Ying, Y.; Ping, J. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Saad, M.; Barhoum, A.; Bechelany, M.; Rizk, M.S. PVC membrane, coated-wire, and carbon-paste ion-selective electrodes for potentiometric determination of galantamine hydrobromide in physiological fluids. Mater. Sci. Eng. C 2018, 89, 140–148. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Petrisor, C.; Aboul-Enein, H.Y. Evaluation of Sensor Response Characteristics. Instrum. Sci. Technol. 1998, 26, 353–362. [Google Scholar] [CrossRef]
- Ozoemena, K.I.; Stefan, R.-I.; Van Staden, J.F.; Aboul-Enein, H.Y. Enantioanalysis of S-perindopril using different cyclodextrin-based potentiometric sensors. Sens. Actuators B Chem. 2005, 105, 425–429. [Google Scholar] [CrossRef]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical methods for the analysis of clinically relevant biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef] [PubMed]
- Baptista, F.R.; Belhout, S.A.; Giordani, S.; Quinn, S.J. Recent developments in carbon nanomaterial sensors. Chem. Soc. Rev. 2015, 44, 4433–4453. [Google Scholar] [CrossRef] [PubMed]
- Albert, E.L.; Abdullah, C.A.C.; Shiroshaki, Y. Synthesis and characterization of graphene oxide functionalized with magnetic nanoparticle via simple emulsion method. Results Phys. 2018, 11, 944–950. [Google Scholar] [CrossRef]
