Short- and Long-Term Effects of a Scapular-Focused Exercise Protocol for Patients with Shoulder Dysfunctions—A Prospective Cohort
Abstract
:1. Introduction
- After 4-weeks of treatment, the protocol would lead to an amelioration in both groups in pain and function (decrease in the shoulder pain and disability index (SPADI) [32] levels with a minimal clinically important difference (MCID) ranging from 8 to 13 points) [33]; decrease in the numeric pain rating scale (NPRS) level of at least a MCID of 2.17 points [34]; and decrease in the disabilities of the arm, shoulder, and hand (DASH) levels with a MCID of 10.2 points [35].
- Primary outcome ameliorations (pain reduction and function improvement) made at the 4-week assessment would be retained at the 2-year assessment in both groups.
2. Materials and Methods
2.1. Study Design
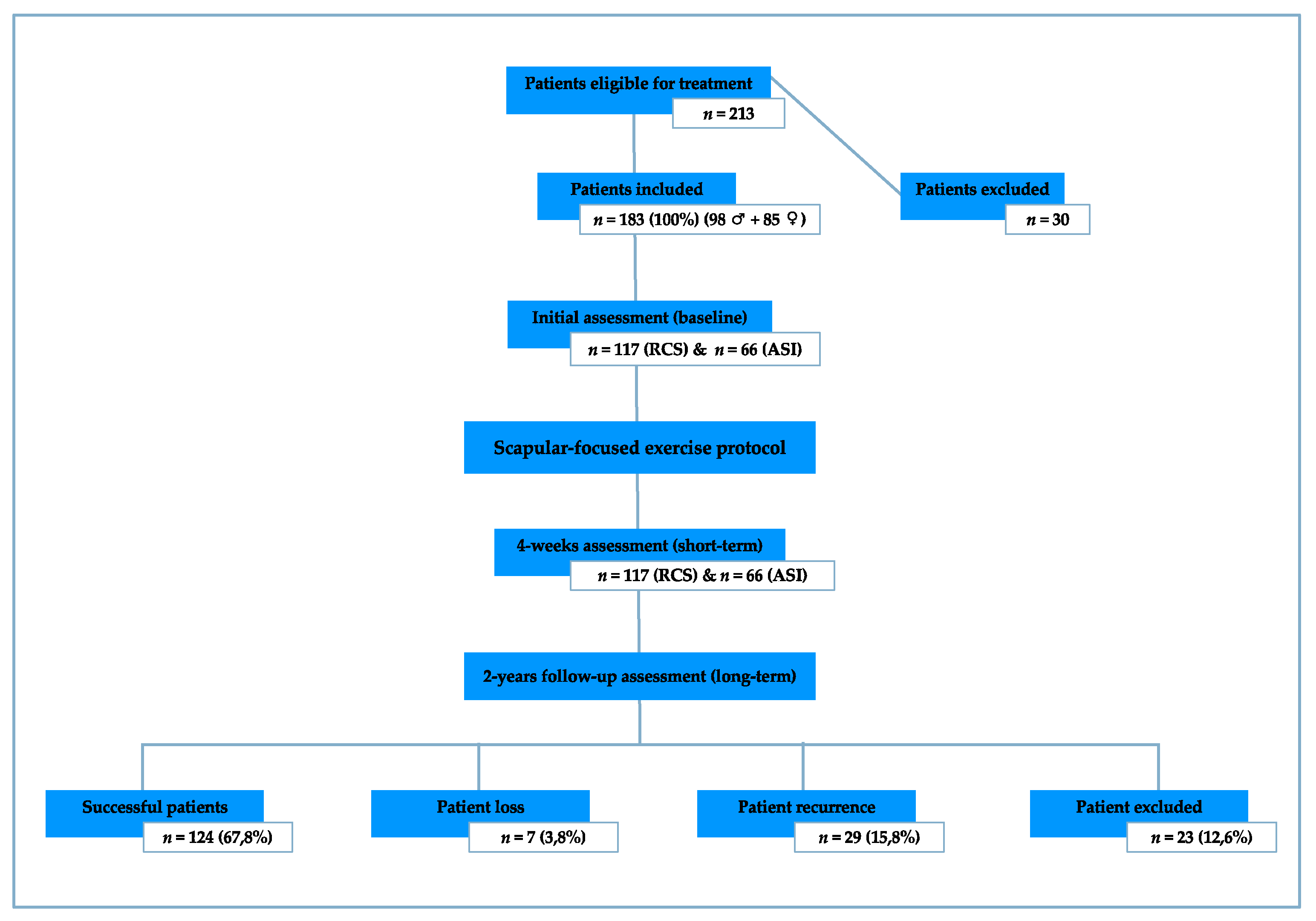
2.2. Sample
2.3. Diagnostic Criteria
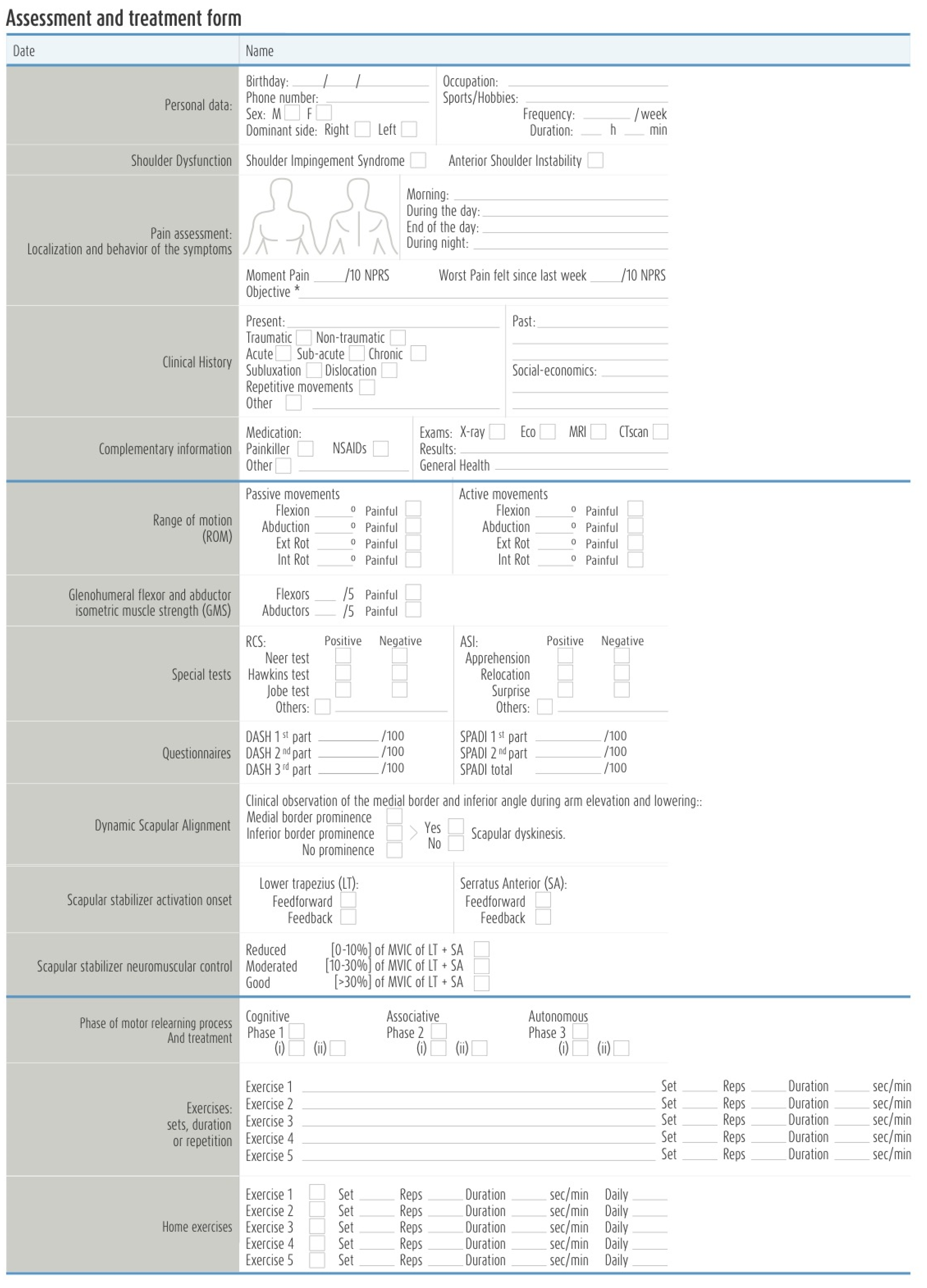
2.4. Testing Procedure
2.5. Treatment Protocol
2.6. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Detailed Description of the Scapular-Focused Exercise Protocol
| Muscle | Placement of the Electrodes | Position | Normalization: Muscular Action to Measure the Maximum Voluntary Isometric Contraction |
|---|---|---|---|
| Upper Trapezius [76,77] | Between C7 spinous process and the lateral tip of the acromion | Sitting position with no back support. Shoulder abducted to 90° (no abduction in the case of pain) with the neck side-bent to the same side, rotated to the opposite side | Pressure applied to extend the head above the elbow (or to shoulder elevation in the case of pain) |
| Lower Trapezius [76] | At 2/3 on the line from the root of the spine of the scapula to the 8th thoracic vertebra | Sitting position with no back support Arm raised above the head in line with the lower trapezius muscle | Pressure applied against the arm elevation |
| Serratus Anterior [76,77] | Vertically along the mid-axillary line at the 6th rib through the 8th rib | Sitting position with no back support. Shoulder abducted to 125° in the scapular plane | Pressure applied above the elbow and at the inferior angle of the scapula attempting to de-rotate the scapula |
| Anterior Deltoid [76] | At one finger width distal and anterior to the acromion | Sitting position with no back support. Place the humerus in a slight external rotation to increase the effect of gravity on the anterior fibers | Pressure applied on the antero-medial surface of the arm, against abduction and flexion |

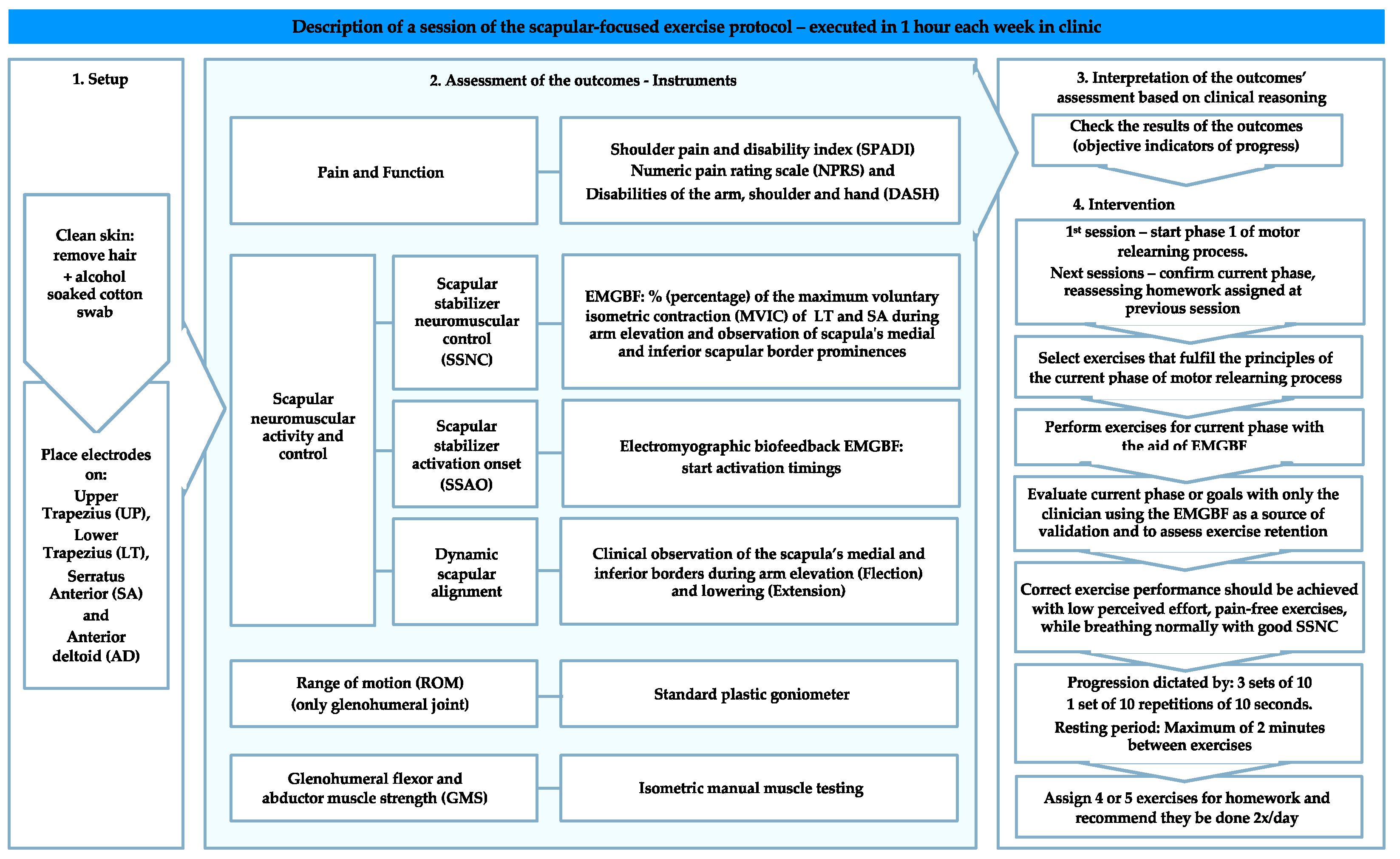
| Motor Relearning Phase | Phases Description and Purpose | Progression |
|---|---|---|
| Phase 1 | Facilitate patient pain-free awareness and dynamic control of scapulothoracic neutral zone through its stabilizers’ co-activation, namely, LT and SA, with a minimum participation of UT (or other scapulothoracic, glenohumeral, and spinal muscles) | (i) Patient should be able to activate scapular stabilizer muscles and dissociate their activation from other scapulothoracic, glenohumeral, and spinal muscles without pain provocation; (ii) Be capable of moving the scapula from different postural orientations and positions to its neutral zone (maintaining this position) through the co-activation of its scapular stabilizers in low-load exercises without pain provocation. |
| Phase 2 | Progressively integrate scapular neuromuscular activity and control skills gained in Phase 1 during pain-free directional shoulder movements. It is currently accepted that the scapula axis of rotation changes with the increasing arm elevation and plane of movement [79]. This implies the integration of LT and SA co-activation with other scapulothoracic force generators, such as UT, and simultaneous coordination with glenohumeral muscles. | (i) Maintain scapulothoracic neutral zone by activating its stabilizers while raising (fexion) the arm (<30°) in different elevation planes, the primary aim of this stage being the focus on the scapular neuromuscular activity and control setting phase [80,81]; (ii) Arm elevation movements (>30°) should be chosen so that their primary elevation plane or direction matches that of symptom producing movements and progressively explored through the available pain-free glenohumeral ROM (concentrically and eccentrically). |
| Phase 3 | Expected learning transfer of motor skills acquired in Phases 1 and 2 to functional activities. | (i) Fragmenting daily living activities into less complex achievable movements that can be progressively trained; (ii) and During normal function, occupational, recreational, and sports activities. |
| Progression Guidelines: | |
|---|---|
| Exercise complexity | Two possible sources: (i) Mechanical load, which included exercise variations that required greater arm elevation angles or the use of weights;(ii) Task or motor planning-control difficulty, which involved tasks and exercises in which it is necessary to incorporate both feedforward and feedback mechanisms of motor performance [58,84]. |
| Feedback from the EMGBF | Provided during all sessions to facilitate the best performance at each step. However, to progress to the next exercise or phase, the patient had to demonstrate their capability to reproduce the same performance without visual feedback. At this stage, EMGBF was used by the clinician to confirm the correct exercise performance. |
| Perceived effort | Although a high-perceived effort is acceptable at the beginning of each phase or while increasing exercise complexity, correct exercise performance should be achieved with low perceived effort, pain-free exercise performance, and with normal breathing. |
| Sets, repetitions and endurance | In the absence of normative data for endurance, exercises for this population were progressed when the patient could perform three sets of 10 repetitions or hold the specified position for one set of 10 repetitions of 10 s with no pain, low perceived effort (although a high-perceived effort is acceptable at the beginning of each phase or while increasing exercise complexity), normal breathing, and good SSNC. Note, while this arbitrary performance criteria was effective for this population, the number of sets, repetitions or holding time goal for progression will vary with different patient groups according to sport, work, and lifestyle requirements. |
| Resting time between exercises | Although patients were encouraged to rest the least time possible between exercises, they could rest for a maximum of 2 min between exercises (especially high-loaded) but not between sets or repetitions [65]. |

References
- Lewis, J. Rotator cuff related shoulder pain: Assessment, management and uncertainties. Man. Ther. 2016, 23, 57–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diercks, R.; Bron, C.; Dorrestijn, O.; Meskers, C.; Naber, R.; de Ruiter, T.; Willems, J.; Winters, J.; van der Woude, H.J. Guideline for diagnosis and treatment of subacromial pain syndrome. Acta Orthop. 2014, 85, 314–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, K.; Growse, A.; Korda, L.; Wray, E.; MacDermid, J.C. The effectiveness of rehabilitation for nonoperative management of shoulder instability: A systematic review. J. Hand Ther. 2004, 17, 229–242. [Google Scholar] [CrossRef]
- Michener, L.; McClure, P.; Karduna, A. Anatomical and biomechanical mechanisms of subacromial impingement syndrome. Clin. Biomech. 2003, 18, 369–379. [Google Scholar] [CrossRef]
- Bae, Y.; Lee, G.; Shin, W. Effect of motor control and strengthening exercises on pain, function, strength and the range of motion of patients with shoulder impingement syndrome. J. Phys. Ther. Sci. 2011, 17, 687–692. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Savin, D.; Shah, N.; Bronsnick, D.; Goldberg, B. Scapular Winging: Evaluation and Trearment. J. Bone Joint Surg. Am. 2015, 97, 1708–1716. [Google Scholar] [CrossRef]