- Sutter, J.; Radu, A.; Peper, S.; Bakker, E.; Pretsch, E. Solid-contact polymeric membrane electrodes with detection limits in the subnanomolar range. Anal. Chim. Acta 2004, 523, 53–59. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Rizk, M.S.; Badr, I.H.A. Potentiometric determination of ciprofloxacin in physiological fluids using carbon paste and nano-composite carbon paste electrodes. Electroanalysis 2017, 29, 1172–1179. [Google Scholar] [CrossRef]
- Psomas, G. Mononuclear metal complexes with ciprofloxacin: Synthesis, characterization and DNA-binding properties. J. Inorg. Biochem. 2008, 102, 1798–1811. [Google Scholar] [CrossRef] [PubMed]
- Rkik, M.; Ben Brahim, M.; Samet, Y. Electrochemical determination of levofloxacin antibiotic in biological samples using boron doped diamond electrode. J. Electroanal. Chem. 2017, 794, 175–181. [Google Scholar] [CrossRef]
- Radi, A. Determination of levofloxacin in human urine by adsorptive square-wave anodic stripping voltammetry on a glassy carbon electrode. Talanta 2002, 58, 319–324. [Google Scholar] [CrossRef]
- Tang, L.; Tong, Y.; Zheng, R.; Liu, W.; Gu, Y.; Li, C.; Chen, R.; Zhang, Z. Ag nanoparticles and electrospun CeO2-Au composite nanofibers modified glassy carbon electrode for determination of levofloxacin. Sens. Actuators B Chem. 2014, 203, 95–101. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, L.; Zhang, J. Electrochemical sensor for levofloxacin based on molecularly imprinted polypyrrole–graphene-gold nanoparticles modified electrode. Sens. Actuators B Chem. 2014, 192, 642–647. [Google Scholar] [CrossRef]