- Bateman, M.; Smith, B.; Osborne, S.; Wilkes, S. Physiotherapy treatment for atraumatic recurrent shoulder instability: Early results of a specific exercise protocol using pathology-specific outcome measures. Shoulder Elbow 2015, 7, 282–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, L.; Lafosse, L.; Garrigues, G. Global Perspectives on Management of Shoulder Instability: Decision Making and Treatment. Orthop. Clin. N. Am. 2019, 51, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Warby, S.; Ford, J.; Hahne, A.; Watson, L.; Balster, S.; Lenssen, R.; Pizzari, T. Comparison of 2 Exercise Rehabilitation Programs for Multidirectional Instability of the Glenohumeral Joint: A Randomized Controlled Trial. Am. J. Sports Med. 2018, 46, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Galace De Freitas, D.; Marcondes, F.; Monteiro, R.; Gonçalves Rosa, S.; de Moraes Barros Fucs, P.M.; Yukio Fukuda, T. Pulsed electromagnetic field and exercises in patients with shoulder impingement syndrome: A randomized, double-blind, placebo-controlled clinical trial. Arch. Phys. Med. Rehabil. 2014, 95, 345–352. [Google Scholar] [CrossRef]
- Beaudreuil, J.; Lasbleiz, S.; Richette, P.; Seguin, G.; Rastel, C.; Aout, M.; Vicaut, E.; Cohen-Solal, M.; Lioté, F.; de Vernejoul, M.-C.; et al. Assessment of dynamic humeral centering in shoulder pain with impingement syndrome: A randomised clinical trial. Ann. Rheum. Dis. 2011, 70, 173–179. [Google Scholar] [CrossRef]
- Cools, A.; Borms, D.; Castelein, B.; Vanderstukken, F.; Johansson, F.R. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Warby, S.; Pizzari, T.; Ford, J.; Hahne, A.J.; Watson, L. The effect of exercise-based management for multidirectional instability of the glenohumeral joint: A systematic review. J. Shoulder Elbow Surg. 2014, 23, 128–142. [Google Scholar] [CrossRef] [PubMed]
- McClure, P.; Greenberg, E.; Kareha, S. Evaluation and management of scapular dysfunction. Sports Med. Arthrosc. 2012, 20, 39–48. [Google Scholar] [CrossRef]
- Kibler, W.; Ludewig, P.; McClure, P.; Uhl, T.L.; Sciascia, A. Scapular Summit 2009: Introduction, 16 July 16, 2009, Lexington, Kentucky. J. Orthop. Sports Phys. Ther. 2009, 39, A1–A13. [Google Scholar] [CrossRef] [PubMed]
- Kibler, W.; Ludewig, P.; McClure, P.; Michener, L.A.; Bak, K.; Sciascia, A.D. Clinical implications of scapular dyskinesis in shoulder injury: The 2013 consensus statement from the 'scapular summit'. Br. J. Sports Med. 2013, 47, 877–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratcliffe, E.; Pickering, S.; McLean, S.; Lewis, J. Is there a relationship between subacromial impingement syndrome and scapular orientation? A systematic review. Br. J. Sports Med. 2014, 48, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Struyf, F.; Nijs, J.; Mollekens, S.; Jeurissen, I.; Truijen, S.; Mottram, S.; Meeusen, R. Scapular-focused treatment in patients with shoulder impingement syndrome: A randomized clinical trial. Clin. Rheumatol. 2013, 32, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Haahr, J.P.; Østergaard, S.; Dalsgaard, J.; Norup, K.; Frost, P.; Lausen, S.; Holm, E.A.; Andersen, J.H. Exercises versus arthroscopic decompression in patients with subacromial impingement: A randomised, controlled study in 90 cases with a one year follow up. Ann. Rheum. Dis. 2005, 64, 760–764. [Google Scholar] [CrossRef] [Green Version]
- Holmgren, T.; Oberg, B.; Sjöberg, I.; Johansson, K. Supervised strengthening exercises versus home-based movement exercises after arthroscopic acromioplasty: A randomized clinical trial. J. Rehabil. Med. 2012, 44, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Reijneveld, E.; Noten, S.; Michener, L.; Cools, A.; Struyf, F. Clinical outcomes of scapular-focused treatment in patients with subacromial pain syndrome: Systematic review. Br. J. Sports Med. 2017, 51, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Bateman, M.; Osborne, S.; Smith, B. Physiotherapy treatment for atraumatic recurrent shoulder instability: Updated results of the Derby Shoulder Instability Rehabilitation Programme. J. Arthrosc. Joint Surg. 2019, 6, 35–41. [Google Scholar] [CrossRef]
- Eshoj, H.; Rasmussen, S.; Frich, L.; Hvass, I.; Christensen, R.; Boyle, E.; Lund Jensen, S.; Søndergaard, J.; Søgaard, K.; Juul-Kristensen, B. Neuromuscular Exercises Improve Shoulder Function More Than Standard Care Exercises in Patients With a Traumatic Anterior Shoulder Dislocation. Orthop. J. Sports Med. 2020, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juul-Kristensen, B.; Larsen, C.M.; Eshoj, H.; Clemmensen, T.; Hansen, A.; Bo Jensen, P.; Boyle, E.; Søgaard, K. Positive effects of neuromuscular shoulder exercises with or without EMG-biofeedback, on pain and function in participants with subacromial pain syndrome—A randomised controlled trial. J. Electromyogr. Kinesiol. 2019, 48, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, Z.; Baskurt, F.; Gelecek, N.; Özkan, M.H. The effectiveness of scapular stabilization exercise in the patients with subacromial impingement syndrome. J. Back Musculoskelet. Rehabil. 2011, 24, 173–179. [Google Scholar] [CrossRef] [PubMed]
- DeMey, K.; Danneels, L.; Cagnie, B.; Cools, A. Scapular muscle rehabilitation exercises in overhead athletes with impingement symptoms: Effect of a 6-week training program on muscle recruitment and functional outcome. Am. J. Sports Med. 2012, 40, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Lin, J.J.; Leon Guo, Y.; Wang, W.T.; Chen, Y.-J. EMG biofeedback effectiveness to alter muscle activity pattern and scapular kinematics in subjects with and without shoulder impingement. J. Electromyogr. Kinesiol. 2013, 23, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.; Søgaard, K.; Chreiteh, S.; Holtermann, A.; Juul-Kristensen, B. Neuromuscular control of scapula muscles during a voluntary task in subjects with subacromial impingement syndrome. A case-control study. J. Electromyogr. Kinesiol. 2013, 23, 1158–1165. [Google Scholar] [CrossRef]
- Worsley, P.; Warner, M.; Mottram, S.; Gadola, S.; Veeger, H.E.J.; Hermens, H.; Morrissey, D.; Little, P.; Cooper, C.; Carr, A.; et al. Motor control retraining exercises for shoulder impingement: Effects on function, muscle activation, and biomechanics in young adults. J. Shoulder Elbow Surg. 2013, 22, e11-9. [Google Scholar] [CrossRef] [Green Version]
- Dorrestijn, O.; Stevens, M.; Winters, J.; van der Meer, K.; Diercks, R.L. Conservative or surgical treatment for subacromial impingement syndrome? A systematic review. J. Shoulder Elbow Surg. 2009, 18, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Struyf, F.; Cagnie, B.; Cools, A.; Baert, I.; Van Brempt, J.; Struyf, P.; Meeus, M. Scapulathoracic muscle activity and recruitment timing in patients with shoulder impingement symptoms and glenohumeral instability. J. Electomiogr. Kinesiol. 2014, 24, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Roach, K.; Budiman-Mak, E.; Songsiridej, N.; Lertratanakul, Y. Development of a Shoulder Pain and Disability Index. Arthritis Care Res. 1991, 4, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; MacDermid, J.; Woodhouse, L. Measuring Shoulder Function: A Systematic Review of Four Questionnaires. Arthritid. Rheum. 2009, 61, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Michener, L.; Snyder, A.; Leggin, B. Responsiveness of the numeric pain rating scale in patients with shoulder pain and the effect of surgical status. J. Sports Rehabil. 2011, 20, 115–128. [Google Scholar] [CrossRef]
- Hudak, P.; Amadio, P.; Bombardier, C. Development of an Upper Extremity Outcome Measure: The DASH (Disabilities of the Arm, Shoulder and Hand)[Corrected]. The Upper Extremity Collaborative Group (UECG). J. Sports Rehabil. 2011, 20, 115–128. [Google Scholar]
- Ludewig, P.; Cook, T. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys. Ther. 2000, 80, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L. Rotator cuff tendinopathy/subacromial impingement syndrome: Is it time for a new method of assessment? Br. J. Sports Med. 2009, 43, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, C.-J.; Jan, M.-H.; Lin, Y.-F.; Wang, T.-Q.; Liu, J.-J. Scapular kinematics and impairment features for classifying patients with subacromial impingement syndrome. Man. Ther. 2010, 15, 547–551. [Google Scholar] [CrossRef]