| Sensor | Percent Compositions (wt/wt%) (wt/wt)% | Response Characteristics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IP | PVCM | Gr | Additive | Plasticizer | C.R. (M) | LOD (M) | Slope | RSD (%) | |
| 1 | - | 33.0 | - | 1.0 St-TFPMB | 66.0 TCP | 10−2–10−5 | 1.0 × 10−5 | 50.0 | 0.43 |
| 2 | - | 33.0 | - | 1.0 St-TFPMB | 66.0 DOP | 10−2–10−5 | 1.0 × 10−5 | 47.5 | 2.11 |
| 3 | 5.0 | 31.7 | - | - | 63.3 DOP | 10−2–10−4 | 2.1 × 10−5 | 43.0 | 3.95 |
| 4 | 5.0 | 31.3 | - | 1.0 St-TFPMB | 62.7 DOP | 10−2–10−4 | 2.5 × 10−5 | 45.5 | 2.26 |
| 5 | 5.0 | 26.0 | - | 1.0 St-TFPMB + 1.0 CD | 67.0 DOP | 10−2–10−4 | 7.8 × 10−5 | 43.0 | 2.33 |
| 6 | - | - | 49.5 | 1.0 St-TFPMB | 49.5 TCP | 10−2–10−5 | 1.0 × 10−5 | 51.5 | 3.7 |
| 7 | 5.0 | - | 47.0 | 1.0 St-TFPMB | 47.0 TCP | 10−2–10−4 | 1.0 × 10−5 | 46.5 | 0.7 |
| 8 | 5.0 | - | 47.0 | 1.0 St-TFPMB | 47.0 DOP | 10−2–10−5 | 8.4 × 10−5 | 44.0 | 4.1 |
| 9 | 5.0 | - | 44.0 | 1.0 St-TFPMB + 3.0 MWCNTs | 47.0 TCP | 10−2–10−5 | 1.0 × 10−5 | 46.5 | 1.5 |
| 10 | 5.0 | - | 44.0 | 1.0 St-TFPMB + 3.0 rGO | 47.0 TCP | 10−2–10−5 | 1.0 × 10−5 | 47.7 | 3.7 |
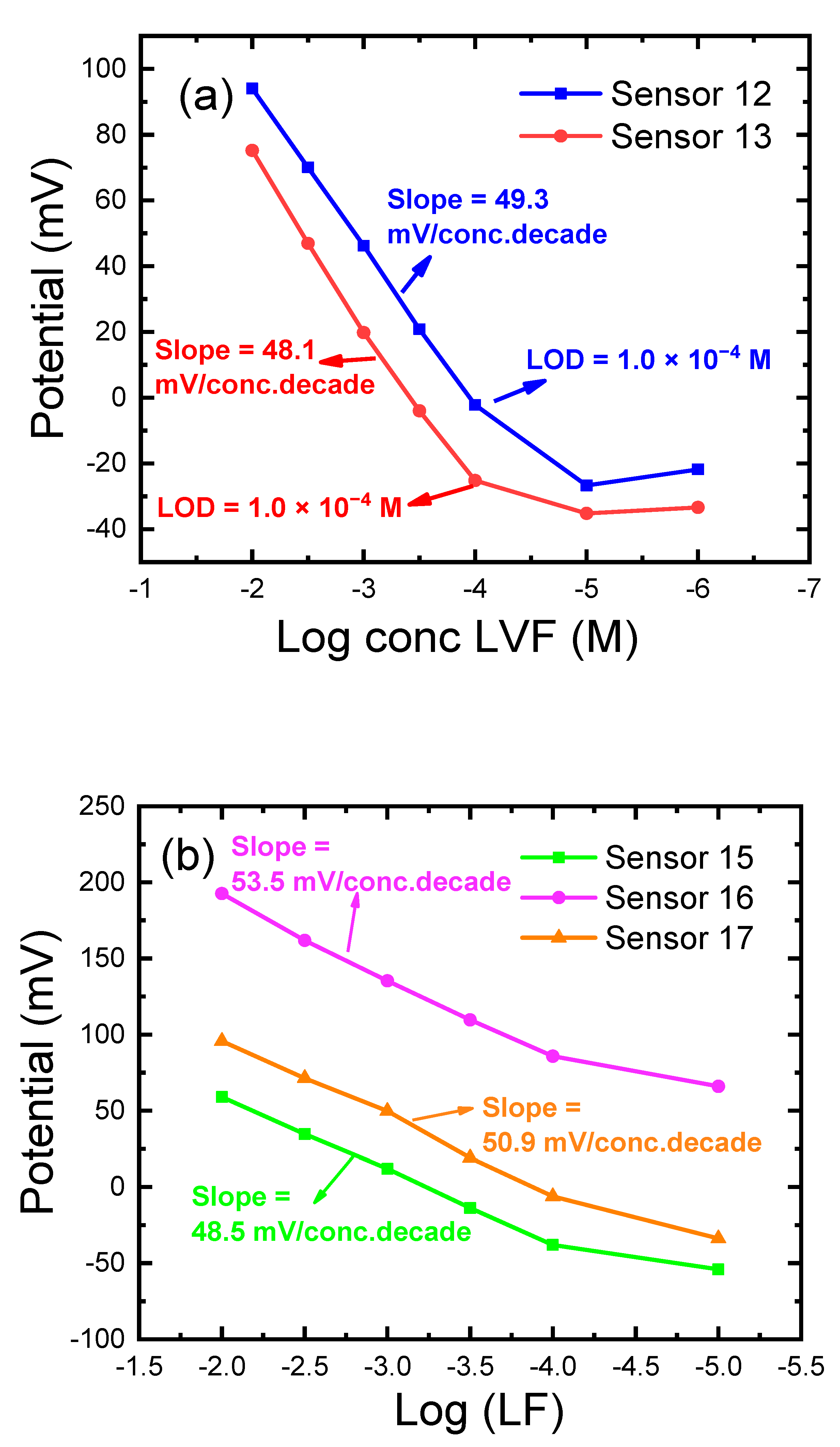
| 11 | 5.0 | - | 44.0 | 1.0 St-TFPMB + 3.0 GO | 47.0 TCP | 10−2–10−5 | 1.0 × 10−5 | 49.3 | 4.8 |
| 12 | 5.0 | Coated* | 44.0 | 1.0 St-TFPMB + 3.0 MWCNTs | 47.0 TCP | 10−2–10−4 | 1.0 × 10−4 | 48.1 | 0 |