- Dong, W.; Goost, H.; Xang-Bo, L.; Burger, C.; Paul, C.; Wang, Z.-L.; Zhang, T.-Y.; Jiang, Z.-C.; Welle, K.; Kabir, K. Treatment for Shoulder Impingement Syndrome. A PRISMA Systematic Review and Network Meta-Analysis. Medicine 2015, 94, e510. [Google Scholar] [CrossRef]
- Haik, M.; Alburquerque-Sendín, F.; Silva, C.; Siqueira-Junior, A.L.; Ribeiro, I.L.; Camargo, P.R. Scapular-kinematics pre-and post-thoracic thrust manipulation in individuals with and without shoulder impingement symptoms: A randomised controlled study. J. Orthop. Sports Phys. Ther. 2014, 44, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Tate, A.; McClure, P.; Young, I.; Salvatori, R.; Michener, L.A. Comprehensive impairment-based exercise and manual therapy intervention for patients with subacromial impingement syndrome: A case series. J. Orthop. Sports Phys. Ther. 2010, 40, 474–493. [Google Scholar] [CrossRef] [PubMed]
- Neer, C. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: A preliminary report. J. Bone Joint Surg. Am. 1972, 54, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.; Kennedy, J. Impingement syndrome in athletes. Am. J. Sports Med. 1980, 8, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Jobe, F.W.; Moynes, D. Delineation of diagnostic criteria and a rehabilitation program for rotator cuff injuries. Am. J. Sports Med. 1982, 10, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, E.J.; Goode, A.; Campbell, S.; Morin, A.; Tamaddoni, M.; Moorman, C.T., III; Cook, C. Physical examination tests of the shoulder: A systematic review with meta-analysis of individual tests. Br. J. Sports Med. 2008, 42, 80–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, K.; Callanan, M.; Walton, J.; Paxinos, A.; Murrell, G.A.C. Shoulder instability: Management and rehabilitation. J. Orthop. Sports Phys. Ther. 2002, 32, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, E.J.; Goode, A.; Cook, C.; Michener, L.; Myer, C.A.; Myer, D.M.; Wright, A.A. Which physical examination tests provide clinicians with the most value when examining the shoulder? Update of a systematic review with meta-analysis of individual tests. Br. J. Sports Med. 2012, 46, 964–978. [Google Scholar] [CrossRef] [Green Version]
- Huskisson, E. Measurement of pain. J. Rheumatol. 1982, 9, 768–769. [Google Scholar] [CrossRef]
- Norkin, C.; White, D. The shoulder. In Measurement of Joint Motion: A Guide to Goniometry, 4th ed.; F.A. Davis Company: Philadelphia, PA, USA, 2009; pp. 57–90. [Google Scholar]
- Kolber, M.; Hanney, W. The reliability and concurrent validity of shoulder mobility measurement using a digital inclinometer and goniometer: A technical report. Int. J. Sports Phys. Ther. 2012, 7, 306–313. [Google Scholar]
- Celik, D.; Dirican, A.; Baltaci, G.; Layman, J. Intrarater reliability of assessing strength of the shoulder and scapular muscles. J. Sport Rehabil. 2012, 21, 1–5. [Google Scholar] [CrossRef]
- Kendall, F.; McCreary, E.; Provance, P. Upper extremity and shoulder girdle strength tests. In Muscles: Testing and Function, with Posture and Pain, 4th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 1993; pp. 235–298. [Google Scholar]
- Shumway-Cook, A.; Woolacott, J. Motor learning and recovery of function. In Motor Control: Theory and Practical Applications, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Comerford, M.; Mottram, S. Functional stability re-training: Principles and strategies for managing mechanical dysfunction. Man. Ther. 2001, 6, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.A.; Rivett, D.A. Clinical Reasoning: Fast and Slow Thinking in Musculoskeletal Practice. In Clinical Reasoning in Musculoskeletal Practice, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 2–31. [Google Scholar]
- Salamh, P.; Lewis, J. It is time to put special tests for rotator cuff-related shoulder pain out to posture. J. Orthop. Sports Phys. Ther. 2020, 50, 222–225. [Google Scholar] [CrossRef]
- Hodges, P. Pain and motor control: From the laboratory to rehabilitation. J. Electromyogr. Kinesiol. 2011, 21, 220–228. [Google Scholar] [CrossRef]
- Glover, S. Separate visual representations in the planning and control of action. Behav. Brain Sci. 2004, 27, 3–78. [Google Scholar] [CrossRef] [Green Version]
- Roy, J.; Moffet, H.; Hébert, L.; Lirette, R. Effect of motor control and strengthening exercises on shoulder function in persons with impingement syndrome: A single-subject study design. Man. Ther. 2009, 14, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Moffet, H.; McFadyen, B.; Lirette, R. Impact of movement training on upper limb motor strategies in persons with shoulder impingement syndrome. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2009, 17, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aurin, A.; Latash, M. Directional specificity of postural muscles in feed-forward postural reactions during fast voluntary arm movements. Exp. Brain Res. 1995, 103, 323–332. [Google Scholar] [CrossRef]
- Ludewig, P.; Reynolds, J. The association of scapular kinematics and glenohumeral joint pathologies. J. Orthop. Sports Phys. Ther. 2009, 39, 90–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraes, G.; Faria, C.; Teixeira-Salmela, L. Scapular muscle recruitment patterns and isokinetic strength ratios of the shoulder rotator muscles in individuals with and without impingement syndrome. J. Shoulder Elbow Surg. 2008, 17, S48–S53. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; Tucker, K. Moving differently in pain: A new theory to explain the adaptation to pain. Pain 2011, 152, S90–S98. [Google Scholar] [CrossRef]
- Crow, J.; Pizzari, J.; Buttifani, D. Muscle onset can be improved by therapeutic exercise: A systematic review. Phys. Ther. Sport 2011, 12, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.; Hodges, P. Persistence of improvements in postural strategies following motor control training in people with recurrent low back pain. J. Electromyogr. Kinesiol. 2008, 18, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; van Dillen, L.R.; McGill, S.; Brumagne, S.; Hides, J.A.; Moseley, G.L. Integrated clinical approach to motor control interventions in low back and pelvic pain. In Spinal Control: The Rehabilitation of Back Pain; Churchill Livingstone: London, UK, 2013; pp. 244–306. [Google Scholar]
- Berkovitch, Y.; Shapira, J.; Haddad, M.; Keren, Y.; Rosenberg, N. Current clinical trends in first time traumatic anterior shoulder dislocation. Merit. Res. J. Med. Med. Sci. 2013, 1, 7–13. [Google Scholar]
- Lewis, J. Subacromial impingement syndrome: A musculoskeletal condition or a clinical illusion? Phys. Ther. Rev. 2011, 16, 388–398. [Google Scholar] [CrossRef]
- Steuri, R.; Sattelmayer, M.; Elsig, S. Effectiveness of conservative interventions including exercise, manual therapy and medical management in adults with shoulder impingement: A systematic review and meta-analysis of RCTs. Br. J. Sports Med. 2017, 18, 1340–1347. [Google Scholar] [CrossRef] [Green Version]
- Hanrraty, C.; McVeigh, J.; Kerr, D.; Basford, J.R.; Finch, M.B.; Pendleton, A.; Sim, J. The effectiveness of physiotherapy exercises in subacromial impingement syndrome: A systematic review and meta-analysis. Semin. Arthritis. Rheum. 2012, 42, 297–316. [Google Scholar] [CrossRef]
- Haik, M.; Alburquerque-Sendín, F.; Moreira, R.; Pires, E.D.; Camargo, P.R. Effectiveness of physical therapy treatment of clearly defined subacromial pain: A systematic review of randomised controlled trials. Br. J. Sports Med. 2016, 50, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Bury, J.; West, M.; Chamorro-Moriana, G.; Littlewood, C. Effectiveness of scapula-focused approaches in patients with rotator cuff related shoulder pain: A systematic review and meta-analysis. Man. Ther. 2016, 25, 35–42. [Google Scholar] [CrossRef]