| 13 | 5.0 | Coated* | 44.0 | 1.0 St-TFPMB + 3.0 GO | 47.0 TCP | 10−2–10−4 | 1.0 × 10−4 | 50.2 | 4.2 |
| 14 | - | - | 30.3 | 1.0 St-TFPMB | 30.0 TCP + 38.7 PVCP | 10−2–10−4 | 1.0 × 10−5 | 43.3 | 0 |
| 15 | 5.0 | - | 27.8 | 1.0 St-TFPMB | 30.0 TCP + 36.2 PVCP | 10−2–10−4 | 5.6 × 10−5 | 48.5 | 6.8 |
| 16 | 5.0 | - | 27.0 | 1.0 St-TFPMB + 1.0 CD | 30.0 TCP + 36.0 PVCP | 10−2–10−4 | 6.3 × 10−5 | 53.5 | 4.4 |
| 17 | 10.0 | - | 24.5 | 1.0 St-TFPMB + 1.0 CD | 30.0 TCP + 33.5 PVCP | 10−2–10−4 | 2.8 × 10−5 | 50.9 | 4.5 |
| Sensors | pH | Slope | Detection Limit | Linear Dynamic Range | RSD |
|---|---|---|---|---|---|
| mV/conc. Decade | M | M | % | ||
| CPE (Sesnor 11) | 2.2 | 35.4 | 3.16 × 10−5 | 10−2–10−5 | 0.2 |
| 4.1 | 49.3 | 1.0 × 10−5 | 10−2–10−4 | 4.8 | |
| C-CPE (Sesnor 13) | 2.2 | 41.8 | 2.5 × 10−5 | 10−2–10−4 | 5.14 |
| 4.1 | 50.2 | 1.0 × 10−4 | 10−2–10−4 | 4.2 | |
| P-CPE (Sesnor 16) | 2.2 | 32.0 | 3.98 × 10−5 | 10−2–10−4 | 4.47 |
| 4.1 | 53.5 | 6.3 × 10−5 | 10−2–10−4 | 4.4 |
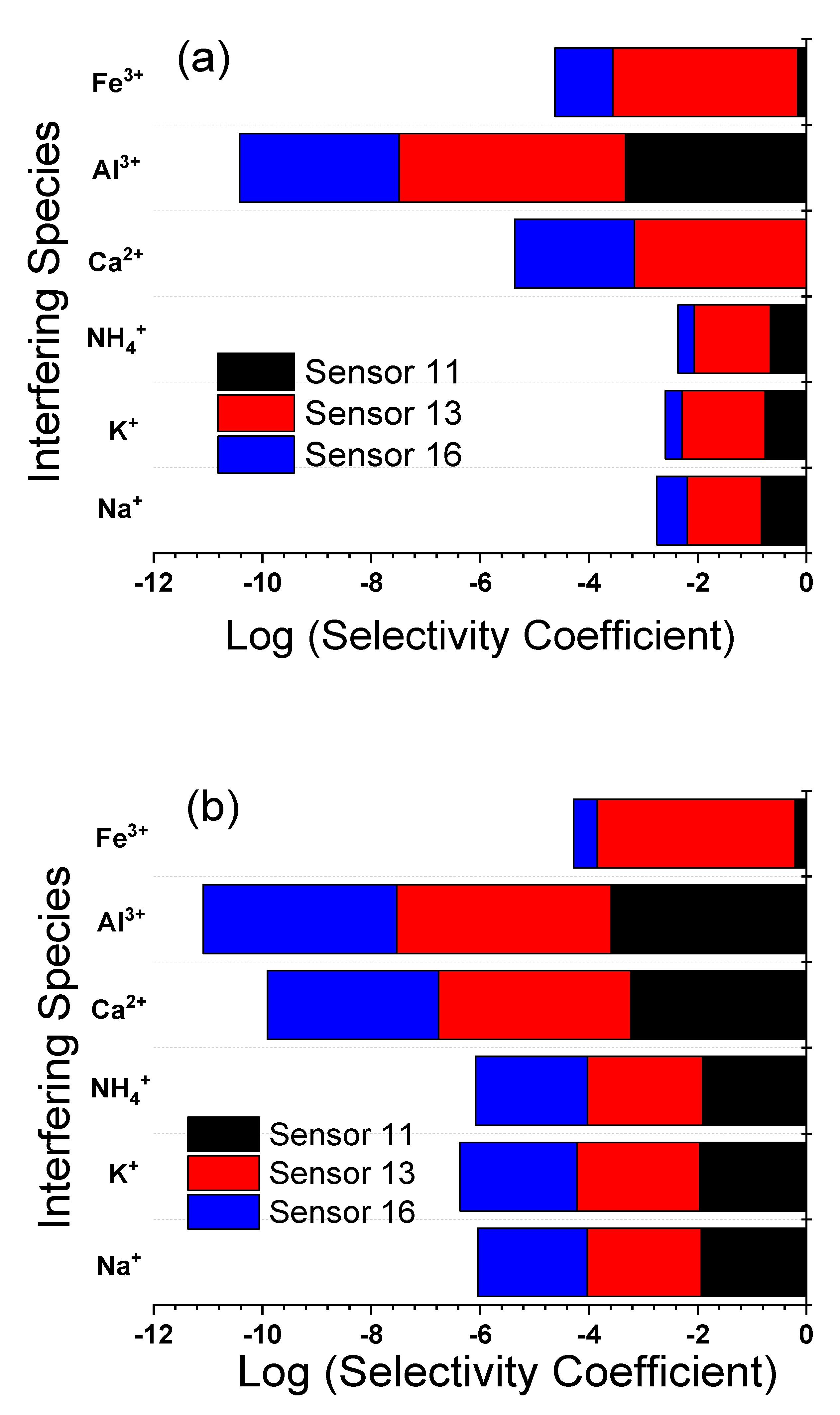
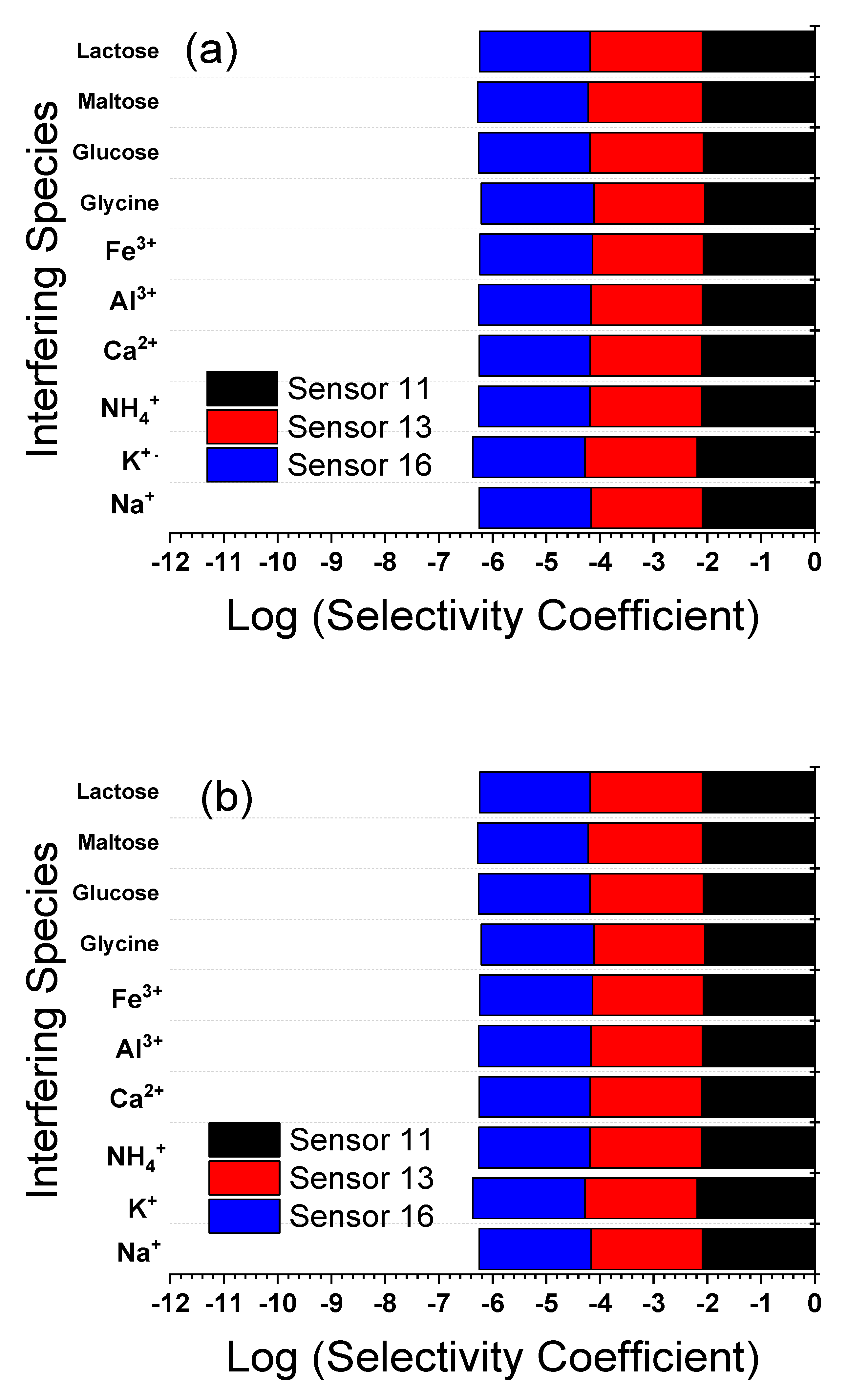
| Interferent | CPE (Sensor 11) | C-CPE (Sensor 13) | P-CPE (Sensor 16) | |||
|---|---|---|---|---|---|---|
| SSM | MPM | SSM | MPM | SSM | MPM | |
| Na+ | −0.83 | −2.09 | −1.36 | −2.07 | −0.56 | −2.09 |
| K+ | −0.76 | −2.19 | −1.53 | −2.09 | −0.30 | −2.09 |
| NH4+ | −0.66 | −2.10 | −1.40 | −2.09 | −0.30 | −2.07 |