- Matias, R.; Jones, M. Incorporating Biomechanical Data in the Analysis of a University Student With Shoulder Pain and Scapula Dyskinesis. In Clinical Reasoning in Musculoskeletal Practice; Elsevier: Amestradam, The Netherlands, 2019; pp. 483–503. [Google Scholar]
- Ferreira, A.L.; dos Santos, C.; Matias, R. A kinematic biofeedback-assisted scapular-focused intervention reduces pain, and improves functioning and scapular dynamic control in patients with shoulder dysfunction. Gait Posture 2016, 49, 277. [Google Scholar]
- Ekstrom, R.; Soderberg, G.; Donatelli, R. Normalization procedures using maximum voluntary isometric contractions for the serratus anterior and trapezius muscles during surface EMG analysis. J. Electromyogr. Kinesiol. 2005, 15, 418–428. [Google Scholar] [CrossRef]
- Hermens, H.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hagg, G. Eurpoean Recommendations for Surface Electromyography—Results of the Seniam Project. SENIAM 8, 2nd ed.; Roessingh Research and Development: Enschede, The Netherlands, 1999; pp. 27–30. [Google Scholar]
- Gamboa, H.; Matias, R.; Araújo, T.; Veloso, A. Electromyography onset detection: New methodology. J. Biomech. 2012, 45, S494. [Google Scholar] [CrossRef]
- Bagg, S.; Forrest, W. Electromyographic study of the scapular rotators during arm abduction in the scapular plane. Am. J. Phys. Med. 1986, 65, 111–124. [Google Scholar] [PubMed]
- Borsa, P.; Timmons, M.; Sauers, E. Scapular-positioning patterns during humeral elevation in unimpaired shoulders. J. Athl. Train. 2003, 38, 12–17. [Google Scholar] [PubMed]
- Inman, V.; Sauders, J.; Abbott, L. Observations of the function of the shoulder joint. Clin. Orthop. Relat. Res. 1996, 330, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.; Arena, R.; Riebe, D.; Thompson, P. Behavioral Theories and Strategies for promoting exercise. In ACSM's Guidelines for Exercise Testing and Prescription, 9th ed.; Wolkers Kluwer / Lippincott Williams & Walkins: Baltimore, MD, USA, 2014; pp. 355–365. [Google Scholar]
- Baechle, T.; Earle, R. Training variation: Periodization. In Essentials of Strength Training and Conditioning, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2000; pp. 513–527. [Google Scholar]
- Desmurget, M.; Grafton, S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn. Sci. 2000, 4, 423–431. [Google Scholar] [CrossRef]
| RCS Group (n = 117) | ASI Group (n = 66) | ||
|---|---|---|---|
| Age (mean (SD)) | 41.1 (12.2) | 26.7 (10.3) ** | |
| Sex (%) | Female | 48 (41.0) | 37 (56.1) |
| Male | 69 (59.0) | 29 (43.9) | |
| Origin of symptoms (%) | Trauma | 30 (25.6) | 32 (48.5) ** |
| Non-traumatic | 18 (15.4) | 0 (0.0) ** | |
| Overuse | 69 (59.0) | 27 (40.9) ** | |
| Sub or Dislocation | 0 (0.0) | 7 (10.6) ** | |
| Length of symptoms (%) | Acute (0–2 weeks) | 3 (2.6) | 6 (9.1) |
| Sub-acute (2–6 weeks) | 19 (16.2) | 13 (19.7) | |
| Chronic (+6 weeks) | 95 (81.2) | 47 (71.2) | |
| Symptomatic side (%) | Dominant | 80 (68.4) | 53 (80.3) * |
| Non-Dominant | 34 (29.0) | 9 (13.6) * | |
| Bilateral | 3 (2.6) | 4 (6.1) * | |
| Outcome | Goal | Instrument | MCID | Assessment Procedures | |
|---|---|---|---|---|---|
| Pain and Function | Determine pain intensity between assessment moments and measure and monitor function and symptoms over time | SPADI [32] | ranging from 8 to 13 points [33] | Filling in the SPADI questionnaire | |
| NPRS [48] | 2.17 [34] | Patient asked to report the worst pain felt in the last week | |||
| DASH [35] | 10.2 [33] | Filling in the DASH questionnaire | |||
| Scapular neuromuscular activity and control | SSNC | Assess the muscular percentage of MVIC activity of LT, SA, and UT during arm elevation and lowering | EMGBF, PhysiopluxTM system version 1.06 | N/A | Actively raise (Flexion) then lower (Extension) the arm at a controlled self-paced velocity through maximum painless ROM in the sagittal, scapular, and frontal planes from a natural standing position for one set of three repetitions with a 20-s pause between repetitions |
| SSAO | Assess muscular activation onset during rapid active shoulder elevation | EMGBF, PhysiopluxTM system version 1.06 | N/A | Actively raise (Flexion) the arm as rapidly as possible, without exacerbating pain or discomfort, to a maximum arm elevation angle of 45° in the sagittal, scapular, and frontal planes from a natural standing position for one set of three repetitions with a 20-s pause between repetitions | |
| Dynamic Scapular Alignment | Detect scapular dyskinesis | Clinical observation of the scapular medial and inferior border [14] | N/A | Clinical observation of the scapular medial and inferior border behavior during the arm elevation (Flexion) and lowering (Extension) | |
| ROM | Assess glenohumeral ROM | Standard goniometer [49] | N/A | Normative ROM assessment with a standard goniometer | |
| GMS | Assess glenohumeral flexor and abductor muscle strength | Isometric manual muscle testing [52] | N/A | Measured in a sitting position with the arm at 90° in the sagittal and frontal planes, respectively. Manual resistance was applied against the forearm with the elbow extended. | |
| RCS Group | ASI Group | ||||||
|---|---|---|---|---|---|---|---|
| Initial (n = 117) | 4-Weeks (n = 117) | 2-Year Follow-Up (n = 93) | Initial (n = 66) | 4-Weeks (n = 66) | 2-Year Follow-Up (n = 54) | ||
| SPADI (0–100) | 42.07 ± 18.64 | 9.03 ± 8.21 ** | 8.62 ± 15.12 | 32.74 ± 19.50 ‡‡ | 4.80 ± 5.66 **‡‡ | 7.24 ± 15.78 ‡ | |
| NPRS (0–10) (Worst Pain felt) | 5.85 ± 1.97 | 1.58± 1.29 ** | 1.46± 2.05 | 5.27 ± 2.34 | 0.91 ± 1.16 **‡‡ | 1.21 ± 1.96 | |
| DASH 1st part (0–100 point) | 33.55 ± 16.53 | 7.63 ± 6.85 ** | 7.51 ± 12.92 | 28.47 ± 15.48 ‡ | 4.93 ± 5.78 **‡‡ | 4.37 ± 9.02 ‡ | |
| DASH 2nd part (0–100 point) | 10.69 ± 19.25 | 2.83 ± 6.84 ** | 1.58 ± 7.54 * | 8.60 ± 18.16 | 1.80 ± 4.63 ** | 0.22 ± 1.17 * | |
| DASH 3rd part (0–100 point) | 45.88 ± 29.01 | 12.50± 14.27 ** | 10.00± 17.59 | 53.80 ± 31.01 ‡ | 9.66 ± 12.61 ** | 8.15 ± 16.47 * | |
| SSNC | Diminished (poor or moderate) | 117 (100.00) | 78 (66.67) ** | 61 (65.59) * | 66 (100.00) | 39 (59.09) ** | 22 (47.74) * |
| Good | 0 (0.00) | 39 (33.33) ** | 32 (34.41) * | 0(0.00) | 27 (40.91) ** | 32 (59.26) * | |
| SSAO (ms) | Feedback | 59 (50.43) | 22 (18.80) ** | 18 (19.35) | 32 (48.48) | 8 (12.12) * | 7 (12.96) |
| Feedforward | 58 (49.57) | 95 (81.20) ** | 75 (80.65) | 34 (51.52) | 58 (87.88) * | 47 (87.04) | |
| Dynamic Scapular Alignment | “YES” scapula dyskinesis (IB, MB or both prominences) | 117 (100.00) | 85 (72.65) ** | 49 (52.69) * | 100 (100.00) | 43 (65.15) ** | 32 (59.26) * |
| “NO” scapula dyskinesis (no prominences) | 0 (0.00) | 32 (27.35) ** | 44 (47.31) * | 0 (0.00) | 23 (34.85) ** | 22 (40.74) * | |
| ROM | Decreased | 102 (87.18) | 13 (11.11) ** | 9 (9.68) | 51 (77.27) | 1 (1.52) ** | 1 (1.85) |
| Normal | 15 (12.82) | 104 (88.89) ** | 84 (90.32) | 15(22.73) | 65 (98.48) ** | 53 (98.15) | |
| GMS | Decreased | 114 (97.44) | 30 (25.64) ** | 19 (20.43) | 65 (98.48) | 13 (19.70) ** | 8 (14.81) |
| Normal | 3 (2.56) | 87 (74.36) ** | 74 (75.57) | 1 (1.52) | 53 (80.30) ** | 46 (85.19) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, C.; Jones, M.A.; Matias, R. Short- and Long-Term Effects of a Scapular-Focused Exercise Protocol for Patients with Shoulder Dysfunctions—A Prospective Cohort. Sensors 2021, 21, 2888. https://doi.org/10.3390/s21082888
dos Santos C, Jones MA, Matias R. Short- and Long-Term Effects of a Scapular-Focused Exercise Protocol for Patients with Shoulder Dysfunctions—A Prospective Cohort. Sensors. 2021; 21(8):2888. https://doi.org/10.3390/s21082888
Chicago/Turabian Styledos Santos, Cristina, Mark A. Jones, and Ricardo Matias. 2021. "Short- and Long-Term Effects of a Scapular-Focused Exercise Protocol for Patients with Shoulder Dysfunctions—A Prospective Cohort" Sensors 21, no. 8: 2888. https://doi.org/10.3390/s21082888
APA Styledos Santos, C., Jones, M. A., & Matias, R. (2021). Short- and Long-Term Effects of a Scapular-Focused Exercise Protocol for Patients with Shoulder Dysfunctions—A Prospective Cohort. Sensors, 21(8), 2888. https://doi.org/10.3390/s21082888