| Ca2+ | −−2.46 | −2.10 | −3.16 | −2.08 | −2.2 | −2.07 |
| Al3+ | −3.33 | −2.09 | −4.16 | −2.08 | −2.93 | −2.09 |
| Fe3+ | −0.16 | −2.07 | −3.40 | −2.07 | −1.06 | −2.10 |
| Glycine | - | −2.05 | - | −2.06 | - | −2.10 |
| Glucose | - | −2.07 | - | −2.12 | - | −2.07 |
| Maltose | - | −2.09 | - | −2.13 | - | −2.06 |
| Lactose | - | −2.09 | - | −2.09 | - | −2.06 |
| Interferent | CPE (Sensor 11) | C-CPE (Sensor 13) | P-CPE (Sensor 16) | |||
|---|---|---|---|---|---|---|
| SSM | MPM | SSM | MPM | SSM | MPM | |
| Na+ | −1.93 | −2.09 | −2.10 | −2.07 | −2.01 | −2.09 |
| K+ | −1.96 | −2.19 | −2.26 | −2.09 | −2.15 | −2.09 |
| NH4+ | −1.91 | −2.10 | −2.11 | −2.09 | −2.06 | −2.07 |
| Ca2+ | −3.23 | −2.10 | −3.53 | −2.08 | −3.15 | −2.07 |
| Al3+ | −3.59 | −2.09 | −3.94 | −2.08 | −3.56 | −2.09 |
| Fe3+ | −0.21 | −2.07 | −3.64 | −2.07 | −0.43 | −2.10 |
| Glycine | - | −2.05 | - | −2.06 | - | −2.10 |
| Glucose | - | −2.07 | - | −2.12 | - | −2.07 |
| Maltose | - | −2.09 | - | −2.13 | - | −2.06 |
| Lactose | - | −2.09 | - | −2.09 | - | −2.06 |
| Samples | LF Conc. | CPE (Sensor 11) | C-CPE (Sensor 13) | P-CPE (Sensor 16) |
|---|---|---|---|---|
| Pure solution | 10−3 M | 100.1 ± 0.1 | 99.8 ± 0.1 | 99.7 ± 0.2 |
| 10−4 M | 100.3 ± 0.2 | 100.2 ± 0.3 | 100.1 ± 0.3 | |
| Pharm. solution | 10−3 M | 99.8 ± 0.2 | 95.0 ± 0.3 | 99.1 ± 0.5 |
| 10−4 M | 94.4 ± 0.5 | 93.0 ± 0.5 | 94.4 ± 0.3 | |
| serum solution | 10−3 M | 99.7 ± 0.5 | 99.5 ± 0.8 | 98.5 ± 0.6 |
| 10−4 M | 93.5 ± 0.8 | 99.6 ± 0.7 | 99.2 ± 0.3 | |
| Urine solution | 10−3 M | 99.2 ± 0.9 | 101.0 ± 0.7 | 95.5 ± 0.7 |
| 10−4 M | 95.5 ± 0.8 | 94.4 ± 0.9 | 93.5 ± 0.8 |
| Samples | LF Conc. | CPE (Sensor 11) | C-CPE (Sensor 13) | P-CPE (Sensor 16) |
|---|---|---|---|---|
| Pure solution | 10−3 M | 99.7 ± 0.1 | 101.3 ± 0.1 | 100.1 ± 0.1 |
| 10−4 M | 99.3 ± 0.1 | 99.7 ± 0.2 | 99.2 ± 0.2 | |
| Pharm. solution | 10−3 M | 99.0 ± 0.1 | 89.2 ± 0.5 | 93. 3 ± 0.3 |
| 10−4 M | 98.1 ± 0.3 | 94.4 ± 0.3 | 100.0 ± 0.2 | |
| serum solution | 10−3 M | 98.7 ± 0.7 | 100.0 ± 0.6 | 87.1 ± 0.6 |
| 10−4 M | 95.6 ± 0.4 | 95.5 ± 0.5 | 100.0 ± 0.5 | |
| Urine solution | 10−3 M | 98.4 ± 0.4 | 100.0 ± 0.8 | 100.0 ± 0.7 |
| 10−4 M | 94.5 ± 0.9 | 95.5 ± 0.8 | 100.1 ± 0.8 |
| Comparison | This work | Rizka et al. [30] | AbdelGhani et al. [31] | ||||
|---|---|---|---|---|---|---|---|
| CPE (Sensor 11) | C-CPE (Sensor 13) | P-CPE (Sensor 16) | Le-Re | Le-FI | CPE | SPE | |
| Slope, mV/conc. Decade | 49.3 | 50.2 | 53.5 | 50.3 | 48.5 | 51.5 | 44.0 |
| Detection limit, M | 1.0 × 10−5 | 7.3 × 10−5 | 6.3 × 10−5 | 1.0 × 10−4 | 1.0 × 10−4 | 5.0 × 10−5 | 2.5 × 10−5 |
| Linear dynamic range, M | 10−2–10−5 | 10−2–10−4 | 10−2–10−4 | 10−2–10−4 | 10−2–10−4 | 10−2–10−4 | 10−2–10−4 |
| Lifetime, days | 14 | 14 | 5 | - | - | 7 | 7 |
| −0.21 | −3.64 | −0.43 | −1.18 | −1.67 | −0.66 | −1.78 | |
| −3.23 | −3.35 | −3.15 | −2.29 | −1.39 | −3.08 | −3.31 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Haleem, F.M.; Mahmoud, S.; Abdel-Ghani, N.E.T.; El Nashar, R.M.; Bechelany, M.; Barhoum, A. Polyvinyl Chloride Modified Carbon Paste Electrodes for Sensitive Determination of Levofloxacin Drug in Serum, Urine, and Pharmaceutical Formulations. Sensors 2021, 21, 3150. https://doi.org/10.3390/s21093150
Abdel-Haleem FM, Mahmoud S, Abdel-Ghani NET, El Nashar RM, Bechelany M, Barhoum A. Polyvinyl Chloride Modified Carbon Paste Electrodes for Sensitive Determination of Levofloxacin Drug in Serum, Urine, and Pharmaceutical Formulations. Sensors. 2021; 21(9):3150. https://doi.org/10.3390/s21093150
Chicago/Turabian StyleAbdel-Haleem, Fatehy M., Sonia Mahmoud, Nour Eldin T. Abdel-Ghani, Rasha Mohamed El Nashar, Mikhael Bechelany, and Ahmed Barhoum. 2021. "Polyvinyl Chloride Modified Carbon Paste Electrodes for Sensitive Determination of Levofloxacin Drug in Serum, Urine, and Pharmaceutical Formulations" Sensors 21, no. 9: 3150. https://doi.org/10.3390/s21093150
APA StyleAbdel-Haleem, F. M., Mahmoud, S., Abdel-Ghani, N. E. T., El Nashar, R. M., Bechelany, M., & Barhoum, A. (2021). Polyvinyl Chloride Modified Carbon Paste Electrodes for Sensitive Determination of Levofloxacin Drug in Serum, Urine, and Pharmaceutical Formulations. Sensors, 21(9), 3150. https://doi.org/10.3390/s21093150








